Abstract
Leaf-cutter ants cultivate and feed on the mutualistic fungus, Leucoagaricus gongylophorus, which is threatened by parasitic fungi of the genus Escovopsis. The mechanism of Escovopsis parasitism is poorly understood. Here, we assessed the nature of the antagonism of different Escovopsis species against its host. We also evaluated the potential antagonism of Escovopsioides, a recently described fungal genus from the attine ant environment whose role in the colonies of these insects is unknown. We performed dual-culture assays to assess the interactions between L. gongylophorus and both fungi. We also evaluated the antifungal activity of compounds secreted by the latter on L. gongylophorus growth using crude extracts of Escovopsis spp. and Escovopsioides nivea obtained either in (1) absence or (2) presence of the mutualistic fungus. The physical interaction between these fungi and the mutualistic fungus was examined under scanning electron microscopy (SEM). Escovopsis spp. and E. nivea negatively affected the growth of L. gongylophorus, which was also significantly inhibited by both types of crude extract. These results indicate that Escovopsis spp. and E. nivea produce antifungal metabolites against the mutualistic fungus. SEM showed that Escovopsis spp. and E. nivea maintained physical contact with the mutualistic fungus, though no specialised structures related to mycoparasitism were observed. These results showed that Escovopsis is a destructive mycoparasite that needs physical contact for the death of the mutualistic fungus to occur. Also, our findings suggest that E. nivea is an antagonist of the ant fungal cultivar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Throughout evolution, ants in the tribe Attini (hereafter named “attines”) developed the habit to cultivate fungi for food. The genera Atta and Acromyrmex, known as leaf-cutter ants, practice the most derived fungiculture within the attines, characterized by the use of fresh leaves and flowers as substrate for the growth of the fungal partner (Schultz and Brady 2008). The fungus cultivated by these insects is a basidiomycete named Leucoagaricus gongylophorus (Agaricales: Agaricaceae). In this association, ants provide protection and plant substrate required for the development of their partner. In turn, the fungus is the primary source of nutrients for the ant larvae and queens. L. gongylophorus is grown by leaf-cutter ants in fungus gardens, which consist of the mycelium of the mutualistic fungus and fragments of the plant substrate foraged by workers (Weber 1972).
Due to the intense foraging habits, it is common that a plethora of microorganisms enter the ant colonies, which may be potential antagonists for the mutualistic fungus (Pagnocca et al. 2012). Although ants have developed strategies to control the development of other microorganisms in the fungus gardens, the presence of an extensive variety of yeasts, filamentous fungi and bacteria is widely known (Möller 1893; Carreiro et al. 1997; Currie et al. 1999; Rodrigues et al. 2008; Suen et al. 2010). Such microbes may play important roles, including promotion of plant material degradation in the fungus garden by bacteria (Suen et al. 2010). Yeasts and bacteria may also act in the protection of the ant colony by producing antimicrobial compounds against antagonistic fungi (Santos et al. 2004; Rodrigues et al. 2009). In addition, filamentous fungi present on the substrate foraged by ant workers may be considered a threat to the fungus garden (Rodrigues et al. 2008). On the other hand, it is also likely these microbes are only transients, with no ecological function for the ant-fungus association (Pagnocca et al. 2012).
Among the microbes found in attine ant gardens, asexual fungi in the genus Escovopsis (Ascomycota: Hypocreales) are specialised parasites of the fungus cultivated by the ants (Currie et al. 1999). A study by Currie et al. (2003) provided evidences for the ancient evolutionary history that Escovopsis shares with these insects and their mutualistic fungus. Such co-diversification with the ants and their fungal partners contributed to the appearance of broad phylogenetic patterns of specificity leading to different Escovopsis groups specialised on parasitizing different mutualistic fungi (Currie et al. 2003; Gerardo et al. 2006; Birnbaum and Gerardo 2016). Moreover, Man et al. (2016) showed that the Escovopsis genome has also undergone significant changes during evolution to be adapted to the mycoparasitic lifestyle (e.g. reduced genome by loss of genes involved in plant material metabolism, when compared with close fungal relatives). In addition, a new genus of filamentous fungus named Escovopsioides was recently found in the fungus gardens of leaf-cutter ants (Augustin et al. 2013). This genus is phylogenetically related with Escovopsis but differs in morphological characteristics by the absence of colony pigmentation and the presence of langeniform phialides on terminal and intercalary globose vesicles. Until now, there is only one described species in the genus, Escovopsioides nivea (Augustin et al. 2013). However, information on its ecological role in the fungus garden remains elusive.
