Abstract
The effect of single versus multiple mating on longevity and fecundity as well as the number of matings required to maximize a female’s reproductive success of the predatory mite Kampimodromus aberrans Oudemans were studied under laboratory conditions. Newly emerged adult females of the stock colony of K. aberrans were placed individually on a bean leaf disc, and maintained at 25°C and 16:8 LD. A young male remained with a female for limited periods or continuously. Mating was a requisite for oocyte maturation and oviposition. Females which mated three to four times during their life and females in continuous presence of males, laid significantly and considerably more eggs than single-mated females. Virgin females lived the longest, and those in continuous presence of males the shortest. In all cases and irrespective of the number of matings, the sex ratio of the offspring was male-biased in the first three to four days of oviposition period, and female-biased in later days.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In insects and mites, females usually require one or very few matings in order to maximize their fitness, whereas males mate many times and their fitness increases with mate rate (Thornhill and Alcock 1983). However, in many cases females can mate many times and this may result in an increased fecundity and egg production. The favorable effect of multiple mating on female fitness may be due among others, to the sperm itself or to different accessory substances which are transferred during mating. By contrast, multiple mating may decrease female fitness due to the cost of mating that includes energy cost, risk of physical injury and parasite infection (Radwan and Rysińska 1999; Arnqvist and Nilsson 2000; Kolodziejczyk and Radwan 2003; Liana 2005).
The effect of multiple mating in the predatory mites of the family Phytoseiidae has been poorly studied. Several phytoseiid species require multiple mating, whereas in others a single mating suffices to maximize their reproductive output (Putman 1962; Prasad 1967; Elbadry and Elbenhawy 1968; Laing 1969; Zaher and Shehata 1971; Amano and Chant 1978a; Schulten et al. 1978; Hoy and Smilanick 1979; Overmeer et al. 1982; Momen 1993; Tsunoda and Amano 2001).
The phytoseiid mite Kampimodromus aberrans (Oudemans) is an important biological control agent of several plant-feeding mites such as Eotetranychus carpini (Oudemans) (Duso 1989; Schausberger 1997; Tixier et al. 1998, 2006; Kreiter et al. 2002) and Phytoptus avellanae Nalepa (Ozman-Sullivan 2006). In Greece, it is found on various forest and orchard trees as well as on non-cultivated plants and occasionally on vine (Papadoulis 1993; Koveos unpublished data). Few laboratory studies have been carried out on the life-history parameters of K. aberrans when fed on certain phytophagous mites and/or pollens (Schausberger 1997; Kasap 2005; Ozman-Sullivan 2006). The mating behavior of K. aberrans, and more specifically the movements of both females and males and the time spent in the different phases of mating, as well as the presence and number of spermatophores in the female spermathecae after mating were described by Pappas et al. (2005). In the present work, we studied the effects of single and multiple mating of females of K. aberrans on its fecundity and longevity. The rational behind these experiments was to determine whether multiple mating is beneficial for K. aberrans females, as well as the minimum number of mating required to maximize reproductive success under specific laboratory conditions. Such data may be useful for future mass rearing of K. aberrans and use in biological control programs.
Materials and methods
The mite stock colony
The laboratory stock colony of K. aberrans was established one month before starting the experiments with approximately 500 individuals collected from apple trees grown on the campus of the Aristotle University of Thessaloniki, during May 2003. Mites were maintained on detached bean leaves (Phaseolus vulgaris L.) placed on wet cotton wool in plastic cups with water and fed on Typha sp. pollen at 25°C and a photoperiod of 16:8 LD, as described by Pappas et al. (2005).
Experimental rearing units
For the experiments the mites were maintained individually on bean leaf discs 20 mm in diameter, deposited on wet cotton wool inside open cylindrical cells 22 mm in diameter of a polystyrene multiwell tissue culture plate (Corning®, NY), as described by Broufas and Koveos (2000). A sufficient quantity of Typha sp. pollen was placed on the leaf discs where a few cotton threads served as shelter and oviposition sites for the mites. Every three to four days the experimental mites were transferred with a camel hair brush to fresh leaf discs.
