Abstract
A complexity (orientation and shape of stimuli) in the mental rotation (MR) task often affects reaction time (RT) and response accuracy, but the nature of such reflections in neuroscientific research is commonly undocumented. A number of studies have explored the effect of complexity and subsequently noted down the differences in performance. However, a few studies explored complexity (in the term of angular disparity) and cognitive strategies with respect to correct responses only. In contrast, the present study investigated frontal alpha desynchronization with reference to the complexity and proportions of correct and incorrect responses. Behavioral and neurophysiological responses were investigated to understand the switching between strategies (Analytic vs. Holistic). Results showed longer response time with respect to increased complexity. Frontal alpha desynchronization increased for difficult trials and incorrect responses, suggesting a higher utilization of cognitive resources at the frontal region during the MR task. Higher left frontal desynchronization reflected a trading off between strategies for difficult trials. Taken together, these findings suggest that the effect of stimuli complexity is more nuanced than implied by a simple hemispheric dichotomy for frontal cortex and discuss possible future directions to better understand the multitudinous brain mechanisms involved in MR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Classic Mental Rotation (MR) task requires participants to compare two figures and to decide whether they are similar or not (Shepard and Metzler 1971). The task involves identical figures in the different orientation which participants rotate mentally until it aligns to match the reference figure (Shepard and Metzler 1988). The typical behavioral outcome is Response Time (RT) and response accuracy. Behavioral descriptors depend on the angle of rotation between two figures known as angular disparity. A linear increase in RTs with increasing angular disparity is usually observed (Shepard and Cooper 1982). This rectilinear function indicates that individuals mentally rotate the image in the same continuous trajectory as if they are physically rotating it. Though relevant research in this particular field is rapidly growing, it seems nevertheless noteworthy that, compared to other mental ability constructs such as working memory, the MR is only at the initial stages of a long search for potential cognitive and neural mechanisms. At present researchers are working towards understanding MR ability in various forms due to its huge applications. Such applications cover jobs of higher cognitive processing using spatial intelligence and abstract reasoning such as scientific data analysis, maneuvering flights, devising methods for drug delivery etc. The future direction in MR will be focused on identifying different cognitive strategies used and their corresponding neural correlates along with flexibility in switching between the identified cognitive strategies (Tomasino and Gremese 2016). The present study investigated the effect of complexity on frontal desynchronisation to address switching between strategies during MR task.
An increasing number of studies have examined angular disparity and RT for understanding the underlying neural correlates involved in the MR task including sex differences (Boone and Hegarty 2017; Parsons et al. 2004), hemispheric lateralization (Heil and Jansen-Osmann 2008), dimensionality (2D vs. 3D) (Neubauer et al. 2010), and strategy switching (Gardony et al. 2017). Besides angular disparity, a complex MR stimulus also affects RT and accuracy. In other words, any change in the two factors viz. angular disparity and complexity of stimuli leads to a variation in the difficulty of the MR task. Constructing a complex MR stimulus includes an increasing number of edges and vertices of the figures and number of arms, their positions, and a significant distortion in orientation. A recent study demonstrated the effect of difficulty in mental rotation in terms of angular disparities, but the authors had not considered the effect stimulus complexity (Gardony et al. 2017). On the other hand, a psychological study attempted to address task difficulty in terms of stimulus complexity (Heil and Jansen-Osmann 2008). Authors varied the stimulus complexity by varying the number of vertices for polygons. Polygons with 5 or 6 vertices were considered as simple stimuli while polygons with 13 or 14 as complex stimuli. However, a lot of research is to be carried out to understand task difficulty as a whole, to establish a relationship between angular disparity and stimulus complexity, specifying the underlying neural mechanisms involved in the MR process. But, still there is no sufficient research to observe the overall effect of task difficulty on behavioral as well as on neural aspects of MR process.
Psychological studies indicate that either stimulus types (various kinds of shapes or complex figures) or different strategies can lead to differences in the performance of mental rotation task (Heil and Jansen-Osmann 2008; Hugdahl et al. 2006; Rubia et al. 2010). For instance, a comparative study (Tomasino and Rumiati 2004) showed that strategy dominated neural activity such as hemispheric lateralization rather than the type of stimulus. Broadly, two types of strategies can be adopted for the mental rotation task viz. holistic strategy and analytic strategy (Heil and Jansen-Osmann 2008; Rubia et al. 2010). In the holistic strategy, participants mentally rotate the stimulus representation as a whole and involve right hemisphere whereas, in analytic strategy, participants mentally rotate the stimulus in a piecemeal fashion and engross left hemisphere (Corballis 1997). To further elaborate the analytic strategy, it includes decomposing the stimulus figure into several pieces, mentally rotating one piece into congruence with the reference figure, and subsequently performing similar rotation on the other segments to confirm their parity (Xue et al. 2017). Given previous evidence for the effect of complex stimulus and adopted strategy, Heil and Jansen-Osmann (2008) reported slower response time for analytic strategy. The slower reaction time was due to over-additive interaction of angular disparity and complexity. Not only the psychological study, but an imaging study (Hugdahl et al. 2006) and an electroencephalography (EEG) study (Gardony et al. 2017) pointed out differences in the responses due to adopted strategies. Gardony et al. (2017) demonstrated the preferential use of analytic strategy, involving visual comparison of key object features when the task difficulty increased. For complex stimuli, noticeable changes were observed in response time where slower response time corresponded with analytic strategy.
