Abstract
High work stress has been consistently associated with disturbed autonomic balance, specifically, lowered vagal cardiac control and increased sympathetic activity, which may lead to increased cardiovascular risk. Stress management procedures have been proposed to reduce autonomic dysfunctions related to work stress in different categories of workers exposed to heightened work demands, while a limited number of studies addressed this issue in managers. The present study was aimed at evaluating the effectiveness of a respiratory sinus arrhythmia (RSA) biofeedback (BF) intervention on psychological and physiological outcomes, in managers with high-level work responsibilities. Thirty-one managers leading outstanding private or public companies were randomly assigned to either a RSA-BF training (RSA-BF; N = 16) or a control group (N = 15). The RSA-BF training consisted of five weekly 45 min sessions, designed to increase RSA, whereas controls had to provide a daily stress diary once a week. After the training, managers in both groups reported reduced heart rate at rest, lower anxiety levels and improvement in health-related quality of life. More importantly, managers in the RSA-BF group showed increased vagal control (as indexed by increased RSA), decreased sympathetic arousal (as indexed by reduced skin conductance and systolic blood pressure) and lower emotional interferences, compared to managers in the control group. Results from this study showed that RSA-BF training was effective in improving cardiac autonomic balance at rest. Moreover, findings from this study underline the effectiveness of biofeedback in reducing psychophysiological negative outcomes associated with stress in managers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Job-related stress is quickly becoming one of the most widespread disorders among workers. Working life and job environment are well known sources of harmful psychophysiological stress in different working classes, including craftsmen, skilled workers, clerks and administrative professionals (Aronsson 1999; Chandola et al. 2010).
Heightened job-related stress responses have been associated with elevated job demands, dynamic work, high job authority, conflict and competition, which, in turn negatively affect individual health and well-being.
Stress related to work has also been associated with increased cardiovascular risk (Backé et al. 2012; Kivimäki et al. 2002; Möller et al. 2005; Rosengren et al. 2004; Rozanski et al. 2005). Stress is thought to directly alter the neural cardiovascular regulation (Lucini et al. 2002), and work stress has been shown to affect many physiological systems, including the cardiovascular, endocrine and the immune system (for a review see Chandola et al. 2010), increasing disease susceptibility and progression across a broad spectrum of disorders (Flier et al. 1998; Kiecolt-Glaser and Glaser 1995).
More recently, Lucini et al. (2007) reported that work stress was consistently associated with an altered autonomic profile, characterised by high resting heart rate (HR), elevated blood pressure (BP) and reduced cardiac vagal tone. The study by Vrijkotte et al. (2000) confirmed that individuals with high job strain displayed exaggerated cardiovascular activity. Specifically, men who reported excessive imbalance between job demands and rewards, showed elevated systolic BP and HR and reduced vagal tone, not only during working hours but also during leisure time. These findings suggest that the effects of work stress on cardiovascular health are mediated by excessive sympathetic reactivity during workday and impaired parasympathetic activity during leisure time, leading.
In addition, a negative association between work stressors and heart rate variability has been consistently reported (Collins et al. 2005; Eller et al. 2011b; Hemingway et al. 2005; Hintsanen et al. 2007; Kang et al. 2004). Results indicate that work stress can lead to autonomic imbalance, characterized by vagal withdrawal and sympathetic saturation, which, in turn, may lead to cardiac electrical instability (Eller et al. 2011a; Malik and Camm 1993; Ohsuga et al. 2001).
In spite of the vast literature on occupational stress, fewer studies have investigated the impact of job stress on executives and managers. Managers’ jobs are usually characterised by high job demand, an extremely high pressure to succeed, and elevated responsibilities (Judge et al. 1994; Worrall and Cooper 1995) and massive quantitative workloads (Brett and Stroh 2003), exposing managers to hazardous levels of stress (Mohr and Wolfram 2010; Schieman and Reid 2009). Despite the high level of job satisfaction usually reported by managers and the coping opportunities, given that managers have elevated earnings, job autonomy, non-routine work and schedule control (Mirowsky and Ross 2005), managers still report high levels of stress. In fact, a large study by the Institute of Management and Personal Performance Consultants (Institute of Management 1993) on almost 1000 managers found that despite most of the managers reporting high job satisfaction, 70 % of them had job-related stress and worries. The major source of reported stress was the influence of job on leisure time and affective relationships. Also, job-related stress had a negative influence on mood, job efficacy and perceived health. Another large study of 2737 workers (Dewa et al. 2011) showed that jobs characterised by undefined boundaries between work and home hours, job authority and non-routine work, were associated with increased work-to-home conflict, that in turn reduces the ability to cope with stress. Also, Schieman and Reid (2009) found that professionals and people who are in positions of authority are more likely to be exposed to interpersonal conflicts and work-to-home interference, which may lead to greater levels of stress. Therefore, managers should be considered a high-risk occupational group.
Moreover, managers’ health is crucial for organizations to function. They are in charge of key decisions, and poor health can negatively influence the work and well-being of the employees as well as threaten the whole organization’s effectiveness (Little et al. Nelson 2007). Therefore, it is of crucial importance to identify and develop strategies to manage psychophysiological consequences of work-related stress in managers.
Occupational stress management programmes are designed to prevent or reduce health risks by minimising the negative outcomes of exposure to stressors (Bellarosa and Chen 1997; Richardson and Rothstein 2008). Usually, stress management programmes include behavioural techniques such as progressive muscle relaxation, meditation, and cognitive behavioural skills training (Lamontagne et al. 2007; Richardson and Rothstein 2008).