Mating frequency experiments
In all treatments newly molted virgin females and young males of K. aberrans have been used. For the experiments eggs laid within 12 h by females of the stock colony were transferred individually with a fine hair brush to the bean leaf discs of the rearing units and subsequently maintained at 25°C and 16:8 LD with Typha sp. pollen as food source. Every 12 h the leaf discs were inspected under a stereomicroscope and survivorship as well as the development stage of each individual were recorded. Upon adult emergence newly molted females and males were transferred individually to a fresh leaf disc. Subsequently, in order to test whether multiple mating is required to maximize female fecundity and to determine the required minimum number of mating, the following four treatments were tested.
a. Virgin females. Females were maintained individually without mate throughout their lifespan.
b. Single-mated females. Newly molted females were transferred with a male on a leaf disc of the experimental rearing units. The couple was observed every 5 min under a stereomicroscope (Leica MZ8, magnification 25X) with cool light. In all cases, after the first contact couples through a sequence of stereotypic behavior reached the venter-to-venter copulation position and remained at that stage for approximately 4 h. Based on earlier work, this mating duration was sufficient to complete copulation (Pappas et al. 2005). Following couple’s separation males were removed and females were maintained individually without a mate throughout their further lifespan.
c. Multiple-mated females with males added at intervals. In this treatment—following the procedure described under treatment b—females, after completing copulation, were kept individually and started laying eggs for a limited period of time. Five days after the end of this first egg-laying period a new male was introduced for a second mating to take place. Subsequently, the males were removed and a second oviposition period followed. We chose the time interval of five days to introduce a male because data of our previous work (Broufas et al. 2007), in which females had continued access to a male throughout their lifespan, have shown that if eggs were not laid within 5 days after the end of the oviposition period, no further egg would be laid. The same procedure was repeated three or four times, until the females displayed male-rejection behavior, i.e. females forced males away with their front pair of legs after they touched them or climbed on their dorsum (Hoy and Cave 1985; Pappas et al. 2005). Afterwards, another male was added to remain there till female’s death. If females of K. aberrans require more than one mating to achieve maximum egg production, each mating in this treatment may result in a resumption of egg laying.
d. Multiple-mated females with continuous access to males. Each virgin female was maintained with a male on each leaf disc of the experimental unit. The couple was observed under a stereomicroscope as described in treatment b to ensure a complete first mating. However, in this treatment following couple’s separation the male was not removed, but once a week was replaced by a new one. The purpose was to ensure the presence of young, sexually active males throughout the adult female’s life. The exact rate of mating in this group was not determined. However, for data analysis of this group we used fecundity of only those females which were observed in copula at least two times during their life.
In all treatments 40 females were used. Females that drowned in the water barrier surrounding the leaf discs or died due to improper handling were excluded from data analysis. Furthermore, since age and prior mating history may affect male’s fertility (Amano and Chant 1977) only young six-day-old virgin males were used in our experiments. These males were also reared and maintained individually in the same way as females in the experimental rearing units until use in any of the treatments.
In all cases a sufficient quantity of approximately 2 mg of Typha sp. pollen grains was added every other day on the surface of each leaf disc as food, and a rectangular piece of cotton wool as shelter and oviposition site for the mites. In all treatments, survival and fecundity of females were recorded every 24 h throughout their life.
To record the sex ratio of the progeny, in each treatment eggs were collected daily and transferred separately to a bean leaf disc (4 cm in diameter) on wet cotton wool in a plastic Petri dish (9 cm in diameter) at 25°C and 16:8 LD. Pollen of Typha sp. was added on the leaf surface as food for the hatched mites. When those mites reached the adult stage, their sex was recorded.
Statistical analysis
Differences in longevity and total fecundity between different groups were compared using ANOVA with the procedure of SPSS (2001). Log transformation of survival time and total fecundity were used to equal variances. A mean comparison test (Student-Newman-Keuls) was then applied (SPSS 2001). Pearson’s chi-square test was used to compare progeny sex ratio during oviposition period (Kenneth and Hardy 2002).