Despite our understanding of the evolutionary history of Escovopsis, little is known about the mechanisms of parasitism on its host L. gongylophorus. Escovopsis does not seem to be a competitor for the nutrients of the mutualistic fungus. In the first attempt to elucidate the nature of this parasitism by Reynolds and Currie (2004), Escovopsis weberi presented the best growth in the presence of the mutualistic fungus. These authors showed that E. weberi did not obtain a good development when inoculated on Petri dishes containing plant material as a source of nutrients. Additionally, by means of mycoparasitism assays, the authors documented hyphal degradation of the mutualistic fungus before physical interactions with E. weberi hyphae. These results lead the authors to classify this fungus as a necrotrophic mycoparasite.
A recent study by Marfetán et al. (2015) reported the occurrence of specialised hyphal structures by strains of E. weberi such as hook-like protuberances and prolongations with spiky tips to penetrate the host hyphae. They found that the strains able to produce such structures were the most damaging to the host. Then, the authors re-classified Escovopsis as a destructive biotrophic mycoparasite as they interpreted that Escovopsis uptook its nutrients from living cells of the fungus cultivated by the ants and that host death occurred after this parasitic process.
The studies by Reynolds and Currie (2004) and Marfetán et al. (2015) advanced our understanding on the role played by Escovopsis in the fungus gardens. However, information about the parasite’s mode of action on its host considering a broad range of Escovopsis isolates from different leaf-cutter ant species is still lacking. Here, we performed experimental assays and used scanning electron microscopy to evaluate the interactions of Escovopsis spp. and of E. nivea against L. gongylophorus. As there is no information on the potential antagonism of E. nivea, this study is the first to demonstrate the effect of this fungus against the leaf-cutter ant cultivar.
Materials and methods
Fungal isolates
In the present study, we evaluated fungi belonging to the genera Escovopsis (n = 10 isolates) and Escovopsioides (n = 2) obtained from fungus gardens or midden of different species of leaf-cutter ants (Table 1). Morphological and molecular analyses carried out by Rodrigues et al. (2008) and Meirelles et al. (2015) revealed seven undescribed Escovopsis species within the isolates evaluated in the present work. The two Escovopsioides isolates were previously examined by Rodrigues et al. (2008), but in the present study these were identified as E. nivea by morphology. All isolates are stored at −80 °C as conidial suspensions at the Laboratory of Fungal Ecology and Systematics (LESF), UNESP, Rio Claro, São Paulo State, Brazil. Stored conidia were cultured in potato-dextrose agar medium (PDA, Acumedia) supplemented with 150 μg mL−1 of chloramphenicol (Sigma) and incubated at 25 °C for 10 days in the dark. After incubation, pure cultures of all isolates were confirmed by macro- and microscopic characteristics of the colonies.
The strain of L. gongylophorus (FF2006) was isolated from a mature Atta sexdens rubropilosa laboratory colony, kept in the Center for the Study of Social Insects (CEIS) at UNESP. For the isolation of this fungus, garden fragments, without ant workers and brood, were inoculated on PDA medium supplemented with 150 μg mL−1 of chloramphenicol (Sigma) and incubated in the dark at 25 °C for 20 days. Then, pure cultures of the mutualistic fungus were maintained by successive transfers every 20 days on culture medium (in g L−1: 10 glucose, 5 sodium chloride, 5 peptone, 10 malt extract, 15 agar supplemented with oatmeal extract; Pagnocca et al. 1990).