A number of recent studies have included EEG to elucidate the neural basis of cognitive processes (Chandra et al. 2016; Kasabov and Capecci 2015; Lawrence et al. 2014; Roberts and Bell 2000; Sharma et al. 2016, 2017). EEG is an effective technique for measuring temporal resolution; therefore, a multitude of studies use this technique to explain the underlying neural mechanisms involved in the MR task. In a recent study, physiological responses characterizing strategy shifting evoked by the difficulty in the MR task were studied using EEG (Gardony et al. 2017). The authors found that performance in the MR task was negatively correlated with the task difficulty and was reflected by the increased midline frontal theta (4–8 Hz) and reduced parietal alpha power (8–14 Hz). EEG alpha oscillations appear to be sensitive to cognitive performance and mental effort (Klimesch 1999); thus, corresponding changes in the alpha power have been observed in the various studies (e.g. Neubauer et al. (2010); Horst et al. 2013; So et al. 2017).
Of particular relevance to EEG techniques, Event-related synchronization/desynchronization (ERS/ERD) is one of the valuable indexes of cortical deactivation and activation respectively (Pfurtscheller and Aranibar 1977). To date, various studies covering a broad range of cognitive processes include the ERS/ERD method (excellent reviews are given in Klimesch 1999; Klimesch et al. 2006; Neuper and Klimesch 2006) such as mental rotation study (Chen et al. 2013; Klimesch et al. 2006). Specifically, ERD/ERS of the alpha band is sensitive to cognitive task performance (Neubauer et al. 2006; Neubauer and Fink 2009); for example, task involving visual imagery (Salenius et al. 1995) and mental visuospatial manipulation (Williams et al. 1995). Chen et al. (2013) identified the possible neurophysiological basis for the MR process by correlating the alpha ERD with RT in “mentally rotating the hands” task. Similarly, Neubauer et al. (2010) explored the effect of dimensionality (2D vs. 3D), MR training, and hemispheric interaction using alpha ERD. They noticed higher synchronization (decrease in brain activation) in the frontal regions as compared to the other regions following MR training. A relatively lower ERD was associated with faster response time. Not only the classic Shepard-Metzler stimuli, but alphanumeric character stimuli have also shown similar results with alpha ERD. In such a study, Riečanský and Katina (2010) found that late frontal alpha power provided a significant contribution to the prediction of response latency and consequently reduced ERD signified better performance. Considering the importance of alpha ERD in the MR task, we chose alpha ERD in the present study.
Several studies have emphasized the role of the posterior parietal cortex in the MR task (Gogos et al. 2010; Harris and Miniussi 2003), but the role of differential frontal activations is not completely comprehended. While studying cortical regions has been the core of empirical research in the MR task for decades, there is a surprising lack of linking between task difficulty and frontal alpha synchronization. Although we can not negate the role of the parietal region in MR process, we are quite intrigued by how frontal alpha desynchronization diverges with respect to task difficulty as well as response accuracy. A lot of studies of the neural mechanism during the MR task thus far have systematically evaluated differences in the performances, but frontal alpha has not been explored in terms of complexity and performance differences. To bridge the gap in the existing literature, the present study investigated the effect of task difficulty on the adopted strategy leveraging alpha ERD in the frontal region.
The frontal region shows promising changes because of the employed analytic strategy which is quite evident for the complex stimuli (Gardony et al. 2017; Hugdahl et al. 2006). Frontal alpha oscillations have been linked to higher order cognitive processes (Akiyama et al. 2017; Brzezicka et al. 2017; Fink and Benedek 2014; Kolev et al. 2002; Sauseng et al. 2005). For instance, Neubauer et al. (2006) suggested that higher cognitive processes were exclusively correlated with the anterior frontal ERD. Also, So et al. (2017) demonstrated that frontal alpha activity had a close relationship with cognitive performance and mental effort. Similarly, frontal ERD is frequently correlated with the MR task (Gardony et al. 2017; Riečanský and Katina 2010; So et al. 2017). Nevertheless, the potential of quantifying shifting in strategy switching with Frontal EEG has yet to be explored.
