Abstract
Overeating episodes, despite of intentions to control weight, are a common problem among women. Recurring episodes of overeating and dietary failure have been reported to result in higher Body Mass Indexes and to induce severe distress even in non-clinical groups. Based on findings from physiological research on eating behavior and craving, as well as previous biofeedback studies, we derived a cue exposure based EEG neurofeedback protocol to target overeating episodes. The treatment was evaluated in a randomized controlled trial, comparing a neurofeedback group (NFG; n = 14) with a waiting list control group (WLG; n = 13) in a sub-clinical sample of female restrained eaters. At post-treatment, the number of weekly overeating episodes and subsequent distress were significantly reduced in the NFG compared to the WLG (p < .01; r > .50). In a 3 month follow-up, effects in the NFG remained stable. As secondary outcomes, perceived dieting success was enhanced after the treatment. At follow-up, additional beneficial effects on trait food craving were observed. Altogether, we found preliminary evidence for the cue exposure neurofeedback against overeating episodes in female restrained eaters, although specific effects and underlying mechanisms still have to be explored in future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In food rich environments of Western developed countries, temptation of palatable food is not easy to resist. Thus, it is not surprising, that overweight and obesity (Swinburn et al. 2011), as well as eating disorders related to dieting and overeating (Mitchison et al. 2012) have been on the rise throughout the last decades. One common important factor with regard to the development of these problems are episodes of overeating that often precede weight control failures, obesity, and binge-related eating disorders (Klesges et al. 1992; Polivy and Herman 1985). Further, overeating episodes induce distress and negative affect (Stein et al. 2007), which again facilitates future occurrence of overeating to regulate these aroused emotional states, resulting in a vicious circle (Cools et al. 1992; Gluck 2006; Hay and Williams 2013). On a physiological basis, these antecedents can be found in states of tension that is stressful arousal (Freeman and Gil 2004; Jastreboff et al. 2013), especially after confrontation with food cues and in subsequent states of craving (Hill 2007; Pelchat 2002). With regard to the possible negative effects on bodily and mental health, even overeating episodes in sub-clinical groups should be addressed by psychological intervention research.
To date, several psychological interventions that target antecedents of unwanted consumption and overeating have been assessed. With respect to autonomic physiological arousal in food cravers, Meule et al. (2012a) reported preliminary evidence for the effectiveness of heart rate variability biofeedback in altering food craving. However, the researchers reported that the training affected cognitions and attitudes rather than behavior. Using a different physiological measure, Teufel et al. (2013) applied electrodermal biofeedback in obese women, either in a cue exposure setup or in a merely relaxation-based setup. Here, the cue exposure setup enhanced self-efficacy regarding food intake and had higher long term effects than the relaxation-based approach.
Reducing the stressful arousal at its origin in the brain (McEwen 2007; Saletu-Zyhlarz et al. 2004), neurofeedback targeting associated brain responses may constitute a promising approach for the treatment of overeating episodes. In a real time fMRI neurofeedback study among men, Frank et al. (2012) targeted upregulation of the anterior insular cortex as a brain region involved in the processing of food cues. Despite of promising results regarding self-regulation ability, no results of possible effects on eating behavior were provided. Further, more affordable and applicable neurofeedback techniques, such as electroencephalographic (EEG) neurofeedback, might be more appropriate for the treatment of widespread overeating episodes. Yet, in a recent review on neurofeedback in disordered eating, Bartholdy et al. (2013) did not report any studies applying EEG neurofeedback against overeating or binge eating episodes.
With regard to possible spectral ranges that may constitute target frequencies in an EEG neurofeedback protocol, especially fast frequencies in the higher beta range (~18–30 Hz) have been shown to accompany ruminative states of stressful arousal (Andersen et al. 2009; Thompson and Thompson 2007; Seo and Lee 2010). Further, Tammela et al. (2010) reported that EEG activity in the beta range throughout food picture presentation is related to disinhibition in obese women with binge eating disorder. Excess EEG high beta activity has also been shown with respect to drug-induced stressful states of craving (for a review see: Parvaz et al. 2011) which are described as comparable to states of food craving preceding overeating episodes (Sinha and Jastreboff 2013; Styn et al. 2013). Therefore, high beta frequencies are target to inhibition in the reduction of stressful arousal in several neurofeedback protocols (Egner and Gruzelier 2001, 2004; Paquette et al. 2009).
The aim of the present study was to develop and evaluate a neurofeedback protocol based on previous psychophysiological findings. To target a sample which is especially vulnerable to overeating episodes, we chose to address restrained eaters (REs). Peter Herman and colleagues (Herman and Mack 1975; Herman and Polivy 1975) defined restrained eating (RE) as the intention to cognitively restrict caloric intake with the purpose of losing or maintaining weight. Many REs are especially cue-reactive with regard to food cues (Brunstrom et al. 2004), prone to experience food craving, subsequent disinhibition, and finally overeating or binge eating episodes (Polivy and Herman 1985; Polivy et al. 2005; Ruderman 1986; Westenhöfer 1991). With reference to their repeated failures in weight control (Heatheron et al. 1991) and resulting distress (Stein et al. 2007), we concluded that this population constitutes an especially suitable target group for this neurofeedback pilot study. Further, we decided to include females only, because dysfunctional eating behaviors, such as overeating episodes, are generally more prevalent among women compared to men (Provencher et al. 2003).
We hypothesized that a neurofeedback protocol, inhibiting EEG high beta activity after food cue exposure, is effective in reducing the occurrence of overeating episodes. Subsequently, distress associated with binge eating episodes should be alleviated. As secondary outcomes, effects on food craving, perceived dieting success, perceived stress, and well-being were scrutinized to gather insights into possible mechanisms of the training. We expected effects to be stable at a 3 month follow-up. Additionally, we collected qualitative feedback to improve future applications of the intervention.
