Abstract
Little is known about the composition, diversity, and geographical distribution of bacterial communities associated with medicinal plants in arid lands. To address this, a collection of 116 endophytic bacteria were isolated from wild populations of the herb Glycyrrhiza uralensis Fisch (licorice) in Xinyuan, Gongliu, and Tekesi of Xinjiang Province, China, and identified based on their 16S rRNA gene sequences. The endophytes were highly diverse, including 20 genera and 35 species. The number of distinct bacterial genera obtained from root tissues was higher (n = 14) compared to stem (n = 9) and leaf (n = 6) tissue. Geographically, the diversity of culturable endophytic genera was higher at the Tekesi (n = 14) and Xinyuan (n = 12) sites than the Gongliu site (n = 4), reflecting the extremely low organic carbon content, high salinity, and low nutrient status of Gongliu soils. The endophytic bacteria exhibited a number of plant growth-promoting activities ex situ, including diazotrophy, phosphate and potassium solubilization, siderophore production, auxin synthesis, and production of hydrolytic enzymes. Twelve endophytes were selected based on their ex situ plant growth-promoting activities for growth chamber assays to test for their ability to promote growth of G. uralensis F. and Triticum aestivum (wheat) plants. Several strains belonging to the genera Bacillus (n = 6) and Achromobacter (n = 1) stimulated total biomass production in both G. uralensis and T. aestivum under low-nutrient conditions. This work is the first report on the isolation and characterization of endophytes associated with G. uralensis F. in arid lands. The results demonstrate the broad diversity of endophytes associated with wild licorice and suggest that some Bacillus strains may be promising candidates for biofertilizers to promote enhanced survival and growth of licorice and other valuable crops in arid environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endophytic bacteria are ubiquitous microorganisms that reside within healthy plant tissues (Gasser et al. 2011; Malfanova et al. 2011). These microorganisms are often hypothesized to help their hosts by producing resources that afford protection and facilitate survival of the host plant (Sánchez-López et al. 2017). Therefore, a better understanding of plant–microbe interactions with respect to the physicochemical environment may provide insights into the microbial ecology of plant-associated endophytes (Islam et al. 2016; Patil et al. 2016). The plant-associated microbiome consists of distinct microbial communities living in roots, stems, leaves and other tissues. Symbioses between plants and endophytic bacteria are mutually beneficial through the exchange of metabolites and sharing of physiological processes (Reinhold-Hurek and Hurek 2011). The plant provides endophytes with a stable habitat while endophytic bacteria supply nutrients such as fixed nitrogen, soluble potassium, iron, and phosphate, and indole acetic acid (Liu et al. 2016, 2017; Sánchez-López et al. 2017).
In recent years, there has been an increasing interest in the characterisation and bioprospecting of growth-promoting endophytes, broadening the scope for innovative design of biofertilizers for different crops such as soy, canola, lentil, pea, radish, and wheat (Donate-Correa et al. 2005; Egamberdiyeva 2007). These endophytes are part of a broader strategy for sustainable agriculture because they may decrease environmental pollution due to fertilizers and other chemical applications. Endophytic bacteria associated with medicinal plants in arid lands can adopt sophisticated survival strategies to promote plant growth, alleviate abiotic stresses, aid in nutrient acquisition, and enhance systemic resistance and tolerance to disease (Boor 2006; Daffonchio et al. 2015). Nevertheless, despite the considerable body of knowledge of the associations between endophytes and crop plants (Wemheuer et al. 2017), much less is known of the diversity of bacterial communities associated with plants inhabiting arid lands, particularly medicinal plants.
Medicinal plants are receiving global attention because traditional medicines are often effective and easily available alternatives to pure pharmaceuticals. Medicinal plants are used worldwide as remedies for various diseases, including gastrointestinal symptoms, asthma, skin disorders, respiratory and urinary problems, and hepatic disease (Cushnie et al. 2014). It is well known that medicinal plants having an ethnobotanical history may harbor an endophytic microbiome that can synthesize a diverse array of bioactive compounds under conditions that are stressful to the host plant (Bajguz 2007). Bioactive compounds produced during endophyte-herb symbiosis can also affect plant-associated microbial communities and their physiological functions (Strobel et al. 2004).
The genus Glycyrrhiza, commonly known as licorice, comprises approximately 30 species including Glycyrrhiza glabra, Glycyrrhiza uralensis, Glycyrrhiza inflata, Glycyrrhiza aspera, and Glycyrrhiza korshinskii (Lewis et al. 2005). Licorice (Glycyrrhiza uralensis Fisch.) is one of the most widely used plants in food production, and it is also used as traditional Chinese medicine. Licorice contains bioactive compounds such as triterpene saponins, flavonoids, coumarins, and other phenolics (Zhang and Ye 2009), but little is known of their endophytic microflora. A better understanding of endophytic microorganisms adapted to arid lands is of broad importance in arid land ecology and can be exploited for biotechnological applications. Therefore, the objectives of our study were as follows: (1) isolate and identify endophytic bacteria associated with G. uralensis F.; (2) screen them for beneficial activities ex situ; and (3) evaluate their ability to stimulate growth of the host plant and Triticum aestivum. To the best of our knowledge, this is the first report on the isolation, identification, and characterization of endophytic bacteria associated with the medicinal plant G. uralensis F. in an arid environment.
Materials and methods
Sample collection and chemistry analysis
Healthy-looking G. uralensis F. plants were randomly collected in the summer of 2015 from their natural arid habitats in Xinjiang province of China. The study sites were Xinyuan (N 43°23′229′′; E 83°50′248′′), Gongliu (N 43°37′188′′; E 81°48′787′′), and Tekesi (N 43°19′205′′; E 81°48′601′′), each within Xinjiang Province (Fig. 1). Three healthy plants were collected from each site; plants were at least 2 m apart within an area of 100 m2. Whole plants, including root systems (15–20 cm depth), were placed in Zip-loc bags, and stored at 4 °C during transportation to the laboratory. Plants were processed and screened for endophytic bacteria within 48 h of collection.