To bridge the gap in existing literature, the present study investigated the effect of task difficulty on adopted strategy leveraging alpha ERD in the frontal regions. We hypothesized with respect to (1) behavioral outcome and (2) neurophysiological changes:
-
1.
(a) On presenting a complex stimulus, MR process gets slower, since angular disparity and complexity results in an over-additive interaction (Heil and Jansen-Osmann 2008). Thus, as the MR task difficulty increases, participants would have a slower RT and fewer correct responses.
(b) Slower speed for complex stimuli suggests the use of analytic strategy, defining RT as an indicator for shifting between strategies (Heil and Jansen-Osmann 2008). Thus, differences in RT for easy versus difficult trials would demonstrate the type of adopted strategy.
-
2.
(a) Alpha band desynchronization indicates an active state of the cortex while it’s synchronization demonstrates idling of brain (Klimesch 2012). Event-related desynchronization increases when difficulty increases (Klimesch et al. 2006). Thus, increased stimulus complexity would reflect higher frontal alpha desynchronization.
(b) As complexity increases, the strategy is switched from holistic to analytic; consequently, predominant cortical activity is observed in the left frontal regions (Gardony et al. 2017; Hugdahl et al. 2006; Rubia et al. 2010). Thus, for a complex stimulus, participants would show higher left frontal alpha desynchronization, which implies a shifting towards analytic strategy.
Methods
Participants
Behavioral and EEG data were recorded from 30 healthy participants (10 females, mean age = 21.5 years, SD = 2.36) with normal or corrected-to-normal vision. All participants were right-handed and required to sign an informed consent form before starting the experiment. The protocol was approved by the ethical committee at the Institute of Nuclear Medicine and Allied Sciences (INMAS), Delhi.
Mental Rotation Task
The MR stimuli were inspired by Shepard and Metzler figures (Shepard and Metzler 1971). An initial pilot study was conducted to obtain stimuli of varying complexity. The complexity of the stimuli was varied by changing the number of edges, vertices, and rotation with respect to depth and plane of the screen. Fifty students were given a questionnaire consisting of stimuli. They rated the complexity of corresponding stimuli using a Likert scale (1–5).
EEG Signal Acquisition
High-density continuous EEG data were acquired using 64 Ag/AgCl scalp electrodes attached to an elastic cap according to the international 10–20 system (ASA-Lab, ANT B.V., Netherlands). The ground electrode was located 10% anterior to Fz (AFz), with linked earlobes, left and right mastoid, serving as references. Impedance at electrodes size was kept below 5 kΩ. The EEG signals were recorded using eego™sports amplifier at a frequency range of 0.2–70 Hz with a sampling frequency of 1024 Hz. The amplifier had an input impedance ≥ 1 GOhm, sensitivity was set to 6000 μV/cm and common mode rejection ratio was ≥ 100 dB.
Procedure
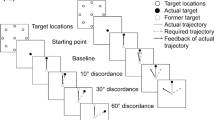
The stimuli were designed in the Unity 5.0 and behavioral outcomes (RT and response accuracy) were stored in a separate text file. A total of 20 stimuli were used with seven angular disparities (z-axis: 0°, ± 30°, ± 60°, ± 90°, ± 120°, ± 150°, 180°). We then grouped the stimuli into 280 pairs, considering twenty stimuli, two difficulty types (simple, complex), and seven angular disparities. Each stimuli pair was 1024 × 768 pixels in size which was separated horizontally by a cross of size 40 × 40-pixel. The complex stimulus was set to have a maximum of eight edges with an angular disparity of at most 120°. All stimuli were presented as a white figure on a black background and on a 17-inch monitor at a viewing distance of 50 cm. A total of 30 trials were presented at random, each consisting of 15 stimuli (7 simple stimuli and 8 complex stimuli) with the 5 s inter-stimulus interval. Each stimulus pair consisted of the pair of 3D figures in which the participant had to decide if they were similar or dissimilar. The participants were required to compare and respond via pressing correct (if the pair of stimuli is similar) or incorrect button (if a pair of stimuli is dissimilar) (Fig. 1). If the participant failed to respond within the allotted 15 s time window, then the 5 s blank screen would appear and proceed to the next stimulus. Later, for analyzing trials, we averaged all the stimuli into one of two types of trials. Trials that consisted of simple stimuli with orientation up to 60° were referred to as easy trials, and those which consisted of complex stimuli with orientation from 90° to 180° were referred to as difficult trials. The participant was seated comfortably in an acoustically and electrically insulated room. Before the actual experiment, a practice session was provided to the participant to get familiarized with the task and response methods. Subsequently, baseline EEG recording was taken for 2 min under eyes open resting condition. Following this, participants completed 30 trials in the random order (counterbalanced between participants), with an inter-trial interval of 10 s (Fig. 2, Table 1). EEG data were recorded simultaneously with the MR task. Trigger signals for the stimulus presentation and the responses were also recorded. Participants were requested to refrain from any unnecessary movement during the task. They were provided 5 min of breaks between sessions. The total task lasted for about 2 h.