Due to the exploratory nature of this pilot study, we decided to conduct a randomized controlled trial using a waiting list control group. With regard to the novelty of this setup, we selected participants in the sub-clinical range of RE only, excluding pathological eating behavior related to overeating and dieting (e.g. manifest bulimia nervosa or binge eating disorder). In line with this decision, overeating episodes in our sample were not required to fulfill DSM-criteria for binge episodes (i.e. larger amount than other people would eat in a comparable time period and loss of control over eating). Instead we primarily aimed at a reduction of subjective overeating episodes as a result of craving, which include undesired ingestion of high calorie food.
Method
Study Design
In this randomized controlled trial, a treatment group (neurofeedback group (NFG)), receiving a neurofeedback-based intervention against overeating episodes was compared to a waiting list group (WLG) at a pre-treatment (T0) and post-treatment (T1) assessment. The occurrence of overeating episodes and distress due to overeating episodes were analyzed as primary outcome measures. Trait-food craving, perceived dieting success, perceived stress, and well-being were assessed as secondary outcome measures. The NFG was additionally invited to a follow-up session (T2) 3 months after the last training session. The research protocol was approved by the local ethics committee.
Sample
Inclusion criteria were self-reported occurrences of overeating episodes, female gender, legal age, and restraint scores ≥12 on the German adaptation of the Restraint Scale (Dinkel et al. 2005). Exclusion criteria were (1) diagnoses of eating disorders within the last 10 years, (2) Body Mass Index (BMI) < 20 kg/m2, (3) alcohol abuse, (4) diabetes mellitus, (5) neurological disorders (e.g. epilepsy), (6) current medication with drugs eliciting weight fluctuations (e.g. cortisone, lithium), (7) current pregnancy, (8) a current weight reduction diet other than long term lifestyle diets (e.g. Weight Watchers, calorie counting, low carb nutrition). Since any unreported eating disorders should be excluded, the Eating Disorder Examination Questionnaire (EDE-Q; Fairburn and Beglin 1994; Hilbert and Tuschen-Caffier 2006) was additionally used for screening. Participants with critical EDE-Q values were interviewed by a trained psychologist and excluded from participation in case of manifest eating disorder symptomatology.
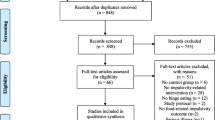
We recruited participants using information leaflets at the University of Wuppertal and in medical offices in the area, e-mail newsletters and website announcements. Sixty-four persons responded to our recruiting attempts and were assessed for eligibility. Twenty-two respondents withdrew before the kick-off event. Three respondents did not meet inclusion criteria, one respondent had to be excluded due to former eating disorder diagnosis and four respondents were excluded due to a BMI < 20. This resulted in a sample of 34 participants, whereof 17 were assigned to the NFG and 17 were assigned to the WLG. In each of the groups, three participants dropped out. Data of n = 1 were excluded from analysis due to an excessive amount of missing data, resulting in samples of n = 14 for the NFG and n = 13 for the WLG post-treatment. At follow-up, n = 2 NFG participants did not respond to our invitation, resulting in a sample of n = 12. Detailed sample characteristics are shown in Table 1. A participant flow diagram according to CONSORT-guidelines is presented in Fig. 1.
Procedure
Women interested in participation received an e-mail containing written information. Further, a web link to an online questionnaire was provided for assessment of inclusion and exclusion criteria. When the requirements were fulfilled, we invited participants to a kick-off event, starting with general information on the study and the neurofeedback method. Participants then filled in informed consent and questionnaires (see “Assessment Instruments”). Thereafter, a psychoeducative presentation on healthy nutrition and overeating episodes was held. At the end of the event, we assessed each participant’s weight to determine weight status.
We used a stratified randomization approach, to ensure comparable weight distributions within the groups. Participants fulfilling inclusion criteria were first classified as normal weight (BMI < 25) or overweight (BMI ≥ 25). Participants in both subgroups were then randomly assigned to either the NFG or WLG. After randomization, the treatment started in the NFG, with a simultaneous 8 week waiting period in the WLG. Treatment was offered to the WLG participants after the waiting period (n = 9 accepted) but data were not included in our analyses. The study was conducted between February and November 2013 in rooms provided by psyrecon GmbH in Wuppertal, Germany.
Neurofeedback Training
Trainers
Two graduate psychology students, experienced in clinical practice, operated the neurofeedback sessions. They were extensively trained by an experienced neurofeedback trainer in terms of the neurofeedback equipment, electrode attachment and software use. All procedures and instructions were standardized based on a treatment manual that we developed for the present trial.
Neurofeedback Protocol
For this pilot study, we chose a relatively low-threshold ten session treatment that would be suitable for a sub-clinical sample, based on session numbers in comparable bio- and neurofeedback studies (Meule et al. 2012a; Teufel et al. 2013; Vernon 2005). The neurofeedback was administered with two sessions during week 1–4 and one weekly session in weeks 5 and 6. Each session lasted approximately 45 min. Sessions started with an adaptation phase (180 s) to ensure participants had the opportunity to calm down and to allow for adjustment of high beta thresholds to the participants’ individual baseline values (ranging from approx. 2–8 µV). This phase was followed by ten alternating phases of cue exposure (30 s) and subsequent relaxation (180 s).