Dual-culture assays
Co-culture assays according to those proposed by Silva et al. (2006) were performed to evaluate the interactions of Escovopsis spp. and E. nivea isolates on L. gongylophorus. Mycelium fragments of 5 mm in diameter of the cultivar, previously grown on the same culture medium as described above at 25 °C, were cut and placed at 1.5 cm distance from the border of Petri dishes containing PDA. Plates were incubated in darkness at 25 °C for 15 days to allow the head-start growth of the cultivar. This was carried out due to the slow growth rate of the cultivar.
All Escovopsis spp. and E. nivea isolates were previously grown on PDA and incubated at 25 °C for 7–10 days. After incubation, mycelium fragments of 5 mm in diameter were cut from the edge of the colony and placed at 3.0 cm apart of the colony of L. gongylophorus. For the control plates, mycelial fragments of the L. gongylophorus cultivar were added instead of the microfungi. Plates were incubated in darkness at 25 °C for 14 days. All interactions between L. gongylophorus and the 12 isolates listed in Table 1 and the control were performed in six replicates.
The effects of each Escovopsis spp. and E. nivea isolates on the growth of the mutualistic fungus were monitored and recorded daily. The experimental plates were scanned in a HP Deskjet F2050 Scanner. The obtained images on days 0, 3, 5, 7, 10 and 14 of incubation were analysed with Image J v.1.38 (Schneider et al. 2012) to measure the area of the mutualistic fungus growth (in cm2). The areas of mycelial growth of L. gongylophorus were statiscally analysed using Repeated Measures ANOVA. The data were checked for normality and homogeneity of variances using the Shapiro–Wilk and Bartlett tests, respectively. Data were transformed by square root or logarithm to achieve normality and homoscedasticity when necessary. Analyses were performed in R v. 3.3.1 (R Core Team 2016).
In addition, we calculated the percentage of inhibition of L. gongylophorus by the filamentous fungi. Growth efficiency (E) of the fungus was calculated by dividing the average area of growth of the colonies after 14 days (FG) by the initial average area of colony growth (IG) by the formula: E = FG/IG. Relative growth efficiency (RE) of L. gongylophorus colonies in the presence of different filamentous fungi was expressed relative to the control of L. gongylophorus by the formula: RE = E/EC, where EC is the efficiency of mutualistic fungus growth in the control (Silva et al. 2006). Differences in growth efficiency within the filamentous fungi were analysed using one-way ANOVA followed by pairwise t test with Bonferroni correction. Analyses were performed in R v. 3.3.1.
Crude extract assays
To investigate the production of antifungal compounds by Escovopsis spp. and E. nivea, two types of extracts were obtained: (1) crude extract in the absence (CEA); and (2) crude extract in the presence (CEP) of the fungus L. gongylophorus. The strategy of adding the fungal cultivar in the latter trial was performed to assess whether Escovopsis spp. and E. nivea produce compounds with inhibitory activity only in the presence of the cultivar.
For the CEA, all Escovopsis spp. and E. nivea isolates were inoculated on PDA and incubated in darkness at 25 °C for 7–10 days. From these cultures, suspensions with approximately 106 conidia mL−1 of Escovopsis spp. (standardised in a Neubauer chamber) were inoculated into Erlenmeyer flasks (125 mL) containing 90 mL of potato-dextrose broth (PDB, Himedia). Flasks were incubated at 25 °C for 14 days, under agitation. Due to the low sporulation of E. nivea on PDA, five fragments (5 mm in diameter) were removed from the mycelium and inoculated in Erlenmeyer flasks and incubated under the same conditions. For the CEP, L. gongylophorus mycelium fragments (5 mm in diameter) were previously inoculated in Erlenmeyer flasks (125 mL) containing 90 mL of PDB. The flasks were incubated at 25 °C for three days, under stirring (120 rpm). After this period, either a suspension of approximately 106 conidia mL−1 of Escovopsis spp. or five mycelial fragments of 5 mm in diameter of E. nivea were inoculated in the Erlenmeyer flasks and further incubated at 25 °C for 14 days under the same stirring conditions. Cultures filtrates (membrane filter of 0.45 μm, Millipore) were used to prepare solid medium (Pagnocca et al. 1990) in 1:1 ratio (v/v), keeping the original concentration of medium compounds.