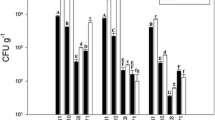
The distribution and identity of 116 culturable endophytes from Glycyrrhiza uralensis F. based on 16S rRNA gene sequences. a A summary of genera present at all sites. b Genus assignments for isolates from different tissues, showing high diversity in the root and low diversity in the leaf. c Genus assignments according to location, showing the lower diversity of isolates from the most arid site, Gongliu. (Color figure online)
Soil samples were taken from each sample collection site and transported to the lab without temperature control and subsequently air dried with 48 h of collection. Soil organic matter was measured by the K2Cr2O7 method (Nelson and Sommers 1996); total N was measured with Kjeltec system 1026 Distilling Unit (Tecator AB, Sweden); soil available phosphorus and potassium were measured by the standard methods used by (Olsen and Sommers 1982; Knudsen et al. 1982). Soil pH was measured in water (soil:water ratio = 1:5); electrical conductivity (EC) was measured using a portable conductivity meter (Cole-Parmer Instrument Company, USA). Soil soluble salts were analyzed using methods described by the Nanjing Institute of Soil Science, Chinese Academy of Sciences (1980).
Isolation, purification, and preservation of the endophytic bacteria
Each plant sample was thoroughly washed with running tap water to remove adhering epiphytes and soil debris. After washing, the samples were separated into leaves, stems, and roots, and then successively cut into 1–2-cm long pieces by using sterile scissors. Tissue samples were separately surface-sterilized in a laminar air flow chamber by immersing them sequentially, with shaking, in 0.1% Tween 20 for a few seconds, 70% ethanol for 3 min, and 5% NaOCl for 5 min. Subsequently, samples were rinsed in sterile distilled water 3–4 times for each step of surface sterilization. After 24 h of drying in a laminar air flow chamber, sterilized parts of the selected plants, including leaves, stems, and roots, were used for the isolation of endophytes. Samples were cut into 0.5 cm long fragments under aseptic conditions and ground in a sterilized blender. About 1 g of tissue homogenate was weighed aseptically and macerated with a sterile mortar and pestle, along with 9 mL sterile distilled water, and then transferred to a sterile polypropylene tube. After that, the tissue homogenate was centrifuged at 2200×g for 5 min. The supernatant was collected and serially diluted to a final concentration of 10−2 and 10−3 and then 100 µL of each dilution was plated in triplicate onto ten different isolation media (Table S2). The agar plates were incubated at 28 °C and monitored every 5 days for microbial growth. Colonies with distinct colony morphology were picked and re-streaked for purification.
The efficiency of surface sterilization was tested by plating 100 μL of the final rinse onto the ten selective isolation media. No microbial growth was detected on the isolation media after 7 days of incubation at 28 °C when the distilled water used in the final rinse of surface-sterilization was plated. This result indicated that the three-step surface sterilization protocol was successful in removing or killing epiphytic bacteria. The subsequent endophytic bacteria obtained during the isolation were, therefore, considered to be true endophytes.
The isolates were stored in the isolation medium with 30% glycerol at − 80 °C.
Genotypic characterization and identification
DNA was extracted from bacterial cells grown in ISP2 broth for 2 days at 28 °C by using Chelex® 100 sodium following the manufacturer’s instructions (Sigma, Shanghai, China). The extracted DNA was dissolved in 20 µL TE buffer and DNA concentration was determined using a NanoDrop2000 spectrophotometer (Thermo Scientific, Waltham, USA) and used as the template for PCR. PCR amplification of the 16S rRNA gene was performed by using primers 27F (5′-CAGAGTTTGATCCTGGCT-3′) and 1492R (5′-AGGAGGTGATCCAGCCGCA-3′) (Lane 1991). DNA was amplified in a BIORAD C1000 Thermal Cycler in a total volume of 25 µL consisting of 1 µL of genomic DNA (approximately 50 ng), 0.5 µL of each primer (10 pmol), 2.0 µL of deoxynucleotide triphosphates (2.5 mM each), 1× PCR buffer, and 1.0 U of Taq DNA polymerase. PCR was performed under the following conditions: initial denaturation step at 95 °C for 6 min, followed by 35 cycles of denaturation at 94 °C for 45 s, annealing at 57 °C for 1 min and extension at 72 °C for 1 min 30 s, with a final extension step at 72 °C for 10 min. The amplified PCR products were analyzed by agarose gel electrophoresis and sequenced using the Sanger method using primers 27F and 1492R. The near-complete 16S rRNA sequences were compared with the GenBank database via BLAST by using the EzBiocloud server (http://www.eztaxon.org) (Chun et al. 2007).
Characterization of potential plant-beneficial traits
Indole acetic acid (IAA) production assay
The ability of bacterial endophytes to produce indole-3-acetic acid (IAA) was tested from bacterial cultures grown in 25 mL of TYC broth (3 g L−1 yeast extract; 5 g L−1 tryptone; and 0.872 g L−1 CaCl2·2H2O) with 0.1% (w/v) l-tryptophan for 5 days at 30 °C at 125 rpm, and were measured based on the colorimetric method. Stationary-phase cultures were centrifuged at 10,000 rpm for 5 min, and 1 mL of supernatant was mixed with Salkowski reagent (2 mL of 0.5 M FeCl3, and 98 mL of 35% HClO4) (1:1 v/v) and incubated in the dark for 30 min (Gordon and Weber 1951). Development of pink color indicated indole production. Results were confirmed by measuring the optical density at 530 nm and comparing with known amounts of IAA using Salkowski reagent and TYC broth with tryptophan and without endophytes as blanks (Li et al. 2012).