Results
Effect of mating frequency on longevity and fecundity
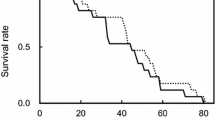
Virgin females lived on average significantly longer than single- or multiple-mated females (F = 26.65; d.f. = 3, 107; P < 0.001) (Table 1). Virgin females did not lay any eggs, whereas the single-mated females laid a low number of eggs. Longevity of females that had access to males three to four times during their adult life and had the same number of matings did not differ from that of single-mated females (F = 19.56; d.f. = 2, 81; P < 0.001). By contrast, females being continuously with males lived significantly and considerably shorter and laid more than twice as many eggs as single-mated ones (Table 1).
Single-mated females laid eggs for approximately 15 days (15.5 ± 0.3 days, n = 24) followed by a long post-oviposition period (66.6 ± 5.6 days, n = 24). Single-mated females lived on average 84.6 days and a few individuals (n = 8) survived for more than 100 d (Fig. 1b). Multiple-mated females with periodic access to males had four distinct oviposition periods of 10.6 ± 0.5 d (n = 27), 10.3 ± 0.6 days (n = 27), 8.7 ± 0.9 days (n = 21) and 7.2 ± 1.3 days (n = 10), respectively following each successive mating (Fig. 1c). The mean number of eggs laid during each of the four successive oviposition periods was 10.8 ± 0.8 (n = 27), 10.6 ± 0.7 (n = 27), 7.3 ± 0.8 (n = 21) and 4.9 ± 0.9 egg/female (n = 10), respectively. Attempts to have the surviving females remated with young males for a fifth time failed, all females ‘rejecting’ their mates and laying no more eggs. Multiple-mated females with continuous access to males had a mean ovipositon period of approximately 26.8 ± 2.9 days (min = 6 d, max = 73 days), whereas post-oviposition period was approximately 11.2 ± 3.5 days (min = 1 d, max = 50 days) (Fig. 1d).
Sex ratio of progeny
The sex ratio of progeny of single-mated or multiple-mated females with continuous access to males was strongly male-biased during the first three to four days of the oviposition period (4.09 < χ2 < 7.66; χ2 0.05;1 = 3.84), but was female-biased after the fourth day of oviposition (4.09 < χ2 < 11.3; χ2 0.05;1 = 3.84) (Fig. 2). In females that mated in three or four distinct periods of their adult life the same trend was observed, i.e. it was male biased in the first days (4.09 < χ2 < 11.3; χ2 0.05;1 = 3.84) and female biased (3.9 < χ2 < 6.8; χ2 0.05;1 = 3.84) later in every post-mating oviposition period.
Sex ratio of the offspring of single-mated (A) and multiple-mated females with periodic (B) or continuous (C) access to males of K. aberrans. The mites were maintained at 25°C and a 16:8 LD. Bars indicate the proportion of male progeny at each different day of the oviposition period. (Open bars: sex ratio was significantly biased. Filled bars: sex ratio was not significantly different from 0.5. Asterisk indicates that in those days either less than 5 eggs or no eggs were laid by females)
Discussion
In (almost) all phytoseiid species studied, copulation is required for egg formation and oviposition (Sabelis 1985a). The present study shows that mating frequency drastically affected fecundity and longevity of K. aberrans females and that three to four matings yield maximum fecundity. Increased fecundity as a result of multiple mating has been reported for certain insect species (Ridley 1988) and several other phytoseiid species such as Amblyseius hibisci Chant (Muma 1964), Euseius gossypi (El Bardy) (Elbadry and Elbenhawy 1968), Euseius brazilli (El Benhawy) (El Benhawy 1975), Typhlodromips swirskii (Athias Henriot) (Ragusa and Swirski 1977; Momen and El-Saway 1993), Neoseiulus bibens (Blommer) (Schulten et al. 1978), Galendromus occidentalis (Nesbitt) (Hoy and Smilanick 1979), Typhlodromus pyri Scheuten (Overmeer et al. 1982), Euseius scutalis (Athias-Henriot) (Bonfour and McMurtry 1987), Neoseiulus barkeri Hughes (Bonde 1989; Momen 1993), Galendromus helveolus (Chant) (Caceres and Childers 1991), Cydnoseius negevi (Swirski and Amitai), Typhlodromus athiasae Porath and Swirski (Momen 1997) and Amblyseius zaheri Yousef and El Brollosy (Saber and Momen 2000).