Before the training period, we asked each participant to name ten specific food items that frequently elicit food craving and episodes of overeating. To ensure individual appeal, pictures of the respective items where either digitally provided or personally selected by the participants at the research facilities. Printed presentation cards of these pictures served as stimuli for the cue exposure phases of the training. During cue exposure, stimuli were presented in random order on a presentation desk in front of the participants. Here, the trainers instructed participants to focus on the picture and imagine the food as vividly as possible (including smell, taste, and consistency). After 30 s the picture was removed for the following relaxation phase. Participants then had to focus on the screen displaying physiological reactions and to try keeping both bars below the thresholds. We asked participants to avoid eating for 3 h prior to each session, to ensure sufficient appeal of the presented stimuli.
In the first session, the therapists explained that high beta activity would decrease in a state of relaxation, and how artifacts would result from heavy movements or speaking. Participants were encouraged to try different techniques of relaxation with the only prerequisite of keeping their eyes open. Thus, each participant was able to develop an individual strategy to efficiently reduce EEG high beta activity according to the feedback presented on the client screen.
Probability of success in high beta regulation was lowered with progress of the training sessions. It was set to 85 % in sessions 1–4, 80 % in sessions 5 and 6, 75 % in sessions 7 and 8, and 70 % in sessions 9 and 10. This procedure was chosen to preserve challenging effects during the training. Throughout each session, the trainer adjusted the threshold for high beta activity whenever probability of success derived more than 5 % from the intended value for more than 1 min of the relaxation phases.
Apparatus
We performed the training using the Mindfield Mindmaster EEG and the corresponding software BioEra Clinical Basic 1.63. The software works in a split screen-mode. The client screen displays bar diagrams of selected EEG frequency ranges, while a trainer screen serves to adjust thresholds and monitor clients’ mean power in different EEG spectra. Probability of successful high beta regulation according to the preset thresholds is displayed on the trainer screen.
For the sessions, participants were seated in a comfortable armchair at a distance of approximately 1 m to a 22″ computer monitor displaying the client screen. After skin preparation with an EEG peeling paste on scalp and earlobes, electrodes were attached, with the target electrode on the vertex position (Cz; Jasper 1958), reference electrode on the left and ground electrode on the right earlobe. Impedance was kept below 5 kΩ. For the intended high beta reduction protocol, EEG high beta activity in the spectral range of 23–28 Hz was selected as feedback frequency. We selected this range of the relatively broad beta-spectrum to prevent down training of beneficial ranges such as sensorimotor rhythm (12–15 Hz) or lower and intermediate beta (16–22 Hz) which are associated with common states of attention. Instead, we aimed at a specific reduction of cortical hyperarousal (Egner and Gruzelier 2004; Thompson and Thompson 2007). After online Fast Fourier Transformation of the raw EEG, power (in µV) of this frequency range was displayed as a bar diagram on the client screen. For control reasons, power of muscular artifacts was shown in addition, as high beta activity may be influenced by muscular electrical activity, due to similar frequency ranges. This assured that participants would not misinterpret artifacts from movements or swallowing as signs of tension. Bar diagrams in a desired range were displayed in green color, and an animated video of a beach landscape at sunset was presented. When any of the bars excessed the preset threshold, the bar turned red and the animation stopped. The initial thresholds were set to 4 µV for high beta and to 1.5 µV for artifacts.
Assessment Instruments
Screening Instruments
All screening instruments were applied prior to randomization, either in the online screening-questionnaire or at the kick-off event.
Restraint Scale
For assessment of RE, the Restraint Scale (RS; Dinkel et al. 2005; Herman and Polivy 1980) was applied. Answer options are provided on 4- or 5-point Likert-scales, ranging from 0 to 3 or 0 to 4 respectively. The sum score ranges from 0 to 34. Evidence for the construct and criterion validities of the German version were reported, and internal consistency showed to be good with α = .83 (Dinkel et al. 2005). In the present study internal consistency was still satisfactory (α = .64). Referring to Dinkel et al. (2005), we used a cut-off score ≥12 to classify respondents as REs. The RS was administered online.
Screening of Inclusion and Exclusion Criteria
Inclusion and exclusion criteria were assessed by an online screening-questionnaire, consisting of seven items with a yes/no format, e.g. “Have you been diagnosed with an eating disorder within the last 10 years? Yes/No”. Concerning medication, participants could indicate a not sure option and type in names of their medicaments. We then checked for possible negative effects of the respective medication. BMI was calculated as weight in kg/(height in m)2.
Eating Disorder Examination Questionnaire
The Eating Disorder Examination Questionnaire (EDE-Q; Fairburn and Beglin 1994; Hilbert and Tuschen-Caffier 2006) was used to screen for undetected, clinically relevant eating disorders. It consists of 28 items with a 7-point rating scale (0 = attribute non-existent; 6 = attribute existent every day/in an extreme degree) referring to symptoms and eating behavior during the previous 28 days. Concurrent validity is reported as good and construct validity as acceptable (Mond et al. 2004). Internal consistency of the German version has been reported as excellent, α = .97 (Hilbert and Tuschen-Caffier 2006), with α = .90 in the present study. We pre-screened diagnostic criteria regarding eating disorders (e.g. vomiting behavior in bulimia nervosa) which were not fulfilled by any participant. An overall mean sum score ≥4 then served as critical value in line with reference scores for female populations (Mond et al. 2006).
Demographics
For demographics, we assessed age and employment status on a questionnaire. The questionnaire further contained questions about smoking (yes/no/occasional) and possible lifestyle diets (such as vegetarism, Weight Watchers, calorie counting or low carb nutrition).
Outcome Measures
Outcome measures were assessed at T0 and T1. In addition, the NFG filled in all outcome measures at follow-up (T2).