Over the last two decades, some studies have reported that a biobehavioral technique, namely cardiovascular biofeedback, can be effective in promoting health and restoring cardiac autonomic balance, reducing sympathetic overreactivity to stress and enhancing cardiac vagal tone (Lehrer et al. 2007; Schwartz and Andrasik 2003; Yucha and Montgomery 2008). Recent studies have also highlighted the effectiveness of biofeedback training aimed at increasing respiratory sinus arrhythmia (RSA), a measure of cardiac vagal modulation, in reducing autonomic nervous system dysregulation, by increasing cardiac vagal tone and baroreflex gain both in patients with cardiovascular diseases and healthy adults (Del Pozo et al. 2004; Lehrer et al. 2003; Nolan et al. 2005; Patron et al. 2013).
Though there is increasing evidence that RSA-BF interventions can enhance vagal modulation and, in turn, reduce the stress response in clinical and non-clinical populations, research has yet to investigate the potential effectiveness of RSA-BF for increasing cardiac vagal tone and lowering sympathetic activation in managers with high status position. In the present study, a RSA-BF training was carried out to enhance vagal cardiac control (as measured by means of RSA) and promote cardiac autonomic balance, in managers with an occupational role characterised by overload, intense job involvement, work demands, ambition and high competitiveness (Gevirtz and Lehrer 2003). The aim of the present study was to evaluate the effectiveness of a short RSA-BF training, compared to a standard stress management intervention, in enhancing psychological and physiological well-being.
Methods
Participants
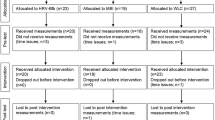
Forty managers were recruited from private (banking group, manufacturing industries and media) and public (health service, education system, local government and military) companies in the north-eastern region of Italy. All participants were males aged 35–73 years (mean age = 48.37 ± 8.71) who were recruited through advertisements in the newsletter of the association of the General Confederation of Italian Industry (Confindustria) and who voluntarily participated in this study.
Participants were in charge either of the whole company (managers) or of departments in organisation managing (middle managers), in a highly competitive work environment. Participants were in active employment status, and had no history of hypertension, heart problems or other chronic mental or neurological diseases. None of the participants were taking medications influencing HR (i.e., beta-blockers or angiotensin-converting enzyme inhibitor), tranquilisers or antidepressants.
Participants were randomly assigned to an RSA-BF training or a control group. Nine managers were unable to complete the training because of their work schedule. Therefore, 31 managers aged 35–67 years (mean age = 47.89 ± 7.98), completed the training, in either RSA-BF (N = 16; mean age = 49.75 ± 9.14) or a control (N = 15; mean age = 45.80 ± 6.20) group. The first group underwent an RSA-BF training (see below) once a week for a total duration of 5 weeks. In the control group, participants were asked to daily record, in a stress diary, events that had triggered a perception of stress, providing details on the causes, intensity and duration of symptoms, as well as the coping strategies adopted. All participants were instructed about the study procedure and gave written informed consent. The study was carried out in accordance with the Declaration of Helsinki, and the study protocol was approved by the ethics committees of the institutions involved.
Measurements and Apparatus
Sociodemographic and Psychological Measures
To collect sociodemographic (age and education) and health behaviour data, including weight, height, physical activity, sleep time, family history of hypertension and cardiovascular disease, a semi-structured interview was conducted by a trained clinical psychologist. In addition, participants filled out two self-report questionnaires:
-
1.
The State-Trait Anxiety Inventory, Y form (STAI-Y; Spielberger et al. 1970, 1996) is a 40-item questionnaire divided in two 20-item subscales measuring state or current (STAI-Y1), and trait or dispositional, anxiety (STAI-Y2). Scores range from 20 to 80, and higher scores reflect greater anxiety levels.
-
2.
The 36-Item Short Form Health Survey (SF-36; Apolone and Mosconi 1998; Gandek and Ware 1993) is a widely used instrument assessing health status. It includes eight multi-item scales (35 items) that evaluate the extent to which the individual’s health limits his or her physical, emotional and social functioning. Specifically, it evaluates physical functioning (ten items), role limitations caused by physical health problems (four items), bodily pain (two items), general health perceptions (five items), vitality (four items), role limitations caused by emotional health problems three items), social functioning (two items), and emotional well-being (five items). Higher scores indicate better health status.
Physiological Recording
Blood volume pulse was recorded through a photoplethysmographic detection sensor (blood volume pulse-Flex/Pro) attached to the right ring finger.
Respiration was recorded by means of two respiration belts with strain gauges/tubes filled with conduction fluid (Respiration-Flex/Pro sensor) worn around the participant’s thorax and abdomen.
Systolic and diastolic BP (SBP and DPB) were recorded by a validated automatic wrist device (NAIS EW272, Matsushita Electrics Works Italia S.r.l.) on the left arm, based upon an oscillometric blood pressure monitoring method. Specifically, three readings were taken at intervals of 1 min, and averaged, according to the recommendations for blood pressure measurement of the American Heart Association (Pickering et al. 2005).
Skin conductance level (SCL) was recorded by means of two Ag/AgCl surface electrodes applied on the first and middle finger of right hand (Skin conductance-Flex/Pro sensor) (Fowles et al. 1981). The probe signal was constant voltage (0.5 V), and no conductive paste was applied on the skin.
All physiological measures were continuously recorded for 4 min at rest during both a pre-training and a post-training session. Data were processed using a FlexComp Infiniti™ encoder and BioGraph Infiniti software (Thought Technology Ltd., Montreal, Quebec). Data were processed via a 14-bit analogue-to-digital converter with a sampling rate of 256 Hz (bandwidth 1–64 Hz) and stored sequentially for analyses in a personal computer (DELL VOSTRO notebook, Intel Core™ 2).