In single-mated Amblyseius andersoni (Chant) (Amano and Chant 1977) and Neoseiulus californicus (McGregor) (Gotoh et al. 2004) the egg laying period is followed by a long non-laying one. This may indicate that females of those species require multiple mating for maximum fecundity. In other phytoseiid species, such as Phytoseiulus persimilis Athias-Henriot (Amano and Chant 1978b; Schulten et al. 1978; Rasmy and Hussein 1996), Amblyseius colimensis Aponte and McMurtry (Aponte and McMurtry 1992) and Euseius alstoniae (Gupta) (Kumari and Sadana 1992) a single mating resulted in maximum egg production. The ecological significance of the inter-specific variation in the number of matings required for maximum fecundity among phytoseiid mites is not clear.
During the first three to four days of oviposition following mating in K. aberrans most eggs gave rise to male progeny. This trend which is not temperature dependent (Broufas et al. 2007) is also common in other phytoseiid species such as P. persimilis, A. andersoni (Amano and Chant 1978b; Schulten et al. 1978) and E. scutalis (Bonfour and McMurtry 1987). According to Sabelis (1985b), the observed increased male progeny production at the beginning of the oviposition period could contribute to the early insemination of females that would afterwards start to disperse in order to find desirable prey. Furthermore, by means of this strategy the maximum reproductive capacity of females that require multiple mating may be reached because the males usually maintain a life-long high capacity of searching and mating and can inseminate different females many times (Amano and Chant 1978b).
Our results indicate that three to four matings are required for females to yield maximum fecundity. Up to that point, the cost of mating in terms of female longevity is negligible. However, female longevity was significantly reduced by the continuous presence of males. This may indicate that more than three to four matings, if occurring, does not contribute to egg production and has a cost for the females in terms of longevity. However, we cannot exclude the possibility that the reduction in female longevity under the continuous presence of males may be due to the struggle and discomfort of females to avoid male “sexual attacks”, or to food competition between females and males. We observed that for a number of days after mating females of K. aberrans were not receptive to mating again and when they contacted males, they forced them away with their front pair of legs and started running on the leaf disc. Further experiments are needed to determine the cost of multiple mating in terms of females’ longevity. In contrast to our results, Ozman-Sullivan (2006) reported a remarkably reduced female longevity for K. aberrans of approximately 11 d when fed on the eriophyoid mite Phytoptus avellanae Nalepa with continuous access to males, which may be due to the different prey and/or experimental procedure. Furthermore, single-mated females of K. aberrans of a Turkish population lived from approximately 16–36 days at 25°C when fed on four different types of food and each female laid approximately 6–19 eggs during its lifetime (Kasap 2005). Differences between our and Kasap’s (2005) results may be due to differences in food, experimental design and/or genetics of the different populations. Food quality and/or availability may severely affect reproduction (Amano and Chant 1977) and thus, optimal food should be used in reproduction studies. In terms of intrinsic rate of increase (r m ) values, Typha sp. pollen was proven to be an excellent nutritious food source of high quality for rearing K. aberrans (Broufas et al. 2007) compared to other pollens or spider mite species tested (Kasap 2005).