Primary Outcome Measures
A self-constructed questionnaire was used for the assessment of overeating episodes and caused distress. Following a definition (overeating episodes induced by craving urges, resulting in consumption of high calorie food without experiencing physiological hunger), subjects were asked to report the number of overeating episodes during the last 7 days and the average distress experienced due to overeating on a 6-point rating scale (0 = not at all, 1 = light, 2 = rather light, 3 = rather strong, 4 = strong, 5 = very strong) with an additional option (not applicable) in case of no episode (= 0).
Secondary Outcome Measures
Food Craving The Food Cravings Questionnaire, trait form (FCQ-T; Cepeda-Benito et al. 2000; Meule et al. 2012b) was applied. The questionnaire contains 39 items on habits and behaviors related to food craving with a 6-point rating scale (1 = never/not applicable, 2 = seldom, 3 = sometimes, 4 = often, 5 = almost always, 6 = always). Convergent and divergent validity of the FCQ-T are given, and internal consistency of the German version has been reported as high (α = .96; Meule et al. 2012b) with α = .96 in the present study. For this study, the sum score was used to assess food craving.
Perceived Dieting Success As a measure of self-regulatory competence we assessed perceived dieting success with the Perceived Self-Regulatory Success in Dieting Scale (PSRS; Fishbach et al. 2003; Meule et al. 2012c). The scale contains three items (one item reverse coded) with a 7-point rating scale (1 = not successful/not difficult; 7 = very successful/very difficult) allowing for a total score in the range of 3–21. Validity of the measure has been demonstrated, for example by negative correlations with BMI, and internal consistency for the German version is satisfactory (α > .70; Meule et al. 2012c), with α = .70 in the present study.
Perceived Stress Perceived stress within the last month was assessed with the Perceived Stress Scale (PSS; Cohen et al. 1983; Cohen and Williamson 1988). The applied version consists in ten items (e.g. “How often have you felt nervous or stressed?”) and a 5-point answer scale (0 = never; 4 = very frequently). Concurrent, convergent, and predictive validity have been reported for the PSS, and internal consistency was reported as good with α > .80 in different samples and cultural backgrounds (e.g. Mitchell et al. 2008; Reis et al. 2010). In the present study the internal consistency was high (α = .89).
Well-Being We applied the German version of the World Health Organization-five well-being index (WHO-5; Psychiatric Research Unit 1998). Its five items assess aspects of well-being within the last 2 weeks on 6-point rating scales (0 = at no time; 5 = all of the time), resulting in a sum score of 0 (very poor well-being) to 25 (excellent well-being). WHO-5 has demonstrated good psychometric properties, e.g. α = .82 in a sample of diabetics (De Wit et al. 2007), reaching α = .88 in the present study. External and internal validity of the WHO-5 have been shown in different populations (e.g. diabetics: De Wit et al. 2007; elderly: Heun et al. 2001).
Subjective Outcomes
A questionnaire was developed to assess general acceptance of the treatment, perceived outcomes, and strategy applicability in daily routine. Further, satisfaction and intentions to recommend the treatment were assessed. The questionnaire consisted of an overall evaluation (“Altogether, how did you experience the neurofeedback training?”) with a 5-point rating scale (1 = very negative; 5 = very positive); five items to assess the treatment effects (e.g. “The neurofeedback training influenced my eating behavior”); two items on perceived changes in behavior (“I perceived changes in behavior due to the neurofeedback training”/“Others perceived changes in my behavior due to the neurofeedback training”); five items on satisfaction and applicability of the training (e.g. “I am satisfied with the neurofeedback training.”/“I would recommend the neurofeedback training to persons experiencing overeating episodes”). Except for the overall evaluation, all items included a 5-point rating scale, 1 = not at all to 5 = very strong.
Statistical Procedure
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) 22.0 for Windows. Single points of missing data were replaced by the participant’s mean value in the respective scale (or subscale, if applicable). For analyses of group differences in demographic data, Chi-square tests were performed for categorical variables and t-tests for continuous variables. For outcome measures, normality of data within the groups at each assessment session was tested by means of Shapiro–Wilk Test. Possible baseline differences in outcome variables were assessed by means of between groups t-tests. For analysis of intervention effects, mixed 2 (group) × 2 (time) ANOVAs were conducted. Post-hoc, Bonferroni corrected pairwise comparisons within and between groups were performed to scrutinize effects. With regard to follow-up analyses, repeated measures ANOVAs were performed for the NFG with Bonferroni-corrected post hoc tests analyzing pairwise differences between T0, T1, and T2. Some data showed deviations from normality and skewness or curtosis. However, we will report ANOVA results, as ANOVA is robust to violations of normality assumptions when group sizes are comparable and nonparametric re-analyses delivered the same pattern of results. Effect sizes were calculated as r, using z-values to adjust for partly skewness of the data (Fritz et al. 2012). Between group effect sizes were calculated as \(r = \left| {\frac{z}{\sqrt N }} \right|\) for intervention outcomes. Effect sizes for within subjects follow-up analyses, were calculated as \(r = \left| {\frac{z}{{\sqrt {2n} }}} \right|\). Guidelines for r are that values >.50 account for large effects, values >.30 for a medium effect and values >.10 for a small effect respectively (Coolican 2009, p. 395, as cited in Fritz et al. 2012). Effects were tested at a two-sided significance level of .05.