Assessment
All managers underwent the same assessment protocol approximately 1 week before the training (i.e., pre-training) and approximately within 2 weeks of the end of the training (i.e., post-training), in a laboratory purposely set up at a participant worksite. Participants were asked to abstain from alcohol, caffeinated beverages and smoking for the 3 h preceding physiological recordings. Self-report questionnaires for anxiety symptoms and health status were administered individually at pre- and post-training by a trained psychologist blind to the patient’s group assignment (RSA-BF or control group). After participants completed the questionnaires, they were invited to sit on a comfortable armchair, in a quiet, dimly lit room at a constant temperature (about 21 °C). No support for the legs was employed to avoid the possible confounding effect of body position on cardiac activity. Before starting the physiological assessment all the participants were informed of the physiological measures being monitored (i.e., HR, respiration and BP). After sensors’ placement and adaptation to the laboratory (10 min), BP was measured. Physiological measures were then recorded over a 4-min period at rest and BP was measured again. Managers were then randomly assigned to either the RSA-BF training or the control group.
Training
The RSA-BF training consisted of five weekly sessions, each lasting about 45 min, performed in the same laboratory of the psychophysiological assessment. Participants were asked to abstain from alcohol, caffeinated beverages and smoking for the three preceding the biofeedback training session. RSA-BF was aimed at increasing RSA and, therefore, at opposing autonomic dysregulation, especially vagal inhibition associated with stress (Lehrer et al. 2000). Before starting the first biofeedback session, all the managers in the RSA-BF group were informed about the feedback system, and they were told that augmenting the amplitude of HR changes in phase with breathing would increase respiratory sinus arrhythmia.
Feedback was provided to subjects by means of the same instruments used for psychophysiological assessment. Participants received the feedback on a 15-in. PC display, positioned in front of them at a distance of 50 cm. During biofeedback, trial participants saw on the screen the HR beat-to-beat tachogram (i.e., beats/min) superimposed over the abdominal respiration signal on the same axis (see Fig. 1). Participants were required to synchronise HR and abdominal respiration variations, until the two signals (i.e., HR and abdominal respiration) covaried in phase, thus leading to the maximal amplitude of RSA. Participants were also suggested to breathe slowly (see also Lehrer et al. 2003). The on-line moving feedback display (the graph representing the tachogram and abdominal respiration curves) was updated at successive 30-s periods. Also, a stepped bar increasing from left to right was applied to represent feedback for subject’s performance. Specifically, each step increase on the bar corresponded to a period of 10 s during which RSA was above the threshold (based on the RSA mean value recorded during pre-training assessment). During each RSA-BF session, participants were reminded not to breathe too deeply to avoid hyperventilation. No pacing stimulus was provided during the training sessions. After sensors’ placement, each session started with a resting period of 3 min, followed by two 6-min biofeedback trials. Each biofeedback trial was followed by a 1-min rest. Every session ended with a resting period of 3 min.
Screenshot from a session of biofeedback. On the screen (duration 30 s) are representations of abdominal breathing (grey line) and heart rate tachogram (black line). The task given to the patient was: “Try to synchronise heart rate with abdominal breathing”. Also, a stepped bar was included on the screen to represent feedback for subject’s performance. Each step increase on the bar corresponded to a period of 10 s during which RSA value was above the threshold
Participants in the control group were asked to keep a daily stress diary for the duration of 5 weeks and consign it weekly to the clinical psychologist. The stress diary is a valuable stress management tool, designed to enhance awareness and coping skills to deal with stressful situations. Specifically, the stress diary records daily situations and events that triggered a perception of stress, reports objective information on causes (circumstances, people or trigger events) and, provide details on how each event made them think and feel, including psychophysiological symptoms (such as tachycardia, breathing speed up, tension headache, difficulty concentrating, and feeling more anxious and fearful). Participants were also asked to report their coping strategies in relation to stress, describing how they reacted and if their reaction were appropriate and useful.
Data Reduction and Analysis
Data reduction and analysis were performed on physiological signals recorded over a 4-min period at rest during pre- and post-assessment sessions.
Given that the electrical and mechanical activities of the heart are coupled, photoplethysmography can be used to determine the normal-to-normal intervals (or interbeat intervals, IBI), which correspond to the reciprocal of HR. After recording, the raw blood volume pulse signal was visually inspected and corrected for movement artefacts and for detection of ectopic beats, then, the IBI series (heartbeat were automatically identified by an algorithm based on the detection of the point of maximum deviation in the blood volume pulse signal). Then, IBI series data were exported in the Kubios-HRV 2.0 (Kuopio, Finland) software and additionally corrected for artefacts with a piecewise cubic spline interpolation method that generates missing or corrupted values into the IBI series. HR was then calculated as the reciprocal of IBI.
RSA recorded for 4 min at rest was used as a reasonable and reliable measure of cardiac vagal tone (Grossman and Taylor 2007). The mechanisms that produce RSA comprehend the interaction between cardiac and respiratory responses (Grossman 1983), hence respiration can confound the relationship between cardiac vagal tone and RSA (Gevirtz and Lehrer 2003; Grossman and Taylor 2007). Therefore, in order to correct for respiration influence, the exact respiration rate range (i.e., maximum minus minimum respiration rate, expressed in cycles/min) was calculated for each participant at rest, and converted in Hz (i.e., from cycles/min to cycles/s). Then, a frequency domain analysis, specifically a fast Fourier transformation (using the Welch’s periodogram method, window width 256 s, 50 % overlap), was applied to the variation of IBI occurring within the specific respiration rate range for each participant (Aysin and Aysin 2006; Grossman and Taylor 2007). RSA epochs were averaged for each assessment phase (i.e., pre-training and post-training assessments, see below). RSA values were expressed in ms2.
RSA and skin conductance signals were normalized using the natural logarithm (lnRSA) and logarithm to base 10 transformation [log (SCL + 1)], respectively.
Respiration rate was obtained by means of the BioGraph Infiniti software which automatically calculated respiration rate from differences in chest expansion in the raw signal waveform.