EEG Signal Processing
A total of 450 (15*30) data epochs were recorded from each participant. The EEG data were segmented into time intervals, each consisting of data from stimulus onset to the response made. Using the EEGLAB toolbox (Delorme and Makeig 2004), the baseline noise was removed, and the remaining data were re-referenced to the mean. Power-line interference was removed by a 50 Hz notch filter. For artefact rejection, we used the Independent Component Analysis (ICA) blind source separation method. For further analysis, the alpha band (8–13 Hz) was filtered from the EEG signals. The power contained in a signal was then computed as:
To calculate ERD/ERS of the alpha band, the percentage change in the alpha band power between the baseline condition (120 s prior the beginning of the task, ‘R’) and the stimuli processing interval, from stimulus onset to the response made, (approx. 225 s per trial, ‘A’) was taken. ERD was represented mathematically as (Pfurtscheller 1989, 1992):
Event-related desynchronization is the phasic and localized amplitude attenuation of alpha oscillations. It is usually associated with an individual being mentally active (Kim et al. 2012). On the contrary, event-related synchronization is the phasic and localized increase in alpha oscillations (Pfurtscheller 1992). Positive %ERD value indicates a reduction in alpha power (cortical activation or desynchronization) and negative %ERD value indicates an increment in alpha power (cortical deactivation or synchronization, ERS) (Neubauer et al. 2004). Considering the crucial role of frontal brain regions in the context of strategy shifting, we expected differences to be limited to these regions of the brain. We calculated frontal % ERD-measure by averaging the following 25 electrodes with corresponding responses (correct and incorrect): Fp1, AF3, AF7, F1, F3, F5, F7, FC1, FC3, FC5, FT7, Fp2, AF4, AF8, F2, F4, F6, F8, FC2, FC4, FC6, and FT8.
Statistical Analysis
We considered difficulty (easy vs. difficult trial) as an independent variable while behavioral response (RT and response accuracy) and neurophysiological outcome (%ERD of the alpha band) as the dependent variables. To test whether gender had any effect on the task performance, we applied a standard welch’s two-sample t test on the behavioral outcome. Welch’s t-test is generally preferred when the assumption of homogeneity of variance is not met (Delacre et al. 2017). Paired sample t-test was applied to observe the effect of difficulty on the behavioral outcome. One-way ANOVA was applied to the %ERD values of averaged frontal electrodes to observe the effect of task difficulty on the response accuracy (correct and incorrect responses). To differentiate between accuracy of easy and difficult trials, a paired sample t-test was applied on the frontal %ERD values.
Results
Behavioral Outcome
Total twenty-eight participants were included for behavioral data analysis as two participants (all females) were unable to complete the MR task. A standard Welch’s two-sample t-test was employed to understand the effect of gender on response accuracy and RT. No significant effect of gender was found on response accuracy (t (20.62) = 1.54, p > .05). Difference between gender and RT was also found to be non-significant (t (11.78) = 1.67, p > .05). These results reflected the fact that gender did not have any implication on the outcomes. The descriptive statistics of behavioural responses (i.e., number of responses and reaction time) are shown in Table 2.
Participants showed poor performance in the difficult trials due to an increase in angular disparity of the stimuli. Thus, conforming to hypothesis 1a that as the complexity was increased, participants had a slower response time and fewer correct responses. Paired sample t-test was performed at p = .05 to observe any significant difference between easy versus difficult trials for RT (t (27) = 3.62, p = .042). Results showed that participants responded comparatively slower towards the complex stimuli as to simple stimuli. Also, performance differences were evident when RT for the correct responses was compared to those with the incorrect responses with a t-test at p = .025. The average RT was significantly lower in the correct responses than those of the incorrect responses (t (27) = 5.54, p = .00). Slower response time with increasing complexity was observed for difficult trials. The slower RT indicated the analytic process where the image was parsed into smaller units and then rotated individually. Thus, adhering to the proposition in 1b, differences in RT for easy versus difficult trials demonstrated the type of adopted strategy.