Results
Group Comparison of Baseline Scores
Demographic and screening data showed that mean age, employment status, smoking habits, RS-scores, and BMIs of the NFG and WLG did not differ significantly at baseline (all p > .104; see Table 1). At pre-treatment (T0), the NFG and WLG did not differ significantly in occurrence of overeating episodes [t(25) = −0.33, p = .740] or distress induced by overeating episodes [t(25) = −1.87, p = .073], nor in any secondary measures (all p > .335).
Treatment Outcomes
Descriptives and F-statistics of primary and secondary outcome measures are displayed in Table 2 with an additional visualization of primary outcome results in Fig. 2. Descriptives and F-statistics of the NFG follow-up analysis are shown in Table 3. An overview on descriptives of subjective outcomes in the treatment evaluation is presented in Table 4.
Primary Outcome Measures
Mixed ANOVAs for the number of overeating episodes per week revealed a significant main effect of time (p = .011) and a significant group × time interaction (p = .020). Post-hoc analysis showed a significant reduction of overeating within the NFG (p = .001) but not within the WLG (p = .857). At post-treatment the NFG reported less overeating episodes than the WLG (p = .001) with a large between group effect size (r = .64).
A comparable pattern was identified for distress induced by overeating episodes. Here, a significant main effect of time (p = .002) as well as a significant time × group interaction (p < .001) were found. A significant reduction of distress induced by overeating episodes was observed within the NFG (p < .001) but not within the WLG (p = .729). Between groups comparison showed a significant difference post-treatment (p = .005) with a large effect size (r = .54).
Secondary Outcome Measures
For perceived dieting success, a significant main effect of time (p = .029) with a trend towards significance (p = .091) in the group × time interaction was found. Descriptively, perceived dieting success was enhanced within the NFG (post hoc: p = .007) but not in the WLG. Still, group differences were not significant post-treatment and results have to be regarded critically due to a lack of significant interaction effect.
With regard to well-being there was a significant main effect of time only (p = .029) yielding descriptive, but non-significant improvements of well-being within both groups. For food craving and perceived stress no significant effects were observed in either analysis of the intervention effects.
NFG Follow-up
Short term stability of the neurofeedback effects was assessed at follow-up (T2). Changes in the primary outcome measures remained significant to follow-up (both main effects of time p < .001). From pre-treatment to follow-up, a significant and large reduction in overeating episodes was observable in post hoc comparisons (p = .003, r = .59). The same pattern was found for the reduction of distress induced by overeating (p = .006, r = .56).
For the secondary outcome measures, there still was an observable significant main effect of time for perceived dieting success (p = .034), although post hoc tests showed that significance vanished at follow-up (T0–T2: p = .212, r = .32). However, follow-up analysis now revealed a significant main effect of time for trait food craving (p = .008), caused by a significant reduction in food craving from pre-treatment to follow-up (p = .008) with a medium effect size (r = .40).
Subjective Outcomes
Overall acceptance of the neurofeedback was high. Altogether, 85.7 % of the participants rated the treatment experience as positive or very positive (positive: 71.4 %; very positive: 14.3 %). No single participant rated the treatment experience as negative or very negative. Satisfaction ratings were good, with 14.3 % rating satisfaction as very strong, 35.7 % as strong, whereas 35.7 % reported to be relatively satisfied. Subjective feedback further indicated that 64.3 % of the participants would very strongly or strongly recommend the treatment to people experiencing overeating episodes. Additional results of subjective outcomes are displayed in Table 4. The only negative side effect stated by some participants was drowsiness during the sessions.
Discussion
Overeating episodes are a common problem within the population of REs. Stressful arousal, associated with craving and ruminative conflicts and its physiological correlates, might play a crucial role as antecedents of this eating behavior. For a randomized controlled pilot-study, we developed a ten session neurofeedback protocol, based on previous findings on EEG arousal, which combined cue exposure with subsequent down regulation of EEG high beta activity.
The present study demonstrated that this new training method accounted for significant improvement in overeating-related primary outcome measures. Overeating episodes were significantly reduced within the NFG only. At post-treatment, subjects in the NFG reported less frequent overeating episodes compared with a waiting list group. The same pattern was found for overeating induced distress. These primary effects remained stable at a 3 month follow-up. Large effect sizes underline the relevance of the improvements induced by the training. Further, our outcome measures relate to actual (albeit retrospective) reports on eating behavior, rather than assessing attitudes towards food or latent constructs which are supposed to be related to eating behavior. Therefore, the present results provide high external validity. Participants reported a positive evaluation of the treatment, with high acceptance, satisfaction, and recommendation rates, whereas the drop-out rates were relatively low. Thus, the neurofeedback protocol not only showed good efficacy but also provided a well-accepted approach for the treatment of overeating episodes in a sub-clinical sample of female REs. First evidence in this sample suggests that neurofeedback might help escape the vicious circle of stress and overeating by self-regulation of brainwave patterns, even in a low-threshold treatment consisting of ten sessions.
As secondary outcome, perceived dieting success was descriptively enhanced within the NFG, although group comparisons post treatment did not yield significant differences. The significant main effect on improvement of perceived dieting success was also observable in the follow-up sample. Still, pairwise comparisons between pre-treatment and follow-up did not yield statistical significance. In contrast, a significant reduction in food craving with a medium effect was observed within the NFG at follow-up. This finding might be a result of the relatively small follow-up sample size, where punctual deviations might influence results to a strong degree. But it is also possible, that the treatment first enhanced perceived dieting success by explicitly providing strategies against cue-induced food craving, while food craving in general was reduced in the long run by implicit transfer of these strategies. As a measure of self-regulatory competence, perceived dieting success is very specific and might not always be the predominant goal of women experiencing overeating episodes. Self-regulatory competence on more superordinate levels, for example general self-efficacy or self-regulatory competence regarding bodily responses, should therefore additionally be assessed in future studies on this neurofeedback protocol.