Detection of siderophores
Siderophore production was assayed based on competition for iron (Fe) between ferric complexes of chrome azurol S (CAS). The medium was prepared by adding 100 mL of MM9 salt solution (50 g L−1 NH4Cl; 15 g L−1 KH2PO4; 25 g L−1 NaCl; 500 mL ddH2O) to 750 mL of distilled water, followed by addition of piperazine-N, N0-bis 2-ethanesulfonic acid (PIPES, 32.24 g), and adjusting the pH to 6.0. Then, this was followed by addition of 1.5% agar, and the medium was autoclaved at 121 °C for 20 min. After the medium was cooled to 50 °C, the following filter-sterilized solutions were added: 30 mL of filter-sterilized 10% (wt/vol) casamino acids, 10 mL of 20% (wt/vol) glucose, and 100 mL of dye solution, described below. The blue agar medium was aseptically poured into sterile plates and allowed to solidify. All the bacterial isolates were inoculated into the CAS medium and incubated at 30 °C for 5–7 days. Change of the blue color of the medium surrounding the bacterial growth and appearance of an orange/purple or purple/red halo zone around the colonies in the plates was scored as positive for production of siderophores (Alexander and Zuberer 1991).
The dye solution was prepared by mixing 50 mL of Chrome azurol S solution (0.06 g) with 9 mL of FeCl3·6H2O solution (0.0027 g dissolved in 10 mL of 10 mM HCl). The resulting solution was mixed with 0.073 g of hexadecyltrimethylammonium bromide in 40 mL of ddH2O slowly along the flask wall with enough agitation to mix the solution.
Phosphate solubilization
All endophytic bacterial isolates were screened for solubilization of inorganic phosphate on solid Pikovskya’s medium (0.5 g L−1 yeast extract; 0.5 g L−1 (NH4)2SO4; 10 g L−1 glucose; 0.2 g L−1 NaCl; 0.1 g L−1 MgSO4·7H2O; 0.2 g L−1 KCl; 0.002 g L−1 FeSO4·7H2O; 0.002 g L−1 MnSO4·2H2O) supplemented with Ca3(PO4)2 (5 g L−1) and bromophenol blue (0.025 g L−1) (Pikovskaya 1948; Paul and Sinha 2017). After 7 days of incubation at 30 °C, the formation of yellow halos and/or clearing zones was evaluated. The change of color from blue to yellow or formation of a clear halo around the colonies was indicative of acid production.
Nitrogen fixation and potassium solubilization
The bacterial isolates were screened for the ability to fix nitrogen by using two nitrogen-free media: Ashby’s mannitol agar (0.2 g L−1 KH2PO4; 0.2 g L−1 MgSO4; 0.2 g L−1 NaCl; 5.0 g L−1 CaCO3; 10.0 g L−1 mannitol; 0.1 g L−1 CaSO4; 15.0 g L−1 agar; pH 7.0) and NFC medium (10.0 g L−1 mannitol; 0.2 g L−1 MgSO4·7H2O; 0.2 g L−1 KH2PO4; 0.2 g L−1 NaCl; 0.2 g L−1 CaSO4·2H2O; 5.0 g L−1 CaCO3; 15.0 g L−1 agar; pH 7.2) (Sen and Sen 1965; Rao 1977; Liu et al. 2016). All the test organisms were incubated at 30 °C for 7 days. Nitrogen fixation activity was observed based on colony growth on the agar plates. For screening of potassium-solubilizing ability, bacterial strains were cultured in a synthetic medium containing 5.0 g L−1 sucrose; 0.5 g L−1 MgSO4·7H2O; 2.0 g L−1 Na2HPO4; 0.5 g L−1 (NH4)2SO4; 0.1 g L−1 CaCO3; 0.005 g L−1 FeCl3; 0.2 g L−1 yeast extract; 1.0 g L−1 waste mica (K source); 15.0 g L−1 agar; pH 7.5 (Basak and Biswas 2010). All endophytic bacterial strains were incubated at 30 °C for 7–10 days; a halo around the colonies was scored as positive.
Estimation of proteolytic, lipolytic, and cellulytic activity
Protease activity was assayed with YEM agar medium containing 5% (v/v) skim milk. After incubation for 5–7 days at 30 °C, a clear halo around the bacterial colonies due to hydrolysis of milk indicated a positive reaction (Brown and Foster 1970). Lipase enzyme activity was assayed with modified Sierra lipolysis agar supplemented with beef extract (3 g L−1) and ferrous citrate (0.2 g L−1) (Sierra 1957). After autoclaving, 50 mL of Victoria Blue B solution (0.1 g per 150 mL) and 10 mL of Tween 80 was added to the agar medium. After 5–6 days of incubation at 30 °C, white calcium precipitates around the bacterial colonies indicated a positive reaction (Li et al. 2012). Cellulase enzyme activity was assayed with modified DSMZ medium 65 (http://www.dsmz.de/microorganisms/media_list.php) without CaCO3 and supplemented with carboxymethyl cellulose (5 g L−1; Sigma, Shanghai, China) in place of glucose. After incubation for 5–6 days at 30 °C, plates were stained with a Congo red solution and destained with a NaCl solution (Teather and Wood 1982). A clear or lightly colored halo around the colonies indicated a positive reaction. For all the tests mentioned above, sterile nutrient agar was used as a control for bacterial growth. All experiments were performed twice with three replicates for each isolate.
Plant growth promotion assay
To investigate the direct effects of our strains on plant growth, G. uralensis F., the host plant, and T. aestivum (wheat) were used in this study. Seeds were sterilized by sequential washing in 95% ethanol for 15 min and 25% sodium hypochlorite (NaOCl) for 5 min, followed by rinsing three times in sterilized double distilled water. Sterilized seeds were germinated in agar Petri dishes at 28 °C for 48 h. Five seedlings were transplanted into each pot containing a very low nutrient soil mixture (sand, peatmoss, stone, perlite) (1:1:1:1), with ddH2O used for plant watering (Chelius and Triplett 2000; Dong et al. 2003). A suspension of endophytic bacteria (108 cells/mL) was prepared using DensiCHEK Plus (Biomerieux, St. Louis, USA) and each seedling was inoculated with 5 mL of this suspension. Twelve strains that were positive for at least three plant-beneficial traits were tested individually. The G. uralensis F and T. aestivum seedlings were grown in a growth chamber with day and night temperatures of 25 and 18 °C, respectively, and with a 16/8-h light/dark cycle (BIC-400, Shanghai Boxun industry Co, Ltd Medical Equipment Factory, Shanghai, China). Plants were harvested 45 days after planting. All plants were separated into roots and shoots and rinsed with running tap water three times. Seedlings without inoculation of endophytes were considered as a negative control. Growth parameters such as root length, root dry weight, shoot length, shoot dry weight and total biomass were determined. All experiments were done in triplicate.