After plate preparation, a mycelium fragment of 5 mm in diameter of L. gongylophorus previously grown on the same solid medium supplemented with oatmeal extract (15–20 days) was inoculated in the center of the experimental plates. As control, mycelium fragments of L. gongylophorus were inoculated in plates containing both PDB and solid medium in 1:1 ratio (v/v). L. gongylophorus growth was recorded by scanning the plates after 3, 7, 14 and 21 days. Images of the colonies were analysed using Image J software v. 1:38 to measure the growth area (in cm2). Seven replicates were performed for each extract. The mean areas of mycelial growth of L. gongylophorus were evaluated using Repeated Measures ANOVA considering each extract obtained by each fungi and the control. The data were checked using Shapiro–Wilk and Bartlett tests and transformed by square root or logarithm to achieve normality and homoscedasticity when necessary. Analyses were performed in R v. 3.3.1.
Scanning electron microscopy (SEM)
Four Escovopsis spp. isolates (LESF017, LESF019, LESF043, and LESF045) and the two E. nivea isolates (LESF596 and LESF599) were used to evaluate the physical hyphae–hyphae interactions against L. gongylophorus. In Petri dishes containing water agar, a mycelium fragment of 5 mm in diameter of each hypocrealean filamentous fungus was inoculated at a distance of 1.5 cm from the mycelial fragment (of the same size) of the fungal cultivar. Plates were incubated at 25 °C and monitored every 12 h to determine the time when the test fungus hyphae established contact with the hyphae of L. gongylophorus. When contact occurred, the plates were fixed with vapour of osmium tetroxide and, after 4 days, the fragment of L. gongylophorus mycelium was detached from the plate and transferred to an aluminium support. Subsequently, the samples were dehydrated in acetone baths with increasing concentrations of 50, 75, 90, 95 and 100%. After dehydration at critical point (Balzers CPD030), the material was stuck with double-stick adhesive tape on stubs and metallised with gold Sputtering (Balzers SCD050). Then, the material was examined in a scanning electron microscope (Hitachi TM3000). As control, we observed the fungus L. gongylophorus and the hypocrealean fungi cultured separately and treated as described above. We performed five replicates for each assay.
To help distinguish Escovopsis spp. and E. nivea hyphae from the ones of the mutualistic fungus, we carried out a separate experiment. All fungi examined under SEM were cultured in Petri plates as indicated above and after the incubation period, wet-mounts were prepared in 10% KOH and examined under light microscopy (Leica—DM500). We carried out 30 hyphae width measuraments per fungal isolate using Leica Application Suite v.4.0.
Results
Dual-culture assays
All of the 12 isolates significantly inhibited the growth of the mutualistic fungus, when compared to the control (ANOVA, P < 0.01, Table 2). The isolate Escovopsis sp. LESF017 provided the greatest inhibition (78%) of the growth of the ant fungal cultivar and the strain Escovopsis sp. LESF023 presented the least inhibition of the mutualistic fungus (56%). The E. nivea isolates had the lowest percentages of inhibition (45% for LESF599 and 56% for LESF596) when compared to Escovopsis spp. isolates.