Other secondary outcome measures, such as perceived stress and general well-being were not significantly affected by the treatment, although they descriptively showed slight improvements within the NFG. Still, the latter outcome measures are relatively general and influenced by a wide range of factors besides overeating episodes, for example by general life circumstances, interpersonal relationships, or individual working load. Since we did not assess this range of possible influence factors, we cannot control for intervening effects.
Despite of high acceptance ratings, some women reported drowsiness throughout the sessions. In contrast to other neuro- or biofeedback protocols, the sessions included no breaks and relatively long and repetitive phases of relaxation, which might have been too monotonous or demanding for the participants. In future studies, relaxation periods should either be shortened or training sessions should be interrupted by short breaks.
To our knowledge, this is the first study evaluating a cue exposure neurofeedback paradigm to address overeating episodes in REs. Strengths of this study lie in the availability of follow-up data, showing stability of primary outcome effects and developments in secondary outcomes. In contrast to previous eating related biofeedback studies (Meule et al. 2012a; Teufel et al. 2013) the present sample included wide ranges in age, employment status, and normal weight as well as overweight participants. Although the results of this pilot study are promising, the study is subject to limitations.
First of all, due to technical limitations, we were not able to analyze EEG data in terms of psychophysiological learning. Therefore, it remains unclear whether participants have learned to regulate EEG activity, especially in the absence of the feedback signal. Besides the neurofeedback itself, the treatment contained multiple components, such as repeated cue exposure and some relaxation instructions. Both aspects have previously shown to exert beneficial effects in comparable intervention studies (Conklin and Tiffany 2002; Jansen et al. 1992; Manzoni et al. 2009). To determine neurofeedback effects on spontaneous EEG and participants’ control over EEG parameters, more sophisticated psychophysiological measurements and an experimental assessment of pre- and post-treatment EEG data in the absence of feedback should be included in following studies.
We did not compare the neurofeedback to an alternative treatment. Since other researchers have shown efficacy of different treatment methods on food craving or eating-related self-efficacy in different populations (e.g. electrodermal biofeedback: Teufel et al. 2013; or heart rate variability training: Meule et al. 2012a), effects might also be accountable to biofeedback in general or even to the mere intervention experience based on the experience of self-control or expectation. In future studies on this protocol, it could help to assess participants’ initial expectations towards the treatment, to address their impact on outcome and resulting placebo effects implicitly. For a more detailed assessment of specific neurofeedback effects, a consecutive study is planned, comparing the developed protocol with a highly comparable treatment, for example an imagery-based relaxation training combined with cue exposure.
Sample sizes in this study were relatively small, accounting for limited statistical power despite of significant and large effects. Future studies on this protocol should aim at recruiting a larger sample to account for sufficient statistical power. As women are more prone than men to dysfunctional eating and overeating episodes (Provencher et al. 2003), we tested the new method on an exclusively female sub-clinical sample. To assess general effectiveness in terms of overeating symptoms, the method should also be tested in a male sample reporting overeating episodes. Further, the applicability and effectiveness in a clinical sample is yet an unexplored but interesting topic and a field of application for future trials. Finally, future studies on this topic should control for individual characteristics, influencing overeating and treatment effectiveness, such as impulsivity, perfectionism, body-dissatisfaction, affect (Stice 2002), or flexible and rigid eating behavior (Westenhöfer 1991).
The results of this pilot-study need to be confirmed and the distinct beneficial influences of the neurofeedback method yet are to be scrutinized. Still, our first results are promising. Neurofeedback with a high beta reduction protocol is an approach that should be considered in future intervention research against overeating episodes in REs, and might probably also be helpful in other populations prone to food craving, disinhibition and overeating.
References
Andersen, S. B., Moore, R. A., Venables, L., & Corr, P. J. (2009). Electrophysiological correlates of anxious rumination. International Journal of Psychophysiology, 71(2), 156–169.
Bartholdy, S., Musiat, P., Campbell, I. C., & Schmidt, U. (2013). The potential of neurofeedback in the treatment of eating disorders: A review of the literature. European Eating Disorders Review, 21(6), 456–463.
Brunstrom, J. M., Yates, H. M., & Witcomb, G. L. (2004). Dietary restraint and heightened reactivity to food. Physiology & Behavior, 81(1), 85–90.
Cepeda-Benito, A., Gleaves, D. H., Williams, T. L., & Erath, S. A. (2000). The development and validation of the state and trait Food-Cravings Questionnaires. Behavior Therapy, 31(1), 151–173.
Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396.
Cohen, S., & Williamson, G. (1988). Perceived stress in a probability sample of the United States. In S. Spacapan & S. Oskamp (Eds.), The social psychology of health (pp. 31–67). Newbury Park: Sage.
Conklin, C. A., & Tiffany, S. T. (2002). Applying extinction research and theory to cue-exposure addiction treatments. Addiction, 97(2), 155–167.
Coolican, H. (2009). Research methods and statistics in psychology. London: Hodder.
Cools, J., Schotte, D. E., & McNally, R. J. (1992). Emotional arousal and overeating in restrained eaters. Journal of Abnormal Psychology, 101(2), 348–351.
De Wit, M., Pouwer, F., Gemke, R. J., Delemarre-van de Waal, H. A., & Snoek, F. J. (2007). Validation of the WHO-5 Well-Being Index in adolescents with type 1 diabetes. Diabetes Care, 30(8), 2003–2006.