EEG Results
The EEG data of twenty-five participants were processed, as two participants were unable to complete the task and data of three other participants turned out to be noisy. There was no significant effect of gender on the %ERD values for all frontal electrodes, F (4, 20) = 0.39, p > .05; Wilk’s Λ = 0.56, partial η2 = .33. One-way ANOVA was applied on the % ERD values of alpha-band for the four categories, ‘easy-correct’, ‘easy-incorrect’, ‘difficult-correct’, and ‘difficult-incorrect’. The result showed significant differences for %ERD (F (3,23) = 5.50, p < .05, η 2 p = .14), suggesting changes in the frontal alpha desynchronization as the complexity of stimuli increased. A post hoc Tukey test showed that there was a significant reduction in the %ERD for ‘difficult-incorrect’ category at p < .05 and ‘easy-incorrect’ category at p < .05 as compared with the ‘easy-correct’ category. Figures 3 and 4 illustrates the %ERD for categories ‘easy-correct’ and ‘difficult-correct’ trials of the alpha band in the frontal electrodes. Similarly, Figs. 5 and 6 shows the frontal %ERD for categories ‘easy-incorrect’ and ‘difficult-incorrect’ trials. The observations suggested that for the easy-correct category, % ERD shifted towards the negative axis of the plot, indicating lower frontal desynchronization as compared to the easy-incorrect and the difficult-incorrect category. When %ERD of the easy trials were compared with the difficult trials, the easy trials had lower frontal desynchronization. Similarly, when %ERD of the correct responses were compared with the incorrect responses, the correct responses had a lower frontal desynchronization. Thus, conforming to hypothesis 2a, increased stimulus complexity reflected higher frontal desynchronization.
Each category was then compared with the other categories by comparing all 25 frontal electrodes separately by averaging thirty trials using paired sample t-test. When the easy-correct category was compared with the difficult-correct category, significant activity was observed at channel F7 (t(24) = 1.880, p = .05) and FT7 (t(24) = 3.05, p = .014), whereas when the easy-correct category was compared with the difficult-incorrect category, significant activity was observed at channel FC6 (t(24) = 2.368, p = .042), FT7 (t(24) = 2.201, p = .048), and FT8 (t(24) = 1.85, p = .049). For the easy-incorrect category, there was significant reduction in the %ERD for the difficult-correct category (t(24) = 2.356, p = .026) and the difficult-incorrect category (t(24) = 6.992, p = .014).When the easy-incorrect category was compared with the difficult-correct, a significant activity was observed at channel FC1 (t(24) = 2.148, p = .048), whereas when the easy-incorrect category was compared with the difficult-incorrect, a significant activity was observed at channel FC5 (t(24) = 2.54, p = .044). Left frontotemporal and frontocentral electrodes were most active when the easy-correct category was compared with the the difficult-correct and the difficult-incorrect categories.
Considering hemispheric lateralization, %ERD was evaluated with respect to response accuracy and stimulus complexity. One-way ANOVA showed non-significant differences between left and right frontal electrodes, F (3, 46) = 1.25, p > .05; Wilk’s Λ = 0.37, partial η2 = .10. Figure 7 showed the frontal %ERD distribution in all trial types in left and right hemisphere. The plot demonstrated higher left frontal desynchronization for difficult trial. Participants responded slowly in difficult trials (from RT), shifting from holistic to analytic strategy. Thus, as per hypothesis 2b, for complex stimuli, participants showed higher left frontal alpha desynchronization implying a shifting towards analytic strategy.
Discussion
This study explored the effect of complexity on the performance during the MR task. The effect was elucidated using frontal alpha %ERD and attempted to observe switching between strategies. We speculated on possible causes for and implications of behavioral and neurophysiological findings below.
Behavioral outcome suggested that the participants had poor performance in the difficult trials. They had slower RT and fewer correct responses in difficult trials as compared to easy trials. Thus, behavioral results were in concordance with the previous finding (Gardony et al. 2017; Heil and Jansen-Osmann 2008). The slower RT implied that information processing speed in the MR task had significantly diverged which suggested switching between strategies. Previous studies reported that the participants employed analytic strategy as exhibited in the slower RT with increasing stimulus complexity (Gardony et al. 2017; Heil and Jansen-Osmann 2008). Consistent with previous research, we found that as the task difficulty increased, participants switched to the analytic strategy indicated by the statistically significant different RT between difficult trials and easy trials.
Neurophysiological results showed that for easy-correct and easy-incorrect responses, a predominant negative %ERD was found, indicating frontal synchronization. As suggested by the previous studies (Neubauer et al. 2004, 2010), alpha ERD and ERS indicate cortical activation and deactivation respectively. The former studies also showed that the synchronized alpha band activity was found in cortical areas during a relaxed mental state (Pfurtscheller 1992). Reduction in alpha-band synchronization (or increase frontal desynchronization) suggested attentional processing for complex stimuli (Neubauer et al. 2006). When the correct responses were compared against the incorrect responses, %ERD was found to be higher in the incorrect responses compared to the correct responses. Taken together, the observation indicated that the frontal desynchronization was associated with task difficulty.