Statistical analysis
One-way ANOVA with Duncan’s multiple range tests for multiple comparisons was used to compare the means of root length, root dry weight, shoot length, shoot dry weight and total biomass, each separately, between plants inoculated with different microbial species. Before analysis, data homogeneity was tested, and all data were homogeneous. All statistical analyses were conducted using SPSS statistical software (SPSS for Windows, Version 13, Chicago, USA).
Nucleotide accession numbers
Near full-length 16S rRNA gene sequences have been deposited in GenBank under Accession Numbers KY127308–KY127422.
Results
Isolation and Identification of Endophytic Bacteria associated with G. uralensis F.
Endophytic bacterial isolation and corresponding soil chemical analysis was conducted in three regions of Xinjiang Province, China. The three sites each hosted robust, wild populations of G. uralensis F. The soil at each location was sandy, with low organic matter content, low available N, P, and K, high total salt content, and alkaline pH (Table S1). The most extreme soil with regard to low organic matter content, low nutrient content, high salt concentration, and high pH, was at Gongliu.
A total of 116 bacterial isolates were isolated from roots, stems, and leaves of G. uralensis F based on their colony morphologies on different media and were further characterized by 16S rRNA gene sequencing. Based on 16S rRNA gene identity, the 116 isolates were assigned to 20 genera and 35 species (Figs. 1a, S1). All genera belonged to the Firmicutes, Actinobacteria, and Proteobacteria, including the classes Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria. Most of the isolates belonged to the genus Bacillus (65% of total isolates) in the Firmicutes, but the highest genus-level diversity was in the Actinobacteria (n = 11). Other prevalent genera were Brevibacterium, Microbacterium, and Streptomyces (4% each); followed by Kocuria, Micromonospora, Pantoea, and Phyllobacterium (3% each); Stenotrophomonas and Brevundimonas (2% each); and subsequently Achromobacter, Catellatospora, Dietzia, Janibacter, Methylobacterium, Mycobacterium, Nocardioides, Rhodococcus, Staphylococcus, and Starkeya (1% each) (Table S3). The isolation media used in this investigation each supported growth of a high diversity of microorganisms (Fig. S2). The highest number of species was isolated on M10 (n = 13), including members of the genera Bacillus, Brevibacterium, Brevundimonas, Kocuria, Microbacterium, Mycobacterium, Nocardoides, Pantoea, and Stenotrophomonas. The lowest number was obtained on M4 (n = 5).
The diversity of culturable endophytic bacteria was strongly dependent on the plant tissue and sampling location. The highest number of distinct bacterial isolates, selected based on distinct colony morphology, was from root tissue (n = 54), compared to the leaves (n = 34) and stems (n = 28) (Table S3). Similarly, the number of genera associated with roots, stems, and leaves was 14, 9, and 6, respectively (Fig. 1b). Meanwhile, the highest number of isolates was from the Tekesi site (n = 55), followed by Xinyuan (n = 37), and Gongliu (n = 24) (Table S3). Similarly, the number of distinct genera was higher at Tekesi (n = 14) and Xinyuan (n = 12), compared to Gongliu (n = 4) (Fig. 1c). There was a similar drop in higher-level taxa at Gongliu. Almost all isolates from Gongliu belonged to the endospore-forming genus Bacillus (88% of isolates), whereas Proteobacteria were rare (12%), and Actinobacteria were absent (Fig. 1c; Table S3).
Beneficial plant traits of endophytic bacteria
All endophytes isolated from medicinal plant G. uralensis F. were screened for multiple plant growth-promoting traits in order to discover promising endophytic microorganisms (Figs. 2, S3; Table S4). Most strains exhibited one or more plant growth-promoting activities. Among all isolates, about 15% of them synthesized IAA, including members of the genera Achromobacter, Bacillus, Brevibacterium, and Stenotrophomonas. About 23% of the isolates produced siderophores, and these isolates belonged to various species within Bacillus, Achromobacter, and Janibacter. Similarly, about 25% of strains were able to solubilize potassium, including Bacillus, Brevibacterium, and Phyllobacterium isolates. In contrast, only 13% of isolated strains were able to solubilize phosphate, and phosphate solubilization was restricted to Bacillus and Microbacterium isolates. Most of the strains could fix nitrogen (76%), including members of the genera Achromobacter, Bacillus, Brevibacterium, Microbacterium, Mycobacterium, Nocardiodes, Pantoea, Phyllobacterium, Rhodococcus, and Stenotrophomonas.
In addition, the isolates were screened for the presence of hydrolytic enzymes. Most of the strains were able to produce one or more hydrolytic enzymes, but protease, cellulase, and lipase activities were only present in 65, 37, and 47% of isolates, respectively (Figs. 2, S3; Table S4). The strains that could produce all three hydrolytic enzymes belonged to various species within Bacillus and Pantoea.
Effect of selected endophytic bacteria on the growth of two plants
In the present work, all tested isolates showed at least one positive test for the plant growth-promoting properties. To investigate further, twelve strains with multiple plant beneficial traits were selected to evaluate growth enhancement in growth chamber experiments with G. uralensis F and T. aestivum under low-nutrient conditions (Table 1). Plants were harvested 45 days after inoculating the strains, and plant growth parameters were measured. For G. uralensis F. (Figs. 3, S4; Table S5), none of the strains increased root dry weight (Fig. 3a), but eight of the twelve strains significantly increased root length (Fig. 3b), including seven of the eight Bacillus strains, and Achromobacter spanius. For shoots, seven of the strains increased shoot dry weight (Fig. 3c), again including six of the seven Bacillus strains and A. spanius; the same strains, minus one Bacillus isolate, also stimulated shoot length (Fig. 3d). Seven of the strains increased overall biomass (Fig. 3e), including six Bacillus strains in the species B. aryabhatti, B. atrophaeus, B. halotolerans, and B. mojavensis, as well as A. spanius.