Dinkel, A., Berth, H., Exner, C., Rief, W., & Balck, F. (2005). Deutsche Adaptation der Restraint Scale zur Erfassung gezügelten Essverhaltens. Diagnostica, 51(2), 67–74.
Egner, T., & Gruzelier, J. H. (2001). Learned self-regulation of EEG frequency components affects attention and event-related brain potentials in humans. NeuroReport, 12(18), 4155–4159.
Egner, T., & Gruzelier, J. H. (2004). EEG biofeedback of low beta band components: frequency-specific effects on variables of attention and event-related brain potentials. Clinical Neurophysiology, 115(1), 131–139.
Fairburn, C. G., & Beglin, S. J. (1994). Assessment of eating disorders. Interview or self-report questionnaire? International Journal of Eating Disorders, 16(4), 363–370.
Fishbach, A., Friedman, R. S., & Kruglanski, A. W. (2003). Leading us not unto temptation. Momentary allurements elicit overriding goal activation. Journal of Personality and Social Psychology, 84(2), 296–309.
Frank, S., Lee, S., Preissl, H., Schultes, B., Birbaumer, N., & Veit, R. (2012). The obese brain athlete: Self-regulation of the anterior insula in adiposity. PLoS One, 7(8), e42570.
Freeman, L. M. Y., & Gil, K. M. (2004). Daily stress, coping, and dietary restraint in binge eating. International Journal of Eating Disorders, 36(2), 204–212.
Fritz, C. O., Morris, P. E., & Richler, J. J. (2012). Effect size estimates: Current use, calculations, and interpretation. Journal of Experimental Psychology: General, 141(1), 2–18.
Gluck, M. E. (2006). Stress response and binge eating disorder. Appetite, 46(1), 26–30.
Hay, P., & Williams, S. E. (2013). Exploring relationships over time between psychological distress, perceived stress, life events and immature defense style on disordered eating pathology. BMC Psychology, 1, 27. doi:10.1186/2050-7283-1-27.
Heatheron, T. F., Polivy, J., & Herman, C. P. (1991). Restraint, weight loss, and variability of body weight. Journal of Abnormal Psychology, 100(1), 78–83.
Herman, C. P., & Mack, D. (1975). Restrained and unrestrained eating. Journal of Personality, 43(4), 647–660.
Herman, C. P., & Polivy, J. (1975). Anxiety, restraint, and eating behavior. Journal of Abnormal Psychology, 84(6), 666–672.
Herman, C. P., & Polivy, J. (1980). Restrained eating. In A. J. Stunkard (Ed.), Obesity (pp. 208–225). Philadelphia, PA: Saunders.
Heun, R., Bonsignore, M., Barkow, K., & Jessen, F. (2001). Validity of the five-item WHO Well-Being Index (WHO-5) in an elderly population. European Archives of Psychiatry and Clinical Neuroscience, 251(2), 27–31.
Hilbert, A., & Tuschen-Caffier, B. (2006). Eating disorder examination. Deutschsprachige Übersetzung. Münster: Verlag für Psychotherapie, PAG Institut für Psychologie AG.
Hill, A. J. (2007). The psychology of food craving. Proceedings of the Nutrition Society, 66(02), 277–285.
Jansen, A., Broekmate, J., & Heymans, M. (1992). Cue-exposure vs self-control in the treatment of binge eating: a pilot study. Behaviour Research and Therapy, 30(3), 235–241.
Jasper, H. H. (1958). The ten twenty electrode system of the international federation. Electroencephalography and Clinical Neurophysiology, 10, 371–375.
Jastreboff, A. M., Sinha, R., Lacadie, C., Small, D. M., Sherwin, R. S., & Potenza, M. N. (2013). Neural correlates of stress-and food cue-induced food craving in obesity association with insulin levels. Diabetes Care, 36(2), 394–402.
Klesges, R. C., Isbell, T. R., & Klesges, L. M. (1992). Relationship between dietary restraint, energy intake, physical activity, and body weight: A prospective analysis. Journal of Abnormal Psychology, 101(4), 668–674.
Manzoni, G. M., Pagnini, F., Gorini, A., Preziosa, A., Castelnuovo, G., Molinari, E., & Riva, G. (2009). Can relaxation training reduce emotional eating in women with obesity? An exploratory study with 3 months of follow-up. Journal of the American Dietetic Association, 109(8), 1427–1432.
McEwen, B. S. (2007). Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological Reviews, 87(3), 873–904.
Meule, A., Freund, R., Skirde, A. K., Vögele, C., & Kübler, A. (2012a). Heart rate variability biofeedback reduces food cravings in high food cravers. Applied Psychophysiology and Biofeedback, 37(4), 241–251.
Meule, A., Lutz, A., Vögele, C., & Kübler, A. (2012b). Food cravings discriminate differentially between successful and unsuccessful dieters and non-dieters. Validation of the Food Cravings Questionnaires in German. Appetite, 58(1), 88–97.
Meule, A., Papies, E. K., & Kübler, A. (2012c). Differentiating between successful and unsuccessful dieters. Validity and reliability of the perceived self-regulatory success in dieting scale. Appetite, 58(3), 822–826.
Mitchell, A. M., Crane, P. A., & Kim, Y. (2008). Perceived stress in survivors of suicide: Psychometric properties of the perceived stress scale. Research in Nursing & Health, 31(6), 576–585.
Mitchison, D., Hay, P., Slewa-Younan, S., & Mond, J. (2012). Time trends in population prevalence of eating disorder behaviors and their relationship to quality of life. PLoS One, 7(11), e48450. doi:10.1371/journal.pone.0048450.