In conclusion, the present study shows that three to four matings yield maximum total fecundity of K. aberrans whereas continuous access to males and possible further mating do not increase fecundity, but reduce longevity. The positive effects of multiple mating on egg production of K. aberrans may be due to the stimulating effect of mating or to a nutritional effect of male accessory substances and sperm itself as is well documented in a number of insect species (i.e. Arnqvist and Nilsson 2000). In addition, as discussed by Radwan and Witalinski (1991) multiple mating may induce competition between males and thus increase the genetic fitness and diversity of the offspring. Furthermore, through this pattern of reproductive behavior the deleterious effect on the population’s genetic structure of inbreeding or mating with a sterile or a genetically incompatible male is reduced (e.g. Dunn et al. 2005).
The knowledge of the reproductive behavior of K. aberrans could be considered as a prerequisite first step that may help to understand the population dynamics of this species under field conditions. Kreiter et al. (2002) have demonstrated a direct correlation between life stage distribution of K. aberrans and leaf pilosity, the latter offering pollen retention and oviposition sites to females. Thus, we may assume that host plant characteristics and host dependent aggregation on confined plant parts may influence the chance of multiple mating and subsequent increase in K. aberrans populations throughout the year. However, other factors such as the ability of males to inseminate females over their lifetime, as well as the effect of multiple inseminations by various males on fecundity and longevity, maternal age or food quality and / or deprivation could affect the life table parameters and population growth of K. aberrans in the field.
References
Amano H, Chant DA (1977) Life history and reproduction of two species of predacious mites, Phytoseiulus persimilis Athias-Henriot and Amblyseius andersoni (Chant) (Acarina: Phytoseiidae). Can J Zool 55(12):1978–1983
Amano H, Chant DA (1978a) Mating behaviour and reproductive mechanisms of two species of predacious mites, Phytoseiulus persimilis Athias-Henriot and Amblyseius andersoni (Chant) (Acarina: Phytoseiidae). Acarologia 20(2):196–213
Amano H, Chant DA (1978b) Some factors affecting reproduction and sex ratios in two species of predacious mites, Phytoseiulus persimilis Athias-Henriot and Amblyseius andersoni (Chant) (Acarina: Phytoseiidae). Can J Zool 56:1593–1607
Aponte O, McMurtry JA (1992) Mating behavior and reproductive mechanisms of Amblyseius colimensis Aponte and McMurtry (Acari: Phytoseiidae). Bol Entomol Venez 7(1):1–12
Arnqvist G, Nilsson T (2000) The evolution of polyandry: multiple mating and female fitness in insects. Anim Behav 60:145–164
Bonde J (1989) Biological studies including population growth parameters of the predatory mite Amblyseius barkeri (Acarina: Phytoseiidae) at 25°C in the laboratory. Entomophaga 34(2):275–287
Bonfour M, McMurtry JA (1987) Biology and ecology of Euseius scutalis (Athias-Henriot) (Acarina: Phytoseiidae). Hilgardia 55(5):1–23
Broufas GD, Koveos DS (2000) Effect of different pollens on development, survivorship and reproduction of Euseius finlandicus (Acari, Phytoseiidae). Environ Entomol 29(4):743–749
Broufas GD, Pappas ML, Koveos DS (2007). Development, survival, and reproduction of the predatory mite Kampimodromus aberrans (Acari: Phtysoseiidae) at different constant temperatures. Environ Entomol 36(4):657–665
Caceres S, Childers CC (1991) Biology and life tables of Galendromus helveolus (Acari: Phytoseiidae) on Florida citrus. Environ Entomol 20(1):224–229