Student’s t tests for independent groups were performed to compare age, education, body mass index (BMI) and sleep time in the two groups (RSA-BF and control). χ2 was also calculated to test differences between groups in sleep disorders, smoking, physical activity, family history of hypertension and cardiovascular disease.
A mixed-model analysis of variance (ANOVA), with group (RSA-BF and control) as between-subjects factor, and time (pre- and post-training) as within-subjects variable was performed on questionnaire scores (STAI-Y1, STAI-Y2) and physiological measures at rest [HR, lnRSA, thoracic respiration rate, abdominal respiration rate, SBP, DBP, log(SCL + 1)] obtained at pre-training and post-training assessments. Repeated-measures multivariate analysis of variance (MANOVA) with group (RSA-BF or control) as between-subjects factor, and time (pre- and post-training) as within-subjects variable was performed on SF-36 subscales (physical functioning, limitations caused by physical problems, bodily pain, general health, vitality, social functioning, limitations caused by emotional problems and emotional well-being subscales) obtained at pre-training and post-training assessments. Partial eta-squared (η 2 p ) was reported as a measure of the effect size. Significant main effects and interactions (p < .05) were followed by Fisher post hoc comparisons to identify specific differences. Data were analysed using STATISTICA software version 6.1 (StatSoft Inc., Tulsa, OK, USA).
Results
Sociodemographic and Health Behaviour Data
Student’s t tests for independent groups and χ2 analyses revealed no group differences for age (t = 1.40; p = .15), education (t = −0.56; p = .60), BMI (t = 1.52; p = .46) and family history of hypertension (χ2 = 0.0001; p = .99), cardiovascular disease (χ2 = 1.18; p = .28), physical activity level (χ2 = −0.23; p = .80), sleep time (t = 0.13; p = .90) and sleep disorders (χ2 = 1.64; p = .16). The descriptive statistics for each group are reported in Table 1.
Psychological Data
The group by time ANOVA on the STAI-Y2 yielded a time effect (F[1,29] = 10.02; p = .004; η 2 p = .26). These results showed a significant reduction of trait anxiety levels from pre- to post-training. No main effect of group (F[1,29] = 0.05; p = .82; η 2 p = .97) nor interaction group × time (F[1,29] = 0.15; p = .70; η 2 p = .01) were found on trait anxiety scores. The group by time ANOVA on STAI-Y1 yielded no main effect of group (F[1,29] = 0.13; p = .72; η 2 p = .004), time (F[1,29] = 4.03; p = .05; η 2 p = .12), nor interaction group × time (F[1,29] = 0.0001; p = .99; η 2 p = .0001).
The MANOVA analyses examined changes in the SF-36 subscales and indicated a significant multivariate main effect of time (Pillai’s Trace Value = .48; F[8,21] = 2.41, p < .05). There were no significant main effects of group (Pillai’s Trace Value = .29; F[8,21] = 1.01, p = .40) or interactions group × time (Pillai’s Trace Value = .25; F[8,21] = 0.93, p = .52). Univariate analysis showed that there were significant time effects for general health (F[1,29] = 4.51; p = .04; η 2 p = .14), vitality (F[1,29] = 5.29; p = .03; η 2 p = .15) and social functioning (F[1,29] = 5.00; p = .03; η 2 p = .15) subscales. Both groups reported better general health perception, more vitality and better social functioning at post-training compared to pre-training. A significant univariate group × time interaction on limitations caused by emotional problems emerged (F[1,29] = 5.05; p = .03; η 2 p = .15). Post-hoc comparison showed a significant decrease of problems with work or other daily activities as a result of emotional interferences from pre- to post-training in the RSA-BF group only (p = .02).
Physiological Data
The group by time ANOVA on lnRSA at rest showed a significant group × time interaction (F[1,29] = 5.14; p = .03; η 2 p = .15). This interaction is depicted in Fig. 2. Fisher post hoc comparisons showed a significant lnRSA increase from pre- to post-training in the RSA-BF group only (p = .04), whereas no significant difference in lnRSA from pre- to post-training was found in the control group (p = .29). No significant main group (F[1,29] = 0.36; p = .55; η 2 p = .01) or time effect emerged for lnRSA (F[1,29] = 0.52; p = .47; η 2 p = .02). The analyses performed on HR showed a significant time effect (F[1,29] = 8.60; p = .007; η 2 p = .23), yielding a reduction in resting HR from pre- to post-training in both groups (pre-training: 72.15 ± 11.84 bmp; post-training: 67.55 ± 8.57 bmp). No significant main effect of group (F[1,29] = 0.01; p = .91; η 2 p = .0004) nor interaction group × time emerged for HR (F[1,29] = 1.35; p = .25; η 2 p = .04).
The group by time ANOVA on thoracic respiration at rest showed no significant main group (F[1,29] = 0.66; p = .42; η 2 p = .02) or time (F[1,29] = 2.58; p = .12; η 2 p = .08), nor interaction group × time effect (F[1,29] = 1.90; p = .18; η 2 p = .06).
The group by time ANOVA on abdominal respiration at rest showed no significant main group (F[1,29] = 0.23; p = .64; η 2 p = .01) or time (F[1,29] = 0.53; p = .47; η 2 p = .02), nor interaction group × time effect (F[1,29] = 2.48; p = .13; η 2 p = .08). Mean respiration rate recorded during pre- and post-training assessment are reported in Table 2.
The group by time ANOVA on BP measurements yielded no significant DBP differences between groups (F[1,29] = 0.10; p = .75; η 2 p = .003), from pre- to post-training (F[1,29] = 0.48; p = .49; η 2 p = .02), and no effect of interaction was found (F[1,29] = 1.18; p = .29; η 2 p = .04).