The response of Glycyrrhiza uralensis F. 45 days after inoculation with the selected endophytic bacteria, compared with an uninoculated control plant. Asterisks represent p-values from a one-way ANOVA with Duncan’s multiple range test for multiple comparisons. *p < 0.05; **p < 0.01; ***p < 0.001. (Color figure online)
For T. aestivum (Figs. 4, S5; Table S5), four of the Bacillus strains, along with Brevibacterium frigoritolerans and Stenotrophomonas rhizophila, stimulated root dry weight (Fig. 4a), whereas all twelve strains significantly enhanced root length (Fig. 4b). Nine of the strains enhanced shoot dry weight (Fig. 4c), whereas eleven of the twelve strains stimulated shoot length (Fig. 4d), including Bacillus strains, A. spanius, B. frigoritolerans, and S. rhizophila. Ultimately, ten of the twelve strains increased overall biomass (Fig. 4e), again including seven Bacillus strains, A. spanius, B. frigoritolerans, and S. rhizophila.
The response of Triticum aestivum 45 days after inoculation with the selected endophytic bacteria, compared with an uninoculated control plant. Asterisks represent p-values from a one-way ANOVA with Duncan’s multiple range test for multiple comparisons. *p < 0.05; **p < 0.01; ***p < 0.001. (Color figure online)
Discussion
To provide greater insight into plant–endophyte interactions, plant growth-promoting activities were measured in a diverse population of endophytic bacteria isolated from the medicinal plant G. uralensis F. growing in different areas within arid regions in Xinjiang Province, northwest China. In this study, a total 116 strains of different colony morphology were isolated from surface-sterilized root, stem, and leaf tissues of G. uralensis F. The diversity of culturable endophytes differed based on both plant tissue and geographic location in Xinjiang province. The highest diversity was isolated from plant roots, in agreement with other studies (Ma et al. 2013; Liu et al. 2016; Wemheuer et al. 2017). The high bacterial diversity in plant roots may be related to the high bacterial population density in roots, estimated at 108 CFU g−1 in the rhizosphere, compared with 106 CFU g−1 in leaves (Rastogi et al. 2012; Jin et al. 2014). The determination of community structure in different tissues is essential for subsequent application of bacteria as biofertilizers (Szymańska et al. 2016). With regard to geography, a similar diversity of endophytes was obtained from the Xinyuan and Tekesi sites, whereas fewer species were isolated from the Gongliu site. Although all three sites are extreme with regard to low organic content, low N, P, and K content, and high salinity, the Gongliu site was the most extreme. Thus, as has been reported previously, environmental factors such as soil type, nutrient content, and salinity are primary factors influencing both vegetation patterns and the composition of plant-associated microorganisms (Sibanda et al. 2017).
In total, this study resulted in the isolation of 20 genera and 35 species, all belonging to the phyla Firmicutes, Actinobacteria, and Proteobacteria, which are abundant in soils worldwide. In a similar investigation conducted by our group in arid regions of Xinjiang, we isolated 27 and 29 genera from the medicinal plants Ferula songorica and Ferula sinkiangensis, respectively, belonging to the same three bacterial phyla (Liu et al. 2016, 2017). Similarly, Kaplan et al. isolated 31 endophytic strains belonging to four bacterial phyla, Firmicutes, Actinobacteria, Proteobacteria, and Bacteroidetes, and 20 genera from roots of two dominant shrubs found in the Negev Desert (Kaplan et al. 2013). These results confirm the rich microbial diversity in plants grown in arid lands, and the dominance of Firmicutes, Actinobacteria, and Proteobacteria among cultivable endophytes. Although the bacterial communities described in these studies were composed of the same phyla and shared some genera, such as Bacillus, Kocuria, and Micrococcus, they also differed somewhat in microbial composition and structure. This general conclusion is also supported by the finding that similar bacterial taxa have been observed using cultivation-independent techniques (Das et al. 2017; Liu et al. 2016; Jin et al. 2014).
Bacillus was the dominant bacterial genus in the tissus of G. uralensis F., which is consistent with other reports for endophytic bacteria isolated from medicinal plants (Kumar et al. 2012; Wei et al. 2014; Liu et al. 2016, 2017). Bacillus species are widely known for their metabolic activity and various beneficial effects on plant vigor and health. For example, the majority of the registered bacterial products in the European Union Pesticides Database, 2012 are based on species of Bacillus. The genus Streptomyces, which was the second most prevalent genus in our study, is also well known to grow endophytically within medicinal plants, in addition to its well-known capabilities producing novel secondary metabolites (Qin et al. 2012; Sibanda et al. 2017).
Endophytic bacteria are known to exhibit a wide variety of plant growth-promoting activities. Mechanisms of interactions between endophytic bacteria and host plants have been well-documented previously by (Li et al. 2012; Wong et al. 2014; Halo et al. 2015; Islam et al. 2016; Patil et al. 2016; Kandel et al. 2017), and include the production of phytohormones, siderophores, hydrolytic enzymes, phosphate and potassium solubilization, and diazotrophy. Some studies have reported that endophytic bacteria isolated from medicinal plants produced indole-3-acetic acid (IAA) (Khan et al. 2014), which is involved in stimulation of plant growth by stimulating cell enlargement, division, differentiation, and gene regulation (Leveau and Lindow 2005). In our studies, about 15% of the isolates produced IAA.