Considering hemispheric lateralization and adopted strategy in MR task, desynchronization at left frontal region suggested the use of analytic strategy. As indicated by Tomasino and Rumiati (2004), hemispheric lateralization was more influenced by the adopted strategies, either by will or triggering implicitly. Furthermore, there were task-dependent changes in the alpha-frequency synchronization which was reflected by the slower processing speed in difficult trials. The laterality in the frontal lobe thus showed a tendency to switch strategies between holistic and analytic. A plausible explanation for this observation could be that the MR speed was affected by the stimulus complexity which was then countered by switching of strategy from holistic to analytic. Previous studies reported that during the MR task, activation was reported in the parietal region for males and inferior frontal (Hugdahl et al. 2006) and left frontal for females (Rubia et al. 2010). They argued that hemispheric lateralization was due to strategy shifting, wherein males were inclined towards holistic strategy while females towards analytic strategy. The results were further supported by behavioral outcome, where RT was significantly lower in difficult trials as compared to easy trials. In a previously conducted study (Heil and Jansen-Osmann 2008), authors showed that information processing speed for MR task was slower in females with an increase in complexity and that females were more oriented towards analytic strategy. Thus, slower response time indicated the effect of task difficulty on information processing speed which was further indicated by left frontal desynchronization. Left frontal alpha desynchronization might therefore reflect a more focused state of internal attention and was more likely to exhibit analytic strategy. Electrode-based investigation indicated that left frontotemporal and frontocentral electrodes were most active when the easy-correct category was compared with the difficult-correct and the difficult-incorrect categories. The results were supported by previous studies where left frontotemporal and frontocentral regions were associated with analytic strategy (Li et al. 2011; van Hoogmoed et al. 2012). Thus, we could imply that frontotemporal location exhibited higher cortical activation (i.e. higher alpha ERD) during the difficult trial. The results could imply that the activation of left-frontal alpha (alpha desynchronization) was depended on the task difficulty.
Conclusion
The reported studies on the relationship between frontal alpha-band desynchronization and stimulus complexity have opened a window towards understanding neural mechanisms underlying MR. The observed increase in frontal alpha desynchronization during difficult trials could reflect more towards an internally oriented attention which was due to the task complexity. Frontal hemispheric dichotomy also showed task (difficulty) dependent activation such as the higher left frontal desynchronization reflecting analytic strategy. Taken as a whole, however, neuroscientific research on task difficulty and MR is still at an early stage of its development and there are a number of important issues that are in great need to be addressed in future research in order to make the field advance more effectively.
The limitation of study can be an uneven ratio of gender which might affect the strategy switching. Also, it is apparent that the evidence is sparse and is derived from the frontal alpha-band only. Despite limitation in the present study, the present findings have important implications for theory and experimental practice. This study serves as the basis for the existence of the strategy shifting when complexity is increased and can provide a new viewpoint to understand the cognitive processing of an individual.
Besides the consistent use of well-established methods, future research in this field should specifically focus on the time-course of MR using Event Related Potential (ERP) recordings. Sophisticated ERP analysis techniques can be used for the quantification of spatiotemporal signatures of brain oscillations which would yield valuable insights into the cognitive processes involved during MR.
Taken together, theoretical findings indicate generalization of MR ability to various spatial cognition task which in turn reflect individual classification in higher engineering and aptitude tests. It will, therefore, be particularly important in future studies to include, other regions of cerebral cortex such as the parietal lobe, sex differences, developmental factors, and detailed oscillatory coupling at different frequencies. For instance, we can incorporate the parietal region as the center of the investigation to quantify the percentage of the strategy shifting. Consequently, future studies need to be very specific in their definitions of the construct under investigation to develop a coherent and consistent picture about the role of strategy and individual differences. Moreover, the developmental trajectory of cognitive strategy could be studied in future work to understand the preference for a particular strategy during the certain span of life.
References
Akiyama, M., Tero, A., Kawasaki, M., Nishiura, Y., & Yamaguchi, Y. (2017). Theta-alpha EEG phase distributions in the frontal area for dissociation of visual and auditory working memory. Scientific Reports, 7, 42776.
Boone, A. P., & Hegarty, M. (2017). Sex differences in mental rotation tasks: Not just in the mental rotation process! Journal of Experimental Psychology. Learning, Memory, and Cognition, 43(7), 1005.
Brzezicka, A., Kamiński, J., Kamińska, O. K., Wołyńczyk-Gmaj, D., & Sedek, G. (2017). Frontal EEG alpha band asymmetry as a predictor of reasoning deficiency in depressed people. Cognition and Emotion, 31(5), 868–878.