Analysis on SBP showed a significant group × time effect (F[1=29] = 4.37; p = .04; η 2 p = .13). Post-hoc comparisons displayed a significant SBP reduction from pre to post-training in the RSA-BF group only (p < .001), whereas no significant SBP difference was found in the control group from pre- to post-training (p > .52) (see Fig. 3). A significant time effect emerged for SBP (F[1,29] = 9.11; p = .005; η 2 p = .24), yielding a reduction in resting SBP from pre- to post-training. No significant main group effects emerged for SBP (F[1,29] = 0.21; p = .65; η 2 p = .01).
The analysis performed on SCL yielded a significant group × time interaction (F[1,29] = 11.61; p = .002; η 2 p = .29). Post-hoc comparison displayed a significant SCL reduction from pre to post-training in the RSA-BF group only (p < .001), whereas in the control group no significant difference was found from pre- to post-training (p > .58) (see Fig. 4). Also, a significant effect of time emerged (F[1,29] = 6.81; p = .01; η 2 p = .19), showing a reduction in resting SCL over time. No significant main effect of group occurred (F[1,29] = 1.76; p = .20; η 2 p = .06).
Discussion
The present study evaluated the effects of RSA-BF training, as compared to keeping a daily stress diary, in a group of high-status-position managers. At the end of the training period, all managers reported reduced HR at rest, lower levels of state and trait anxiety, better general health perception, more energy, less fatigue and better social functioning, compared to pre-training. Importantly, compared to managers in the control group, those who underwent the biofeedback training showed a significant lnRSA increase, and decreases in both SBP and logSCL from pre- to post-training. Also, participants who underwent the biofeedback training were characterised by a reduction of emotional interferences in work or other regular daily activities from pre- to post-training compared to participants in the control group.
In this study, a stress management protocol (i.e., stress diary) was as useful as RSA-BF training in reducing anxiety, and enhancing well-being and health-related quality-of-life, but failed in promoting autonomic regulation. The improvement in self-report but not objective outcomes in the control group by keeping a stress diary is consistent with the placebo effect, and is in line with data obtained with clinical populations (namely asthma patients) (Wechsler et al. 2011).
However, while it is likely that the training period enhanced subjects’ ability to adaptively cope with stress, to lower HR and anxiety, thus increasing health perception and psychosocial functioning, only the RSA-BF training yielded specific effects on lnRSA, SBP and logSCL, indicating a better modulation of ANS activity. These novel findings add to the literature on biobehavioral training for work stress reduction by showing the effectiveness of a short RSA-BF training in modulating psychophysiological consequences of work stress in managers. The higher RSA in individuals who underwent RSA-BF, compared to control subjects, indicates the effectiveness of this biobehavioral training in enhancing vagal cardiac control, which in turn could reduce autonomic dysregulation. In fact, enhanced parasympathetic cardiac control or well-balanced autonomic cardiac control could counteract stress-mediated sympathetic overstimulation associated with exposure to work stress (Wheat and Larkin 2010). In turn, the increased autonomic nervous system balance (namely increased vagal cardiac control, and reduced sympathetic activation), might reduce susceptibility to stress and stress-related cardiovascular disorders.
Indeed, a significant decrease in resting SBP was observed only in participants who underwent the biofeedback training, possibly suggesting an increased baroreflex gain. During the RSA-BF training, the individual is coached to breathe close to a particular resonant frequency, in order to increase HR oscillations by stimulating rhythmically not only the variation in HR, but also, the baroreceptors activity (Lehrer et al. 2000, 2003; Vaschillo et al. 2006). Importantly, it has been shown that the practice of RSA-BF increases the baroreflex gain and improves modulation of various autonomic functions, leading to improved overall adaptability of the cardiovascular system (Lehrer et al. 2000, 2003).
In addition, the reduction in resting SCL only in individuals who underwent the biofeedback training, suggests that the RSA-BF training was effective in reducing general sympathetic activation. In fact, SCL has been consistently reported as an index of sympathetic nervous system activation and is considered as a general measure of autonomic arousal. The overall reduction of resting SBP and SCL in the RSA-BF group indicates that the effects of RSA-BF could be extended to reduce sympathetic activity. These results confirm previous studies reporting the effectiveness of RSA-BF in reducing overall physiological arousal at rest (Gevirtz and Lehrer 2003; Wheat and Larkin 2010) and underline the capability of this biobehavioral intervention in reducing harmful effects resulting from excessive sympathetic activation (Lehrer et al. 1997, 2007; Sherlin et al. 2009).
Also, results from this study suggest that biofeedback training may improve emotional regulation, which is the ability to generate emotional responses of appropriate timing and magnitude, and depends on the individual’s ability to modify physiological arousal in response to situational demands (Appelhans and Luecken 2006; Gross 1998). It could be speculated that an increase in RSA, leading to a more flexible ANS, could be particularly useful for rapid modulation of physiological and emotional states in situations characterised by rapid changes in demands, dynamicity, conflict and competition, such as the managerial job environments.
To summarise, the RSA-BF has been shown to be effective both in increasing cardiac vagal tone and reducing sympathetic activation, and in improving subjects’ ability to modulate emotional response to environmental demands.
Due to the high levels of involvement, competition and responsibility, job strain has important health consequences by increasing the risk of cardiovascular disease (Backé et al. 2012; Belkic et al. 2004; Kivimäki et al. 2002), and major socio-economic costs by reducing the organization’s effectiveness and generating loss of productivity (Béjean and Sultan-Taïeb 2005; Manning et al. 1996). In the last decades a wide variety of stress-management interventions, including diaphragmatic deep-breathing, relaxation and meditation techniques, have been proposed to reduce occupational stress, physiological arousal and job strain (Giga et al. 2003; Ivancevich et al. 1990; Lamontagne et al. 2007; Lazarus and Folkman 1984). While outcome evaluation of these interventions relied mainly on self-reporting measures (Kushnir et al. 1998)—with no objective measurement of the effectiveness in the reduction of psychophysiological activation—in the present study self-report measures of health and well-being along with physiological measures have been taken into account.