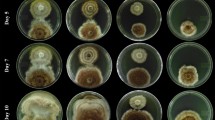
All Escovopsis spp. isolates rapidly overgrew the L. gongylophorus colonies. Similarly, the E. nivea isolates also overgrew the mutualistic fungus. Sporulation of the tested fungi over the colony of the mutualistic fungus was detected in all plates for both Escovopsis spp. and E. nivea. Morphological alterations of the fungal cultivar were observed in all experimental assays when compared to the control, in which the mutualistic fungus showed a gradual and healthy growth (Fig. 1a–c). In general, three types of responses of L. gongylophorus were observed against Escovopsis spp. during the interactions: (1) colour change of the culture medium from light yellow to dark brown in bioassays using the isolates LESF017, LESF019, LESF021, LESF033, LESF039, LESF043 and LESF045. In this category, there was a slight darkening of the edge of the L. gongylophorus colonies (LESF021, LESF033 and LESF039) and the occurrence of gradual degeneration of the mycelium (Fig. 1d–f), and only traces of it were observed at the 14th day of experiment; (2) the isolates LESF040 and LESF041 changed the colour of the culture medium, in the addition the colour of the mutualistic fungus colony changed from whitish to black (Fig. 1j–l). We also observed the degeneration of the fungus mycelium; and (3) the culture medium became reddish in bioassays using the isolate LESF023. However, morphological changes in the mycelium of L. gongylophorus were not observed when confronted with isolate LESF023 (Fig. 1h–i).
Dual-culture assays between the mutualistic fungus Leucoagaricus gongylophorus (FF2006) and filamentous fungi isolated from attine ant gardens. In each plate, the mutualistic fungus was inoculated on the left and all other fungi were inoculated on the right. Control plates were inoculated with the mutualistic fungus and experimental plates with Escovopsis spp. isolates (LESF021, LESF023 and LESF040) and Escovopsioides nivea isolates (LESF599 and LESF596)
The E. nivea isolates also caused the darkening of L. gongylophorus mycelium, especially in areas where there was contact between the hyphae of both fungi (Fig. 1n–o, q–r). We observed changes in colour of the medium from light yellow to orange. The mutualistic fungus apparently showed a slight antibiosis to E. nivea LESF596, however, this has been overcome by the growth of the latter fungus (Fig. 1q–r).
Effects of the crude extracts on the growth of Leucoagaricus gongylophorus
All crude extracts in the absence (CEA) and in the presence (CEP) significantly inhibited the growth of the mutualistic fungus in comparison to the control (ANOVA, P < 0.05, Fig. 2). Comparing the results obtained between treatments with the CEA and CEP for each filamentous fungal isolate, we observed significant differences for eight Escovopsis spp. isolates: LESF017 (F1,40 = 26.25; P < 0.01), LESF019 (F1,40 = 28.54; P < 0 .01), LESF023 (F1,40 = 11.69; P < 0.01), LESF033 (F1,40 = 15.12; P < 0.01), LESF039 (F1,40 = 9.13; P = 0.004), LESF041 (F1,40 = 4.948; P = 0.0318), LESF043 (F1,40 = 18.47; P < 0.01) and LESF045 (F1,40 = 10.56; P = 0.002). Particularly, the mutualistic fungus growth inhibition in treatments with CEP was higher for Escovopsis spp. LESF017, LESF019, LESF023, LESF033, LESF039 and LESF043 (Fig. 2). On the other hand, Escovopsis spp. isolates LESF041 and LESF045 provided the highest inhibition of L. gongylophorus in trials using CEA (Fig. 2). There was no significant differences between the CEA and CEP for E. nivea isolates LESF596 (F1,40 = 0.489; P = 0.488) and LESF599 (F1,40 = 0.762; P = 0.388).
Mycelial growth (mean area ± SE, in cm2) of Leucoagaricus gongylophorus after 21 days in the presence of crude extracts of filamentous fungi (Escovopsis spp. and Escovopsioides nivea, see Table 1) grown in the absence (CEA) and in the presence (CEP) of the mutualistic fungus. Different letters indicate significant statistical differences between control and CEA (minuscule letters) and CEP (capital letters). The * means significant differences between CEA and CEP on growth inhibition with P < 0.05 (** are significant with P < 0.01 and *** are highly significant with P < 0.001)
Assessment of the interaction between the hypocrealean fungi and the mutualistic fungus
Escovopsis spp. isolates grew rapidly towards the mutualistic fungus within 24 h. Both E. nivea isolates grew slower towards the mutualistic fungus, taking about 3–7 days to establish contact with the fungus L. gongylophorus. Interesting results were found during the incubation period of the control plates (i.e. hypocrealean fungi inoculated in water agar in the absence of the mutualistic fungus). We observed that all Escovopsis spp. isolates did not grow in water agar plates, while both E. nivea isolates grew normally in this culture medium.