Chandra, S., Sharma, G., Sharma, M., Mittal, A. P., & Jha, D. (2016). Workload regulation by Sudarshan Kriya: an EEG and ECG perspective. Brain Informatics, 4, 1–13.
Chen, X., Bin, G., Daly, I., & Gao, X. (2013). Event-related desynchronization (ERD) in the alpha band during a hand mental rotation task. Neuroscience Letters, 541, 238–242.
Corballis, M. C. (1997). Mental rotation and the right hemisphere. Brain and Language, 57(1), 100–121.
Delacre, M., Lakens, D., & Leys, C. (2017). Why psychologists should by default use Welch’s t-test instead of Student’s t-test. International Review of Social Psychology, 30(1), 92–101.
Delorme, A., & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21.
Fink, A., & Benedek, M. (2014). EEG alpha power and creative ideation. Neuroscience and Biobehavioral Reviews, 44, 111–123.
Gardony, A. L., Eddy, M. D., Brunyé, T. T., & Taylor, H. A. (2017). Cognitive strategies in the mental rotation task revealed by EEG spectral power. Brain and Cognition, 118, 1–18.
Gogos, A., Gavrilescu, M., Davison, S., Searle, K., Adams, J., Rossell, S. L., et al. (2010). Greater superior than inferior parietal lobule activation with increasing rotation angle during mental rotation: An fMRI study. Neuropsychologia, 48(2), 529–535.
Harris, I. M., & Miniussi, C. (2003). Parietal lobe contribution to mental rotation demonstrated with rTMS. Journal of Cognitive Neuroscience, 15(3), 315–323.
Heil, M., & Jansen-Osmann, P. (2008). Sex differences in mental rotation with polygons of different complexity: Do men utilize holistic processes whereas women prefer piecemeal ones? The Quarterly Journal of Experimental Psychology, 61(5), 683–689. https://doi.org/10.1080/17470210701822967.
Horst, A. C., Lier, R., & Steenbergen, B. (2013). Mental rotation strategies reflected in event-related (de) synchronization of alpha and mu power. Psychophysiology, 50(9), 858–863.
Hugdahl, K., Thomsen, T., & Ersland, L. (2006). Sex differences in visuo-spatial processing: An fMRI study of mental rotation. Neuropsychologia, 44(9), 1575–1583.
Kasabov, N., & Capecci, E. (2015). Spiking neural network methodology for modelling, classification and understanding of EEG spatio-temporal data measuring cognitive processes. Information Sciences, 294, 565–575.
Kim, S., Jung, K. H., & Lee, J. H. (2012). Characteristics of alpha power event-related desynchronization in the discrimination of spontaneous deceptive responses. International Journal of Psychophysiology, 85(2), 230–235.
Klimesch, W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Research Reviews, 29(2), 169–195.
Klimesch, W. (2012). Alpha-band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences, 16(12), 606–617.
Klimesch, W., Doppelmayr, M., & Hanslmayr, S. (2006). Upper alpha ERD and absolute power: Their meaning for memory performance. Progress in Brain Research, 159, 151–165.
Kolev, V., Yordanova, J., Basar-Eroglu, C., & Basar, E. (2002). Age effects on visual EEG responses reveal distinct frontal alpha networks. Clinical Neurophysiology, 113(6), 901–910.
Lawrence, L. M., Ciorciari, J., & Kyrios, M. (2014). Cognitive processes associated with compulsive buying behaviours and related EEG coherence. Psychiatry Research: Neuroimaging, 221(1), 97–103.
Li, S., Mayhew, S. D., & Kourtzi, Z. (2011). Learning shapes spatiotemporal brain patterns for flexible categorical decisions. Cerebral Cortex, 22(10), 2322–2335.
Neubauer, A. C., Bergner, S., & Schatz, M. (2010). Two- vs. three-dimensional presentation of mental rotation tasks: Sex differences and effects of training on performance and brain activation. Intelligence, 38(5), 529–539.
Neubauer, A. C., & Fink, A. (2009). Intelligence and neural efficiency. Neuroscience and Biobehavioral Reviews, 33(7), 1004–1023.
Neubauer, A. C., Fink, A., & Grabner, R. H. (2006). Sensitivity of alpha band ERD to individual differences in cognition. Progress in Brain Research, 159, 167–178.
Neubauer, A. C., Grabner, R. H., Freudenthaler, H. H., Beckmann, J. F., & Guthke, J. (2004). Intelligence and individual differences in becoming neurally efficient. Acta Psychologica, 116(1), 55–74.