These endophytes also exhibited activities to solubilize mineral phases of important elements, specifically iron, phosphorous, and potassium. Iron is an abundant element in the earth’s crust, primarily as ferric iron minerals that are not easily solubilized under oxic atmospheres and at circumneutral or alkaline pH, which are predominant in desert environments. About 23% of our isolates produced siderophores, which is important for the reduction of Fe3+ to Fe2+ and transport into the plant (Lemanceau et al. 2009). It has been shown previously that endophytic bacteria can produce different structural types of siderophores such as hydroxamates, catecholate, and citrate-based polycarboxylates (Ahmed and Holmström 2014), but the structures of the siderophores in our isolates were not determined. Many strains also acted as potassium-solubilizing microorganisms (KSM). In our study, about 25% of the isolates solubilized potassium. Potassium is an essential element for plant growth and development because it is responsible for adjusting cellular osmotic pressure, the transportation of compounds in plant tissues, activation of enzymes, and the synthesis of protein and sugar (Basak and Biswas 2010). Phosphorus plays a major role in numerous plant processes including nucleic acid synthesis, photosynthesis, respiration, energy generation, and cell signaling (Vance et al. 2003). Among the tested isolates, about 13% were capable of solubilizing bound phosphorus and making it available for the plant; therefore, these strains could potentially be used as inocula to increase phosphorus uptake by the plant and thus increase the crop yield (Hameeda et al. 2008; Trivedi et al. 2011).
A majority of isolates (76%) fixed nitrogen under the tested conditions, which is important because most arid soils have low fixed nitrogen content. Nitrogen is important for plant growth and productivity because it is an integral element in proteins, nucleic acids, and other essential biomolecules (Bøckman 1997; Zakry et al. 2012; Lin et al. 2012). Many of the endophytes isolated in this study produced hydrolytic enzymes such as protease, cellulase, and lipase. In similar work, it has been demonstrated that endophytic bacteria isolated from the medicinal plants Ferula songorica, Hypericum perforatum, and Ziziphora capital could secrete different hydrolytic enzymes including cellulase, protease, and amylase (Egamberdieva et al. 2017; Liu et al. 2016). Hydrolytic enzymes may play an important role in initial plant infection, allowing for further spreading within tissues. Additionally, these enzymes are industrially relevant, with their potential uses in various industries including pharmaceuticals, bioremediation, and agriculture having been well-established (Chand and Mishra 2003).
The assessment of the effect of selected endophytic bacteria on the growth enhancement of the host plant (G. uralensis F.) and wheat (T. aestivum) in pot experiments was conducted under low-nutrient conditions. Under these conditions, most of the tested Bacillus strains stimulated root length, shoot length, shoot dry weight, and total biomass in the host plant. In contrast, of the three other genera tested, only Achromobacter promoted growth of the host plant. Interestingly, although the endophytes were isolated from G. uralensis F., they stimulated growth of wheat under the tested conditions more effectively. In terms of total biomass, all strains except one significantly stimulated growth. We speculate that these results were probably a response of enhancement to microbial production of the plant hormone IAA. This hormone functions as an important signal molecule in the regulation of plant development by enlarging the root system (Leveau and Lindow 2005). In particular, they stimulate the formation of lateral roots and absorbent root hairs (Giassi et al. 2016). Moreover, it has been suggested that the combination of phosphate-solubilizing, nitrogen-fixing, and phytohormone producing bacteria could provide a balanced nutrition for plants and stimulate growth of various crops (Khan et al. 2014; Nimaichand et al. 2016). Our experiments show that the endophytes differ in their plant growth-promoting activities and implicate the genus Bacillus as a promising target for biofertilizers.
Conclusions
In summary, the present investigation represents the first report on the distribution and bioactivity of endophytic bacteria associated with the medicinal plant G. uralensis F. Our study has led us to conclude that G. uralensis F. represents a rich reservoir for diverse endophytic bacteria, which differ based on the specific tissue and geographic location. The results provide insights about plant beneficial traits of culturable endophytic bacteria associated with the medicinal plant licorice. The genus Bacillus appears to be the most promising bioinoculum for the two tested plants under growth chamber conditions, and they could be a cost-effective source for agro-based biofertilizer agents. These Bacillus strains have various abilities related to plant growth promotion, including solubilization of phosphate, siderophores, potassium, nitrogen fixation, protease, cellulase, lipase, and production of IAA, and were able to promote growth of both licorice and wheat in growth chamber experiments. In light of this broad activity, further evaluation of these strains to identify biologically active compounds and to assess their ability to promote growth of other plants (including medicinal plants) in arid environments is justified.
References
Ahmed E, Holmström SJ (2014) Siderophores in environmental research: roles and applications. Microb Biotechnol 7:196–208
Alexander DB, Zuberer DA (1991) Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils 12:39–45
Bajguz A (2007) Metabolism of brassinosteroids in plants. Plant Physiol Biochem 45:95–107
Basak B, Biswas D (2010) Co-inoculation of potassium solubilizing and nitrogen fixing bacteria on solubilization of waste mica and their effect on growth promotion and nutrient acquisition by a forage crop. Biol Fertil Soils 46:641–648
Bøckman OC (1997) Fertilizers and biological nitrogen fixation as sources of plant nutrients: perspectives for future agriculture. Plant Soil 194:11–14
Boor KJ (2006) Bacterial stress responses: what doesn’t kill them can make them stronger. PLoS Biol 4:e23