Under light microscopy, we observed a wide variation in hyphae width of the hypocrealean fungi and the hyphae of the mutualistic fungus. The latter varied between 2.8 and 14.9 μm in width, while the hyphae width of Escovopsis spp. varied as follow: 4.3–7.1 μm (for LESF017), 2.5–10.9 μm (for LESF019), 4.9–13.7 μm (for LESF034), and 3.7–8.9 μm (for LESF045). Escovopsioides nivea hyphae varied between 3.6 and 8.4 μm (for LESF596) and between 2.5 and 10.9 μm (for LESF599) in width. Hyphae of Escovopsis spp. and E. nivea presented more transversal septa than the hyphae of the mutualistic fungus.
The samples analysed by SEM showed the growth of Escovopsis hyphae towards the mutualistic fungus, making a bridge between the host and the parasite (Fig. 3a). We followed this hyphal bridge to correctly assure which hyphae belonged to the parasite. The presence of gongylidia in pure culture of the mutualistic fungus was also used to distinguish between the hyphae of L. gongylophorus and the inoculated filamentous fungi (Fig. 3b). For all Escovopsis spp. isolates evaluated, we observed that the parasite maintains physical contact with the mutualistic fungal hyphae in the first 24 h (Fig. 3c–d). In Fig. 3d it is possible to observe the coiling of the Escovopsis sp. hyphae in a gongylidium. This event was detected two times out of five replicates. However, no specialised structure of parasitism (i.e., appressorium or hooks) was observed. During this contact, no degeneration of the host hyphae was observed. After 24 h of experiment, the mutualistic fungus inoculum showed significant growth of Escovopsis, with the presence of sporulation by the parasite (Fig. 3e). At this point, the host hyphae could no longer be evidenced in the preparations. Figure 3c–d illustrate the physical contact of L. gongylophorus hyphae with Escovopsis spp. LESF017 and LESF019 hyphae, respectively, and this event was observed in many preparations. A close contact between the hyphae of L. gongylophorus and Escovopsis sp. LESF017 is noted (Fig. 3c). In the E. nivea assays no specialised structure for parasitism was observed.
Scanning electron micrographs of the dual-culture assays between Escovopsis spp. (E) and Leucoagaricus gongylophorus (L). a Escovopsis hyphae (LESF017) making a bridge towards the mutualistic fungus (bar 1 mm). b Gongylidia and hyphae of the mutualistic fungus after 20 days (bar 50 µm). c Direct contact between hyphae of the mutualistic fungus and hyphae of Escovopsis sp. LESF017 (bar 30 µm). d Physical contact of Escovopsis sp. LESF019 hyphae on a gongylide, physical contact of hyphae of L. gongylophorus with hyphae of Escovopsis sp., evidenced by the arrows (bar 30 µm). e Conidiophores of Escovopsis sp. LESF043, overgrowing the mutualistic fungus mycelium (bar 50 µm). Time elapsed from incubation: a, c and d 24 h; e 48 h
Discussion
The study by Currie et al. (1999) demonstrated that Atta colombica gardens succumbed 72 h after intentional infection with Escovopsis spores. This work was pioneering, as it showed the pathogenicity of Escovopsis in leaf-cutter ant colonies. From this study, several research perspectives were opened to understand the biology of this parasite and its use as potential biological control agent for leaf-cutter ants (Reynolds and Currie 2004; Gerardo et al. 2004, 2006; Silva et al. 2006; Folgarait et al. 2011a; Elizondo-Wallace et al. 2014; Marfetán et al. 2015; Man et al. 2016). In addition, Escovopsioides fungi have similar characteristics to those of Escovopsis and are also found in the fungus gardens of leaf-cutter ants and other genera of attine ants (Augustin et al. 2013; Reis et al. 2015). Unlike Escovopsis, little is known about the biology of these fungi. In this context, the present study focused on the effects of these hypocrealean fungi on the ant-cultivated fungus. The potential antifungal extracts of the culture media from various isolates of Escovopsis spp. and E. nivea were analysed in order to understand whether antagonism of these fungi involves natural products, as suggested by the genome filled with genes encoding for mycotoxins and fungal cell wall degrading-enzymes (Man et al. 2016).