Neuper, C., & Klimesch, W. (Eds.). (2006). Event-related dynamics of brain oscillations (Vol. 159). Amsterdam: Elsevier.
Parsons, T. D., Larson, P., Kratz, K., Thiebaux, M., Bluestein, B., Buckwalter, J. G., et al. (2004). Sex differences in mental rotation and spatial rotation in a virtual environment. Neuropsychologia, 42(4), 555–562.
Pfurtscheller, G. (1989). Spatiotemporal analysis of alpha frequency components with the ERD technique. Brain Topography, 2(1), 3–8.
Pfurtscheller, G. (1992). Event-related synchronization (ERS): an electrophysiological correlate of cortical areas at rest. Electroencephalography and Clinical Neurophysiology, 83(1), 62–69.
Pfurtscheller, G., & Aranibar, A. (1977). Event-related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalography and Clinical Neurophysiology, 42, 817–826.
Riečanský, I., & Katina, S. (2010). Induced EEG alpha oscillations are related to mental rotation ability: The evidence for neural efficiency and serial processing. Neuroscience Letters, 482(2), 133–136.
Roberts, J. E., & Bell, M. A. (2000). Sex differences on a mental rotation task: Variations in electroencephalogram hemispheric activation between children and college students. Developmental Neuropsychology, 17(2), 199–223.
Rubia, K., Hyde, Z., Halari, R., Giampietro, V., & Smith, A. (2010). Effects of age and sex on developmental neural networks of visual–spatial attention allocation. NeuroImage, 51(2), 817–827.
Salenius, S., Kajola, M., Thompson, W. L., Kosslyn, S., & Hari, R. (1995). Reactivity of magnetic parieto-occipital alpha rhythm during visual imagery. Electroencephalography and Clinical Neurophysiology, 95(6), 453–462.
Sauseng, P., Klimesch, W., Doppelmayr, M., Pecherstorfer, T., Freunberger, R., & Hanslmayr, S. (2005). EEG alpha synchronization and functional coupling during top-down processing in a working memory task. Human Brain Mapping, 26(2), 148–155.
Sharma, G., Gramann, K., Chandra, S., Singh, V., & Mittal, A. P. (2017). Brain connectivity during encoding and retrieval of spatial information: Individual differences in navigation skills. Brain Informatics, 4(3), 207.
Sharma, G., Salam, A. A., Chandra, S., Singh, V., & Mittal, A. (2016, October). Influence of spatial learning perspectives on navigation through virtual reality environment. Paper presented at the International Conference on Brain and Health Informatics (pp. 346-354). Springer International Publishing.
Shepard, R. N., & Cooper, L. A. (1982). Mental images and their transformations. Cambridge: MIT Press.
Shepard, R. N., & Metzler, J. (1971). Mental rotation of three-dimensional objects. Science, 171(3972), 701–703.
Shepard, S., & Metzler, D. (1988). Mental rotation: Effects of dimensionality of objects and type of task. Journal of Experimental Psychology: Human Perception and Performance, 14(1), 3.
So, W. K., Wong, S. W., Mak, J. N., & Chan, R. H. (2017). An evaluation of mental workload with frontal EEG. PLoS ONE, 12(4), e0174949.
Tomasino, B., & Gremese, M. (2016). Effects of stimulus type and strategy on mental rotation network: An activation likelihood estimation meta-analysis. Frontiers in Human Neuroscience, 9, 693.
Tomasino, B., & Rumiati, R. I. (2004). Effects of strategies on mental rotation and hemispheric lateralization: Neuropsychological evidence. Journal of Cognitive Neuroscience, 16(5), 878–888.
van Hoogmoed, A. H., van den Brink, D., & Janzen, G. (2012). Electrophysiological correlates of object location and object identity processing in spatial scenes. PLoS ONE, 7(7), e41180.
Williams, J. D., Rippon, G., Stone, B. M., & Annett, J. (1995). Psychophysiological correlates of dynamic imagery. British Journal of Psychology, 86(2), 283–300.
Xue, J., Li, C., Quan, C., Lu, Y., Yue, J., & Zhang, C. (2017). Uncovering the cognitive processes underlying mental rotation: an eye-movement study. Scientific Reports, 7(1), 10076.
Acknowledgements
This study was supported by Director, INMAS, DRDO, Delhi. The EEG data acquired by Nidhi Gupta and Shweta Saraswat. Scales used for this study were designed through the support of Delhi University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the INMAS ethical committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, G., Daniel, R., Chandra, S. et al. Effect of Complexity on Frontal Event Related Desynchronisation in Mental Rotation Task. Appl Psychophysiol Biofeedback 44, 235–245 (2019). https://doi.org/10.1007/s10484-019-09436-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10484-019-09436-0