The current findings should be interpreted in light of a number of possible methodological issues. First, this study used a relatively small sample size; therefore, the present findings need to be replicated to better understand the effectiveness of RSA-BF training. Nonetheless, the effect size, which indicates the proportion of the variance in the dependent variable that is related to the independent variable(s), showed medium group × time effects on SBP (η 2 p = .13) and large group × time effects on RSA (η 2 p = .15), SC (η 2 p = .29) and RE (η 2 p = .15) (Cohen 1969). Second, we used photoplethysmography instead of the electrocardiogram that is considered the gold-standard method to measure HR, and RSA. Nonetheless, several studies have reported that the parameters of photoplethysmographic variability are highly correlated with RSA extracted from electrocardiogram (Lu et al. 2008). Third, we did not directly record the activity of the baroreceptors, nor did we compute other important indexes of autonomic balance. Therefore, for a better understanding of the effects of RSA–BF on both parasympathetic and sympathetic activity, in a future study the direct evaluation of the baroreflex gain as well as additional time [root mean square of the successive differences (RMSSD), and standard deviation of normal to normal interval (SDNN)] and frequency domain measures [very low frequency (VLF), low frequency (LF), and high frequency (HF) power] should be considered. Fourth, given that respiration can influence and even confound the relationship between cardiac vagal tone and RSA (Gevirtz and Lehrer 2003; Grossman and Taylor 2007), in a future study, a direct control condition in which subjects are trained to breathe at their own resonance frequency could be included. Finally, although the current study showed that RSA, SBP and SCL are modifiable through RSA-BF in an acute time frame, we did not conduct follow-up evaluations, so we do not know whether the current results will be long-lasting; therefore longer follow-up studies are required to investigate whether the positive effects of biofeedback on cardiac vagal regulation are long-lasting (Wheat and Larkin 2010).
Despite that managers’ health has been suggested to be an important sign of organisational well-being (Quick et al. 2007) managers seem to be underrepresented in research on occupational health (Bingham 2005; Hambrick et al. 2005; Knudsen et al. 2009). The current study, to our knowledge, is the first to investigate, the effectiveness of RSA-BF training in increasing vagal modulation and reducing sympathetic activation in managers. The findings of the present study, even though based on a small group of participants, provide preliminary evidence of the effectiveness of RSA-BF training in enhancing cardiovascular health, by improving cardiac autonomic balance.
RSA-BF represents a useful resource since it has been shown to be a safe and effective intervention in improving emotion regulation and mood, while enhancing psychophysiological flexibility and adaptive responses to stress.
References
Apolone, G., & Mosconi, P. (1998). The Italian SF-36 Health Survey: Translation, validation and norming. Journal of Clinical Epidemiology, 51(11), 1025–1036.
Appelhans, B. M., & Luecken, L. J. (2006). Heart rate variability as an index of regulated emotional responding. Review of General Psychology, 10(3), 229–240. doi:10.1037/1089-2680.10.3.229.
Aronsson, G. (1999). Influence of worklife on public health. Scandinavian Journal of Work, Environment and Health, 25(6), 597–604.
Aysin, B., & Aysin, E. (2006). Effect of respiration in heart rate variability (HRV) analysis. In IEEE engineering in medicine and biology society. Annual conference (vol. 1, pp. 1776–1779).
Backé, E.-M., Seidler, A., Latza, U., Rossnagel, K., & Schumann, B. (2012). The role of psychosocial stress at work for the development of cardiovascular diseases: A systematic review. International Archives of Occupational and Environmental Health, 85(1), 67–79.
Béjean, S., & Sultan-Taïeb, H. (2005). Modeling the economic burden of diseases imputable to stress at work. The European Journal of Health Economics, 6(1), 16–23.
Belkic, K., Landsbergis, Pa, Schnall, P. L., & Baker, D. (2004). Is job strain a major source of cardiovascular disease risk? Scandinavian Journal of Work, Environment and Health, 30(2), 85–128.
Bellarosa, C., & Chen, P. Y. (1997). The effectiveness and practicality of occupational stress management interventions: A survey of subject matter expert opinions. Journal of Occupational Health Psychology, 2(3), 247–262.
Bingham, J. B. (2005). Job demands and job search among high-level managers in the United States and Europe. Group and Organization Management, 30(6), 653–681.
Brett, J. M., & Stroh, L. K. (2003). Working 61 plus hours a week: Why do managers do it? The Journal of Applied Psychology, 88(1), 67–78.
Chandola, T., Heraclides, A., & Kumari, M. (2010). Psychophysiological biomarkers of workplace stressors. Neuroscience and Biobehavioral Reviews, 35(1), 51–57.
Cohen, J. (1969). Statistical power analysis for the behavioral sciences. New York: Academic Press.
Collins, S. M., Karasek, R. A., & Costas, K. (2005). Job strain and autonomic indices of cardiovascular disease risk. American Journal of Industrial Medicine, 48(3), 182–193.
Del Pozo, J. M., Gevirtz, R. N., Scher, B., & Guarneri, E. (2004). Biofeedback treatment increases heart rate variability in patients with known coronary artery disease. American Heart Journal, 147(3), E11–E17.
Dewa, C. S., Thompson, A. H., & Jacobs, P. (2011). Relationships between job stress and worker perceived responsibilities and job characteristics. International Journal of Occupational and Environmental Medicine, 2(1), 37–46.
Eller, N. H., Blønd, M., Nielsen, M., Kristiansen, J., & Netterstrøm, B. (2011a). Effort reward imbalance is associated with vagal withdrawal in Danish public sector employees. International Journal of Psychophysiology, 81(3), 218–224.
Eller, N. H., Kristiansen, J., & Hansen, A. M. (2011b). Long-term effects of psychosocial factors of home and work on biomarkers of stress. International Journal of Psychophysiology, 79(2), 195–202.