Dunn DW, Sumner JP, Goulson D (2005) The benefits of multiple mating to female seaweed flies, Coelopa frigida (Diptera: Coelpidae). Behav Ecol Sociobiol 58:128–135
Duso C (1989) Role of the predatory mites Amblyseius aberrans (Oud.), Typhlodromus pyri Scheuten and Amblyseius andersoni (Chant) (Acari, Phytoseiidae) in vineyards. I. The effects of single or mixed phytoseiid population releases on spider mite densities (Acari, Tetranychidae). J Appl Entomol 107:474–492
Elbadry EA, Elbenhawy EM (1968) Studies on the mating behaviour of the predacious mite Amblyseius gossipi (Acarina, Phytoseiidae). Entomophaga 13(2):159–162
Elbenhawy EM (1975) Biology and feeding behaviour of the predatory mite Amblyseius brazilli (Mesostigmata: Phytoseiidae). Entomophaga 20(4):353–360
Gotoh TK, Yamaguchi K, Mori K (2004) Effect of temperature on life history of the predatory mite Amblyseius (Neoseiulus) californicus (Acari: Phytoseiidae). Exp Appl Acarol 32(1–2):15–30
Hoy MA, Smilanick JM (1979) A sex pheromone produced by immatures and adult females of the predatory mite, Metaseiulus occidentalis, Acarina: Phytoseiidae. Entomol Exp Appl 26:291–300
Hoy MA, Cave FE (1985) Mating behavior in four strains of Metaseiulus occidentalis (Acari: Phytoseiidae). Ann Entomol Soc Am 78:588–593
Kasap I (2005) Life-history traits of the predaceous mite Kampimodromus aberrans (Oudemans) (Acarina: Phytoseiidae) on four different types of food. Biol Control 35(1):40–45
Kenneth W, Hardy ICW (2002) Statistical analysis of sex ratios: an introduction. In: Sex Ratios: Concepts and Research Methods: 48–92. Hardy ICW, Cambridge University Press, Cambridge
Kolodziejczyk M, Radwan J (2003) The effect of mating frequency on female lifetime fecundity in the bulb mite, Rhizoglyphus robini (Acari: Acaridae). Behav Ecol Sociobiol 53:110–115
Kreiter S, Tixier MS, Croft BA, Auger P, Barret D (2002) Plant and leaf characteristics influencing the predaceous mite Kampimodromus aberrans (Acari: Phytoseiidae) in habitats surrounding vineyards. Environ Entomol 31(4):648–660
Kumari M, Sadana GL (1992) Mating behaviour of the predatory mite Amblyseius alstoniae Gupta (Acari: Phytoseiidae). J Insect Sci 5(2):165–166
Laing JE (1969) Life history and life table of Metaseiulus occidentalis. Ann Entomol Soc Am 62(5):978–982
Liana M (2005) First copulation increases longevity and fecundity of Histiostoma feroniarum (Acari: Astigmata: Acaridida) females. Exp Appl Acarol 35:173–181
Momen FM (1993) Effect of single and multiple copulation on fecundity, longevity and sex-ratio of the predacious mite Amblyseius barkeri (Acari: Phytoseiidae). Anz Sch Pfl Umw 66(8):148–150
Momen FM (1997) Copulation, egg production and sex ratio in Cydnodromella negevi and Typhlodromus athiasae (Acari, Phytoseiidae). Anz fur Schadlingskunde 70(2):34–36
Momen FM, El-Saway SA (1993) Biology and feeding behaviour of the predatory mite Amblyseius swirskii (Acari: Phytoseiidae). Acarologia 35:199–204
Muma M (1964) The population of Phytoseiidae on Florida Citrus. Fla Entomol 47(1):5–11
Overmeer WPJ, Doodeman M, Van Zon AQ (1982) Copulation and egg production in Amblyseius potentillae and Typhlodromus pyri (Acari, Phytoseiidae). Z Angew Entomol 93(1):1–11
Ozman-Sullivan SK (2006) Life history of Kampimodromus aberrans as a predator of Phytoptus avellanae (Acari: Phytoseiidae, Phytoptidae). Exp Appl Acarol 38:15–23
Papadoulis G (1993) Contribution in the study of morphology and systematics of Phytoseiidae (Acari: Mesostigmata) in Greece. PhD Dissertation, Agricultural University of Athens, Athens (in Greek)