Our results obtained in the dual-culture assays showed that both Escovopsis spp. and E. nivea significantly inhibited the mycelial growth of L. gongylophorus. Silva et al. (2006) also conducted dual-culture assays with the mutualistic fungus from the same ant species used in the present study. The authors assessed the effects of three isolates of E. weberi and observed that all inhibited the development of L. gongylophorus. Differences were found in virulence of Escovopsis isolates, as reflected by the different percentage of inhibition between isolates ranging from 56 to 78%, as well as the morphological changes (staining of the medium and degeneration of the mutualistic fungal mycelium) (Silva et al. 2006). In tests carried out by Folgarait et al. (2011a), significant differences in inhibition of the mutualistic fungus depending on the Escovopsis strain evaluated were also detected. The authors attribute the observed results to the fact that strains were isolated from different ant species. These results corroborate the findings by Currie (2001) and Elizondo-Wallace et al. (2014), which demonstrated that there are strains of Escovopsis with different virulence.
E. nivea isolates also significantly inhibited the ant cultivar in the dual-culture trials and, as Escovopsis spp., we observed differences in the inhibition percentages (56% for LESF596 and 45% for LESF599). Previous studies reported the presence of Escovopsioides in the gardens of leaf-cutter ants, but the fungus was identified as Moniliella suaveolens or Moniliella-like (Rodrigues et al. 2005, 2008). The authors demonstrated that in colonies of the leaf-cutter ants reared in the laboratory, the fungus garden may be overgrown by this fungus after treatment with insecticides (Rodrigues et al. 2005). This phenomenon is similar to what occurs with the parasite Escovopsis, indicating a probable role of this fungus as antagonist of the mutualistic fungus of the ants. Reis et al. (2015) also verified the occurrence of Escovopsioides in 66.6% of 12 colonies of Atta cephalotes sampled in two different areas in the state of Bahia, Brazil. Escovopsioides had the highest prevalence (>32%) within the alien fungi isolated in both areas. Our results showed that the negative effect of E. nivea on L. gongylophorus and the high incidence of this fungus in stressed colonies (Rodrigues et al. 2005, 2008; Reis et al. 2015) suggest a pathogenic and/or opportunistic role played by Escovopsioides in the gardens of leaf-cutter ants.
Our results obtained in the antifungal evaluation of crude extracts towards the mutualistic fungus showed that Escovopsis spp. and E. nivea inhibited the growth of L. gongylophorus. These results indicate the production of compounds by both fungi providing evidences for a chemical action of Escovopsis as proposed by Currie et al. (2003), Reynolds and Currie (2004) and Folgarait et al. (2011b), and hitherto demonstrate the chemical action of E. nivea. However, the extracts alone were not able to kill the mutualistic fungus, showing the need for a combined action of chemical and physical mechanisms for the establishment of mycoparasitism. Six Escovopsis spp. isolates appeared to have been stimulated for the production of antifungal compounds when they were cultured in the presence of the mutualistic fungus (crude extracts in the presence), as they showed differences in the inhibition of the mutualistic fungus when compared to the extracts obtained in the absence of the fungus L. gongylophorus. The increased inhibition may be due to one of three factors: (1) increased production of compounds by Escovopsis in the presence of the cultivar, (2) increased growth of Escovopsis in the presence of cultivar so that more compounds are produced per unit of volume, or (3) inhibition by compounds produced by the cultivar itself. However, additional studies are necessary to better understand the molecules involved in the chemical process of this interaction.