Flier, J. S., Underhill, L. H., & McEwen, B. S. (1998). Protective and damaging effects of stress mediators. New England Journal of Medicine, 338(3), 171–179.
Fowles, D. C., Christie, M. J., Edelberg, R., Grings, W. W., Lykken, D. T., & Venables, P. H. (1981). Publication recommendations for electrodermal measurements. Psychophysiology, 18(3), 232–239.
Gandek, B., & Ware, J. E. (1993). SF-36 Health Survey: Manual and interpretation guide. Boston, MA: The Health Institute, New England Medical Center.
Gevirtz, R., & Lehrer, P. M. (2003). Resonant frequency heart rate biofeedback. In M. S. F. Andrasik (Ed.), Biofeedback: A practitioner’s guide. New York, NY: Guilford.
Giga, S. I., Cooper, C. L., & Faragher, B. (2003). The development of a framework for a comprehensive approach to stress management interventions at work. International Journal of Stress Management, 10(4), 280–296.
Gross, J. J. (1998). Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology, 74(1), 224–237.
Grossman, P. (1983). Respiration, stress, and cardiovascular function. Psychophysiology, 20(3), 284–300.
Grossman, P., & Taylor, E. (2007). Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology, 74(2), 263–285.
Hambrick, D. C., Finkelstein, S., & Mooney, A. C. (2005). Executive job demands: New insights for explaining strategic decisions and leader behaviors. Academy of Management Review, 30(3), 472–491.
Hemingway, H., Shipley, M., Brunner, E., Britton, A., Malik, M., & Marmot, M. (2005). Does autonomic function link social position to coronary risk? The Whitehall II study. Circulation, 111(23), 3071–3077.
Hintsanen, M., Elovainio, M., Puttonen, S., Kivimaki, M., Koskinen, T., Raitakari, O. T., & Keltikangas-Jarvinen, L. (2007). Effort-reward imbalance, heart rate, and heart rate variability: The cardiovascular risk in young Finns study. International Journal of Behavioral Medicine, 14(4), 202–212.
Institute of Management. (1993). Managers under stress: A survey of management morale in the 90s. (Institute of Management, Ed.). London.
Ivancevich, J. M., Matteson, M. T., Freedman, S. M., & Phillips, J. S. (1990). Worksite stress management interventions. American Psychologist, 45(2), 252–261.
Judge, T. A., Boudreau, J. W., & Bretz, R. D. (1994). Job and life attitudes of male executives. The Journal of Applied Psychology, 79(5), 767–782.
Kang, M. G., Koh, S. B., Cha, B. S., Park, J. K., Woo, J. M., & Chang, S. J. (2004). Association between job stress on heart rate variability and metabolic syndrome in shipyard male workers. Yonsei Medical Journal, 45(5), 838–846.
Kiecolt-Glaser, J. K., & Glaser, R. (1995). Psychoneuroimmunology and health consequences: Data and shared mechanisms. Psychosomatic Medicine, 57(3), 269–274.
Kivimäki, M., Leino-Arjas, P., Luukkonen, R., Riihimäki, H., Vahtera, J., & Kirjonen, J. (2002). Work stress and risk of cardiovascular mortality: Prospective cohort study of industrial employees. British Medical Journal, 325(7369), 857.
Knudsen, H. K., Ducharme, L. J., & Roman, P. M. (2009). Turnover intention and emotional exhaustion “at the top”: Adapting the job demands-resources model to leaders of addiction treatment organizations. Journal of Occupational Health Psychology, 14(1), 84–95.
Kushnir, T., Malkinson, R., & Ribak, J. (1998). Rational thinking and stress management in health workers: A psychoeducational program. International Journal of Stress Management, 5(3), 169–178.
Lamontagne, F., Labbé, A.-C., Haeck, O., Lesur, O., Lalancette, M., Patino, C., et al. (2007). Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Annals of Surgery, 245(2), 267–272.
Lazarus, R. S., & Folkman, S. (1984). Stress, appraisal and coping. Appraisal, and coping (p. 445). Berlin: Springer.
Lehrer, P. M., Carr, R. E., Smetankine, A., Vaschillo, E., Peper, E., Porges, S., et al. (1997). Respiratory sinus arrhythmia versus neck/trapezius EMG and incentive inspirometry biofeedback for asthma: A pilot study. Applied Psychophysiology and Biofeedback, 22(2), 95–109.
Lehrer, P. M., Vaschillo, E., & Vaschillo, B. (2000). Resonant frequency biofeedback training to increase cardiac variability: Rationale and manual for training. Applied Psychophysiology and Biofeedback, 25(3), 177–191.
Lehrer, P. M., Vaschillo, E., Vaschillo, B., Lu, S., Eckberg, D., Edelberg, R., et al. (2003). Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosomatic Medicine, 65(5), 796–805.
Lehrer, P. M., Woolfolk, R. L., & Sime, W. E. (2007). Principles and practice of stress management. New York, NY: The Guilford Press.
Little, L. M., Simmons, B. L., & Nelson, D. L. (2007). Health among leaders: Positive and negative affect, engagement and burnout, forgiveness and revenge. Journal of Management Studies, 44(2), 243–260.
Lu, S., Zhao, H., Ju, K., Shin, K., Lee, M., Shelley, K., & Chon, K. H. (2008). Can photoplethysmography variability serve as an alternative approach to obtain heart rate variability information? Journal of Clinical Monitoring and Computing, 22(1), 23–29.
Lucini, D., Mela, G. S., Malliani, A., & Pagani, M. (2002). Impairment in cardiac autonomic regulation preceding arterial hypertension in humans: Insights from spectral analysis of beat-by-beat cardiovascular variability. Circulation, 106(21), 2673–2679.