Brown MRW, Foster JS (1970) A simple diagnostic milk medium for Pseudomonas aeruginosa. J Clin Pathol 23:172–177
Chand S, Mishra P (2003) Research and application of microbial enzymes—India’s contribution. Biotechnol India II:95–124
Chelius MK, Triplett EW (2000) Immunolocalization of dinitrogenase reductase produced by Klebsiella pneumoniae in association with zea mays L. Appl Environ Microbiol 66:783–787
Chun J, Lee JH, Jung Y, Kim M, Kim S, Kim BK et al (2007) EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol 57:2259–2261
Cushnie TT, Cushnie B, Lamb AJ (2014) Alkaloids: an overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int J Antimicrob Agents 44:377–386
Daffonchio D, Hirt H, Berg G (2015) Plant–microbe interactions and water management in arid and saline soils. In: Lugtenberg B (ed) Principles of plant–microbe interactions. Springer, Cham
Das G, Park S, Baek KH (2017) Diversity of endophytic bacteria in a fern species Dryopteris uniformis (Makino) Makino and evaluation of their antibacterial potential against five foodborne pathogenic bacteria. Foodborne Pathog Dis 14:260–268
Donate-Correa J, León-Barrios M, Pérez-Galdona R (2005) Screening for plant growth-promoting rhizobacteria in Chamaecytisus proliferus (tagasaste), a forage tree-shrub legume endemic to the Canary Islands. Plant Soil 266:261–272
Dong Y, Chelius MK, Brisse S, Kozyrovska N, Kovtunovych G, Podschun R et al (2003) Comparisons between two Klebsiella: the plant endophyte K. pneumoniae 342 and a clinical isolate, K. pneumoniae MGH78578. Symbiosis 35:247–259
Egamberdieva D, Wirth S, Behrendt U, Ahmad P, Berg G (2017) Antimicrobial activity of medicinal plants correlates with the proportion of antagonistic endophytes. Front Microbiol 8:199
Egamberdiyeva D (2007) The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl Soil Ecol 36:184–189
Gasser I, Cardinale M, Müller H, Heller S, Eberl L, Lindenkamp N et al (2011) Analysis of the endophytic lifestyle and plant growth promotion of Burkholderia terricola ZR2-12. Plant Soil 347:125–136
Giassi V, Kiritani C, Kupper KC (2016) Bacteria as growth-promoting agents for citrus rootstocks. Microbiol Res 190:46–54
Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26:192
Halo BA, Khan AL, Waqas M, Al-Harrasi A, Hussain J, Ali L et al (2015) Endophytic bacteria (Sphingomonas sp. LK11) and gibberellin can improve Solanum lycopersicum growth and oxidative stress under salinity. J Plant Interact 10:117–125
Hameeda B, Harini G, Rupela O, Wani S, Reddy G (2008) Growth promotion of maize by phosphate-solubilizing bacteria isolated from composts and macrofauna. Microbiol Res 163:234–242
Islam F, Yasmeen T, Arif MS, Ali S, Ali B, Hameed S et al (2016) Plant growth promoting bacteria confer salt tolerance in Vigna radiata. Plant Growth Regul 1:23–36
Jin H, Yang XY, Yan ZQ, Liu Q, Li XZ, Chen JX et al (2014) Characterization of rhizosphere and endophytic bacterial communities from leaves, stems and roots of medicinal Stellera chamaejasme L. Syst Appl Microbiol 37:376–385
Kandel SL, Firrincieli A, Joubert PM, Okubara PA, Leston ND, McGeorge KM et al (2017) An in vitro study of bio-control and plant growth promotion potential of Salicaceae endophytes. Front Microbiol 8:386
Kaplan D, Maymon M, Agapakis CM, Lee A, Wang A, Prigge BA et al (2013) A survey of the microbial community in the rhizosphere of two dominant shrubs of the Negev Desert highlands, Zygophyllum dumosum (Zygophyllaceae) and Atriplex halimus (Amaranthaceae), using cultivation-dependent and cultivation-independent methods. Am J Bot 100:1713–1725
Khan AL, Waqas M, Kang SM, Al-Harrasi A, Hussain J, Al-Rawahi A et al (2014) Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J Microbiol 52:689–695
Knudsen D, Peterson GA, Pratt PF (1982) Lithium, sodium and potassium. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis part 2: chemical and microbiologicaI properties, 2nd edn. American Society of Agronomy, Soil Science Society of America, Madison, pp 225–246
Kumar P, Khare S, Dubey R (2012) Diversity of Bacilli from disease suppressive soil and their role in plant growth promotion and yield enhancement. N Y Sci J 5:90–111
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–175
Lemanceau P, Bauer P, Kraemer S, Briat JF (2009) Iron dynamics in the rhizosphere as a case study for analyzing interactions between soils, plants and microbes. Plant Soil 321:513–535
Leveau JH, Lindow SE (2005) Utilization of the plant hormone indole-3-acetic acid for growth by Pseudomonas putida strain 1290. Appl Environ Microbiol 71:2365–2371
Lewis G, Schrire B, Mackinder B, Lock M (2005) Legumes of the world. Royal Botanic Gardens, Kew, London
Li L, Sinkko H, Montonen L, Wei GH, Lindstrom K, Rasanen LA (2012) Biogeography of symbiotic and other endophytic bacteria isolated from medicinal Glycyrrhiza species in China. FEMS Microbiol Ecol 79:46–68
Lin L, Li Z, Hu C, Zhang X, Chang S, Yang L et al (2012) Plant growth-promoting nitrogen-fixing enterobacteria are in association with sugarcane plants growing in Guangxi, China. Microbes Environ 27:391–398
Liu YH, Guo JW, Salam N, Li L, Zhang YG, Han J et al (2016) Culturable endophytic bacteria associated with medicinal plant Ferula songorica: molecular phylogeny, distribution and screening for industrially important traits. 3. Biotech 6:209
Liu Y, Guo J, Li L, Asem MD, Zhang Y, Mohamad OA et al (2017) Endophytic bacteria associated with endangered plant Ferula sinkiangensis KM Shen in an arid land: diversity and plant growth-promoting traits. J Arid Land 9:432–445
Ma B, Lv X, Warren A, Gong J (2013) Shifts in diversity and community structure of endophytic bacteria and archaea across root, stem and leaf tissues in the common reed, Phragmites australis, along a salinity gradient in a marine tidal wetland of northern China. Antonie Van Leeuwenhoek 104:759–768
Malfanova N, Kamilova F, Validov S, Shcherbakov A, Chebotar V, Tikhonovich I et al (2011) Characterization of Bacillus subtilis HC8, a novel plant-beneficial endophytic strain from giant hogweed. Microbial Biotechnol 4:523–532
Nanjing Institute of Soil Research, CAS (1980) Analysis of soil physicochemical features. Shanghai Science and Technology Press, Shanghai (in Chinese)
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: Klute A (ed) Methods of soil analysis, Part 2, 2nd edn. Agron. Monogr. 9. ASA. Madison