Mond, J. M., Hay, P. J., Rodgers, B., & Owen, C. (2006). Eating disorder examination questionnaire: Norms for young adult women. Behavior Research and Therapy, 44(1), 53–62.
Mond, J. M., Hay, P. J., Rodgers, B., Owen, C., & Beumont, P. J. V. (2004). Validity of the eating disorder examination questionnaire (EDE-Q) in screening for eating disorders in community samples. Behaviour Research and Therapy, 42(5), 551–567.
Paquette, V., Beauregard, M., & Beaulieu-Prévost, D. (2009). Effect of a psychoneurotherapy on brain electromagnetic tomography in individuals with major depressive disorder. Psychiatry Research: Neuroimaging, 174(3), 231–239.
Parvaz, M. A., Alia-Klein, N., Woicik, P. A., Volkow, N. D., & Goldstein, R. Z. (2011). Neuroimaging for drug addiction and related behaviors. Reviews in the Neurosciences, 22(6), 609–624.
Pelchat, M. L. (2002). Of human bondage: Food craving, obsession, compulsion, and addiction. Physiology & Behavior, 76(3), 347–352.
Polivy, J., Coleman, J., & Herman, C. P. (2005). The effect of deprivation on food cravings and eating behavior in restrained and unrestrained eaters. International Journal of Eating Disorders, 38(4), 301–309.
Polivy, J., & Herman, C. P. (1985). Dieting and binging. A causal analysis. American Psychologist, 40(2), 193–201.
Provencher, V., Drapeau, V., Tremblay, A., Després, J. P., & Lemieux, S. (2003). Eating behaviors and indexes of body composition in men and women from the Quebec family study. Obesity Research, 11(6), 783–792.
Psychiatric Research Unit (1998). WHO (Fünf)—Fragebogen zum Wohlbefinden (Version 1998). http://www.cure4you.dk/354/WHO-5_German.pdf. Accessed 11th Sept 2013.
Reis, R. S., Hino, A. A. F., & Añez, C. R. R. (2010). Perceived stress scale reliability and validity study in Brazil. Journal of Health Psychology, 15(1), 107–114.
Ruderman, A. J. (1986). Dietary restraint: A theoretical and empirical review. Psychological Bulletin, 99(2), 247–261.
Saletu-Zyhlarz, G. M., Arnold, O., Anderer, P., Oberndorfer, S., Walter, H., Lesch, O. M., et al. (2004). Differences in brain function between relapsing and abstaining alcohol-dependent patients evaluated by EEG mapping. Alcohol and Alcoholism, 39(3), 233–240.
Seo, S.-H., & Lee, J.-T. (2010). Stress and EEG. Convergence and hybrid information technologies. In M. Crisan (Ed.), InTech. Available from http://www.intechopen.com/books/convergence-and-hybrid-information-technologies/stress-and-eeg.
Sinha, R., & Jastreboff, A. M. (2013). Stress as a common risk factor for obesity and addiction. Biological Psychiatry, 73(9), 827–835.
Stein, R. I., Kenardy, J., Wiseman, C. V., Dounchis, J. Z., Arnow, B. A., & Wilfley, D. E. (2007). What’s driving the binge in binge eating disorder?: A prospective examination of precursors and consequences. International Journal of Eating Disorders, 40(3), 195–203.
Stice, E. (2002). Risk and maintenance factors for eating pathology: A meta-analytic review. Psychological Bulletin, 128(5), 825–848.
Styn, M. A., Bovbjerg, D. H., Lipsky, S., & Erblich, J. (2013). Cue-induced cigarette and food craving: A common effect? Addictive Behaviors, 38(3), 1840–1843.
Swinburn, B. A., Sacks, G., Hall, K. D., McPherson, K., Finegood, D. T., Moodie, M. L., & Gortmaker, S. L. (2011). The global obesity pandemic: Shaped by global drivers and local environments. The Lancet, 378(9793), 804–814.
Tammela, L. I., Pääkkönen, A., Karhunen, L. J., Karhu, J., Uusitupa, M. I., & Kuikka, J. T. (2010). Brain electrical activity during food presentation in obese binge-eating women. Clinical Physiology and Functional Imaging, 30(2), 135–140.
Teufel, M., Stephan, K., Kowalski, A., Käsberger, S., Enck, P., Zipfel, S., & Giel, K. E. (2013). Impact of biofeedback on self-efficacy and stress reduction in obesity: A randomized controlled pilot study. Applied Psychophysiology and Biofeedback, 38(3), 177–184.
Thompson, M., & Thompson, L. (2007). Neurofeedback for stress management. In P. M. Lehrer, R. L. Woolfolk, & W. E. Sime (Eds.), Principles and practice of stress management (3rd ed., pp. 249–287). New York: The Guilford Press.
Vernon, D. J. (2005). Can neurofeedback training enhance performance? An evaluation of the evidence with implications for future research. Applied Psychophysiology and Biofeedback, 30(4), 347–364.
Westenhöfer, J. (1991). Dietary restraint and disinhibition: Is restraint a homogeneous construct? Appetite, 16(1), 45–55.
Acknowledgments
The authors would like to thank the psyrecon GmbH, Wuppertal, Germany, for providing the neurofeedback equipment and training room, Ralf Stürmer and Gisela Ulmer for supervision of the neurofeedback training,as well as Kamila Lewicki and Rahel Kuttner for conducting the neurofeedback sessions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Schmidt, J., Martin, A. Neurofeedback Reduces Overeating Episodes in Female Restrained Eaters: A Randomized Controlled Pilot-Study. Appl Psychophysiol Biofeedback 40, 283–295 (2015). https://doi.org/10.1007/s10484-015-9297-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10484-015-9297-6