Lucini, D., Riva, S., Pizzinelli, P., & Pagani, M. (2007). Stress management at the worksite reversal of symptoms profile and cardiovascular dysregulation. Hypertension, 49(2), 291–297.
Malik, M., & Camm, A. J. (1993). Components of heart rate variability—What they really mean and what we really measure. The American Journal of Cardiology, 72(11), 821–822.
Manning, M. R., Jackson, C. N., & Fusilier, M. R. (1996). Occupational stress, social support, and the costs of health care. Academy of Management Journal, 39(3), 738–750.
Mirowsky, J., & Ross, C. E. (2005). Education, cumulative advantage, and health. Ageing International, 30(1), 27–62.
Mohr, G., & Wolfram, H.-J. (2010). Stress among managers: The importance of dynamic tasks, predictability, and social support in unpredictable times. Journal of Occupational Health Psychology, 15(2), 167.
Möller, J., Theorell, T., de Faire, U., Ahlbom, A., & Hallqvist, J. (2005). Work related stressful life events and the risk of myocardial infarction. Case-control and case-crossover analyses within the Stockholm heart epidemiology programme (SHEEP). Journal of Epidemiology and Community Health, 59(1), 23–30.
Nolan, R. P., Kamath, M. V., Floras, J. S., Stanley, J., Pang, C., Picton, P., & Young, Q. R. (2005). Heart rate variability biofeedback as a behavioral neurocardiac intervention to enhance vagal heart rate control. American Heart Journal, 149(6), 1137.
Ohsuga, M., Shimono, F., & Genno, H. (2001). Assessment of phasic work stress using autonomic indices. International Journal of Psychophysiology, 40(3), 211–220.
Patron, E., Messerotti Benvenuti, S., Favretto, G., Valfrè, C., Bonfà, C., Gasparotto, R., & Palomba, D. (2013). Biofeedback assisted control of respiratory sinus arrhythmia as a biobehavioral intervention for depressive symptoms in patients after cardiac surgery: A preliminary study. Applied Psychophysiology and Biofeedback, 38(1), 1–9.
Pickering, T. G., Hall, J. E., Appel, L. J., Falkner, B. E., Graves, J., Hill, M. N., et al. (2005). Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association. Circulation, 111(5), 697–716.
Quick, J. C., Macik-Frey, M., & Cooper, C. L. (2007). Managerial dimensions of organizational health: The healthy leader at work. Journal of Management Studies, 44(2), 189–205.
Richardson, K. M., & Rothstein, H. R. (2008). Effects of occupational stress management intervention programs: A meta-analysis. Journal of Occupational Health Psychology, 13(1), 69–93.
Rosengren, A., Hawken, S., Ounpuu, S., Sliwa, K., Zubaid, M., Almahmeed, W. A., et al. (2004). Association of psychosocial risk factors with risk of acute myocardial infarction in 11 119 cases and 13 648 controls from 52 countries (the INTERHEART study): Case-control study. The Lancet, 364(9438), 953–962.
Rozanski, A., Blumenthal, J. A., Davidson, K. W., Saab, P. G., & Kubzansky, L. (2005). The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: The emerging field of behavioral cardiology. Journal of the American College of Cardiology, 45(5), 637–651.
Schieman, S., & Reid, S. (2009). Job authority and health: Unraveling the competing suppression and explanatory influences. Social Science and Medicine, 69(11), 1616–1624.
Schwartz, M. S., & Andrasik, F. E. (2003). Biofeedback: A practitioner’s guide. New York: Guilford Press.
Sherlin, L., Gevirtz, R., Wyckoff, S., & Muench, F. (2009). Effects of respiratory sinus arrhythmia biofeedback versus passive biofeedback control. International Journal of Stress Management, 16(3), 233.
Spielberger, C. D., Gorusch, R., & Lushene, R. (1970). Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press.
Spielberger, C. D., Pedrabissi, L., & Santinello, M. (1996). STAI, state-trait anxiety inventory, Forma Y: Manuale. Firenze: Organizzazioni Speciali.
Vaschillo, E. G., Vaschillo, B., & Lehrer, P. M. (2006). Characteristics of resonance in heart rate variability stimulated by biofeedback. Applied Psychophysiology and Biofeedback, 31(2), 129–142.
Vrijkotte, T. G. M., van Doornen, L. J. P., & de Geus, E. J. C. (2000). Effects of work stress on ambulatory blood pressure, heart rate, and heart rate variability. Hypertension, 35(4), 880–886.
Wechsler, M. E., Kelley, J. M., Boyd, I. O. E., Dutile, S., Marigowda, G., Kirsch, I., et al. (2011). Active albuterol or placebo, sham acupuncture, or no intervention in asthma. The New England Journal of Medicine, 365(2), 119–126.
Wheat, A. L., & Larkin, K. T. (2010). Biofeedback of heart rate variability and related physiology: A critical review. Applied Psychophysiology and Biofeedback, 35(3), 229–242.
Worrall, L., & Cooper, C. (1995). Executive stress in different industrial sectors, structures and sizes of business. Personnel Review, 24(7), 3–12.
Yucha, C., & Montgomery, D. (2008). Evidence-based practice in biofeedback and neurofeedback. Wheat Ridge, CO: AAPB.
Acknowledgments
The study was supported by Mind Room International s.r.l., and its partners Confindustria Vicenza and Banca Popolare di Vicenza (Funding Number 382/3/17/5/5/24/2010). The funding source contributed to the recruiting of participants, but it had no involvement in study design, data collection, analysis and interpretation, the writing of the report and the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Munafò, M., Patron, E. & Palomba, D. Improving Managers’ Psychophysical Well-Being: Effectiveness of Respiratory Sinus Arrhythmia Biofeedback. Appl Psychophysiol Biofeedback 41, 129–139 (2016). https://doi.org/10.1007/s10484-015-9320-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10484-015-9320-y