Nimaichand S, Devi AM, Li WJ (2016) Direct plant growth-promoting ability of actinobacteria in grain legumes. In: Plant growth promoting actinobacteria. Springer, New york, pp 1–16
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis part 2: chemical and microbiologicaI properties, 2nd edn. Soil Science Society of America. American Society of Agronomy, Madison, pp 403–430
Patil SV, Jayamohan NS, Kumudini BS (2016) Strategic assessment of multiple plant growth promotion traits for shortlisting of fluorescent Pseudomonas spp. and seed priming against ragi blast disease. Plant Growth Regul 80:47–58
Paul D, Sinha SN (2017) Isolation and characterization of phosphate solubilizing bacterium Pseudomonas aeruginosa KUPSB12 with antibacterial potential from river Ganga, India. Ann Agrar Sci 15:130–136
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 17:362–370 (in Russian)
Qin S, Chen HH, Zhao GZ, Li J, Zhu WY, Xu LH et al (2012) Abundant and diverse endophytic actinobacteria associated with medicinal plant Maytenus austroyunnanensis in Xishuangbanna tropical rainforest revealed by culture dependent and culture independent methods. Environ Microbiol Rep 4:522–531
Rao S (1977) Soil microorganisms and plant growth. Oxford and IBH Publishing Co, India
Rastogi G, Sbodio A, Tech JJ, Suslow TV, Coaker GL, Leveau JH (2012) Leaf microbiota in an agroecosystem: spatiotemporal variation in bacterial community composition on field-grown lettuce. ISME J 6:1812–1822
Reinhold-Hurek B, Hurek T (2011) Living inside plants: bacterial endophytes. Curr Opin Plant Biol 14:435–443
Sánchez-López AS, Thijs S, Beckers B, González-Chávez MC, Weyens N, Carrillo-González R et al (2017) Community structure and diversity of endophytic bacteria in seeds of three consecutive generations of Crotalaria pumila growing on metal mine residues. Plant Soil 422:51–66
Sen M, Sen SP (1965) Interspecific transformation in Azotobacter. Microbiology 41:1–6
Sibanda T, Selvarajan R, Tekere M (2017) Synthetic extreme environments: overlooked sources of potential biotechnologically relevant microorganisms. Microbial Biotechnol 10:570–585
Sierra G (1957) A simple method for the detection of lipolytic activity of micro-organisms and some observations on the influence of the contact between cells and fatty substrates. Antonie Van Leeuwenhoek 23:15–22
Strobel G, Daisy B, Castillo U, Harper J (2004) Natural products from endophytic microorganisms. J Nat Prod 67:257–268
Szymańska S, Płociniczak T, Piotrowska-Seget Z, Hrynkiewicz K (2016) Endophytic and rhizosphere bacteria associated with the roots of the halophyte Salicornia europaea L. community structure and metabolic potential. Microbiol Res 192:37–51
Teather RM, Wood PJ (1982) Use of Congo red–polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol 43:777–780
Trivedi P, Spann T, Wang N (2011) Isolation and characterization of beneficial bacteria associated with citrus roots in florida. Microb Ecol 62:324–336
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447
Wei L, Shao Y, Wan J, Feng H, Zhu H, Huang H et al (2014) Isolation and characterization of a rhizobacterial antagonist of root-knot nematodes. PLoS ONE 9:e85988
Wemheuer F, Kaiser K, Karlovsky P, Daniel R, Vidal S, Wemheuer B (2017) Bacterial endophyte communities of three agricultural important grass species differ in their response towards management regimes. Sci Rep 7:40914
Wong WT, Tseng CH, Hsu SH, Lur HS, Mo CW, Huang CN et al (2014) Promoting effects of a single Rhodopseudomonas palustris inoculant on plant growth by Brassica rapa chinensis under low fertilizer input. Microbes Environ 29:303–313
Zakry FAA, Shamsuddin ZH, Abdul RK, Zawawi ZZ, Abdul RA (2012) Inoculation of Bacillus sphaericus UPMB-10 to young oil palm and measurement of its uptake of fixed nitrogen using the 15 N isotope dilution technique. Microbes Environ 27:257–262
Zhang Q, Ye M (2009) Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J Chromatog A 1216:1954–1969
Acknowledgements
This work was supported by Xinjiang Uygur Autonomous Region regional coordinated innovation project (Shanghai Cooperation Organization Science and Technology Partnership Program) (No. 2017E01031). Li Li was supported by China Scholarship Council to study in the United States of America (No. 201509655013). Osama A. A. Mohamad was supported by Chinese Academy of Sciences President’s International Fellowship Initiative (No. 2016PB024) and Available Position Talented Young Scientists Program of Ministry of Science and Technology of the People’s Republic of China (No. P-EG-16-01).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no indirect or direct conflict of interest.
Ethical approval
This article does not contain any studies related to human participants or animals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10482_2018_1062_MOESM1_ESM.tiff
Figure S1. Bacterial species distribution of culturable endophytic isolates from Glycyrrhiza uralensis F based on 16S rRNA gene sequences for all sites. Supplementary material 1 (TIFF 764 kb)
10482_2018_1062_MOESM2_ESM.tiff
Figure S2. Distribution of endophytic isolates and species isolated from Glycyrrhiza uralensis F based on different media. Supplementary material 2 (TIFF 688 kb)
10482_2018_1062_MOESM3_ESM.tiff
Figure S3. Confirmation of plant growth promotion traits by a color change or halo zone on the selective medium (A: Protease; B: Cellulase; C: Lipase; D: Nitrogen; E: Phosphorus; F: Potassium; G: Siderophore; H: IAA). Supplementary material 3 (TIFF 2689 kb)
10482_2018_1062_MOESM4_ESM.tiff
Figure S4. Effect of endophytic isolates on the growth of Glycyrrhiza uralensis F compared with an uninoculated control (CK). Supplementary material 4 (TIFF 4374 kb)
10482_2018_1062_MOESM5_ESM.tiff
Figure S5. Effect of endophytic isolates on the growth of Triticum aestivum compared with an uninoculated control (CK). Supplementary material 5 (TIFF 3684 kb)
Rights and permissions
About this article
Cite this article
Li, L., Mohamad, O.A.A., Ma, J. et al. Synergistic plant–microbe interactions between endophytic bacterial communities and the medicinal plant Glycyrrhiza uralensis F.. Antonie van Leeuwenhoek 111, 1735–1748 (2018). https://doi.org/10.1007/s10482-018-1062-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-018-1062-4