Pappas ML, Broufas GD, Koveos DS (2005) Mating behaviour of the predatory mite Kampimodromus aberrans (Acari: Phytoseiidae). Exp Appl Acarol 36(3):187–197
Prasad V (1967) Biology of the predatory mite Phytoseiulus macropilis in Hawaii (Acarina: Phytoseiidae). Ann Entomol Soc Am 60(5):905–908
Putman WL (1962) Life history and behaviour of the predacious mite Typhlodromus caudiglans Schuster (Acarina: Phytoseiidae) in Ontario, with notes on the prey of related species. Can Entomol 94:163–177
Radwan J, Witalinski W (1991) Sperm competition. Nature 352:671–672
Radwan J, Rysińska M (1999) Effect of mating frequency on female fitness in Caloglyphus berlesei (Astigmata: Acaridae). Exp Appl Acarol 23:399–409
Ragusa S, Swirski E (1977) Feeding habits, post-embryonic and adult survival, mating, virility and fecundity of the predacious mite Amblyseius swirski (Acarina: Phytoseiidae) on some coccids and mealybugs. Entomophaga 22(4):383–392
Rasmy H, Hussein H (1996) Effect of mating on egg production in two species of predatory mites, Agistemus exsertus Gonzalez and Phytoseiulus persimilis Athias-Henriot. Anz Sch Pfl Umw 69:88–89
Ridley M (1988) Mating frequency and fecundity in insects. Biol Rev 63:509–549
Sabelis MW (1985a) Reproduction. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control, vol 1B. Elsevier, Amsterdam, pp 73–82
Sabelis MW (1985b) Sex allocation. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control, vol 1B. Elsevier, Amsterdam, pp 83–94
Saber SA, Momen FM (2000) Effects of mating factors on reproduction and sex-ratio of the predacious mite Amblyseius zaheri yours. and El-Bor (Acari, Phytoseiidae). J Pest Sci 73(4):113–115
Schausberger P (1997) Inter- and intra-specific predation on immatures by adult females in Euseius finlandicus, Typhlodromus pyri and Kampimodromus aberrans (Acari, Phytoseiidae). Exp Appl Acarol 21:131–150
Schulten GGM, Vanarendonk RCM, Russell VM, Roorda FA (1978) Copulation, egg production and sex ratio in Phytoseiulus persimilis and Amblyseius bibens (Acari: Phytoseiidae). Entomol Exp Appl 24:145–153
SPSS (2001) SPSS base 11.0 for Windows user’s guide. SPSS, Chicago
Thornhill R, Alcock J (1983) The evolution of insect mating systems. Harvard Universtiy Press, Cambridge
Tixier M, Kreiter S, Auger P, Weber M (1998) Colonization of Languedoc vineyards by phytoseiid mites (Acari: Phytoseiidae): influence of wind and crop environment. Exp Appl Acarol 22:523–542
Tixier MS, Kreiter S, Cheval B, Guichou S, Auger P, Bonafos R (2006) Immigration of phytoseiid mites from surrounding uncultivated areas into a newly planted vineyard. Exp Appl Acarol 39(3–4):227–242
Tsunoda T, Amano H (2001) Female receptivity behavior in multiple matings of a predacious mite, Amblyseius womersleyi Schicha (Acari: Phytoseiidae). Appl Entomol Zool 36(3):393–397
Zaher MA, Shehata KK (1971) Biological studies on the predatory mite Typhlodromus pyri Scheuten (Acari: Phytoseiidae) with the effect of prey and non-prey substances. Z Angew Entomol 64:389–394
Acknowledgements
Many thanks are due to Professor Emeritus M. E. Tzanakakis for critically reading the manuscript and two anonymous reviewers for their valuable comments on an earlier version of the manuscript. This study was partially funded by a fellowship from the Alexander Onassis Public Benefit Foundation to Maria Pappas.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pappas, M.L., Broufas, G.D. & Koveos, D.S. Effect of mating frequency on fecundity and longevity of the predatory mite Kampimodromus aberrans (Acari: Phytoseiidae). Exp Appl Acarol 43, 161–170 (2007). https://doi.org/10.1007/s10493-007-9112-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-007-9112-z