In the antagonism tests on water agar, in which the mutualistic fungus was used as a sole source of nutrients for the hypocrealean fungi, Escovopsis spp. grew rapidly towards its host (after 24 h), as also noted by Reynolds and Currie (2004). The fact that all Escovopsis spp. isolates have not grown on the control plate, but have grown into the mutualistic fungus is explained by the Escovopsis being attracted by chemical signals secreted by the mutualistic fungus, supporting the hypothesis of specificity of this interaction (Gerardo et al. 2004, 2006; Folgarait et al. 2011b). This was particularly observed in the SEM preparations, in which Escovopsis did not exibit radial growth, but grew direct towards the mutualistic fungus, forming a bridge between the fungi. Within 48 h, Escovopsis spp. surpassed the growth of the mutualistic fungus, both in dual-culture and in the antagonism assays used for SEM. On the other hand, E. nivea grew in water agar in the absence of the host, and when in the presence of the L. gongylophorus the hyphae contacted the colony of the host only after three to seven days. Therefore, it is likely that E. nivea is not stimulated by metabolites produced by the mutualistic fungus, which stimulate the growth of Escovopsis spp. (Gerardo et al. 2006), or the evolutionary history shared by E. nivea and L. gongylophorus was different than that shared with Escovopsis. Further experiments are needed to assess whether there is any specificity in the interaction between L. gongylophorus and E. nivea.
In the dual-culture trials, we also observed the darkening of the L. gongylophorus colonies, from withish cream to dark brown. Savoie et al. (1998) reported the same type of darkening in colonies of Lentinula edodes (Order Agaricales) in the interaction zone with Trichoderma in dual cultures due to the production of laccases by L. edodes, when it rejects the Trichoderma attack (antagonism response). Enzymes such as laccases and peroxidases are secreted by white-rot fungi during lignin degradation, but are also used by these fungi in the presence of other antagonist fungi in detoxification of antifungal compounds as a defensive mechanism (Tsujiyama and Minami 2005; Folgarait et al. 2011b). Evaluating the interaction of other Escovopsis spp. strains and other fungi, not associated with the gardens of ants with the mutualistic fungus, may help understanding potential pathogen resistance of L. gongylophorus.
According to the mode of action of fungal mycoparasites, they can be classified as: biotrophic or necrotrophic. The former gets their nutrients from living host cells through specialised structures or simply their hyphae can stay in close contact with the host mycelium. Generally this relationship does not cause major damage to the host, thus this interaction is thought to be a balanced parasitism (Barnett 1964). On the other hand, necrotrophic mycoparasites get their nutrients from the dead host, usually killing it first and then invading host cells to obtain nutrients (Barnett 1964; Jeffries 1995). Following this classification, E. weberi was first considered to be a necrotrophic mycoparasite by Reynolds and Currie (2004). These authors observed the degeneration (death) of the host hyphae without either the occurrence of physical contact between fungi or the formation of any specialised structure to parasitism by E. weberi. Later, a study by Marfetán et al. (2015) recorded the formation of specialised structures in some E. weberi strains involved in the parasitic process and the latter death of the host, leading these authors to reclassify E. weberi as a destructive biotrophic mycoparasite. The fact that Escovopsis is either necrotrophic or biotrophic is related to the terminology used by the two previous studies. Reynolds and Currie (2004) based their conclusions in the types of mycoparasitism described by Jeffries (1995), who considers the host-parasite interface as an important marker to classify mycoparasites. On the other hand, Marfetán et al. (2015) based their conclusions following Boosalis’s (1964) concept of mycoparasitism, who divided biotrophic mycoparasistes into two categories: destructive and balanced. Despite differences in terminology, our results from SEM analyses, dual-culture and crude extract assays support that Escovopsis acts by contact and also secreting inhibitory compounds.
Similarly to Reynolds and Currie (2004) and Márfetan et al. (2015), we also observed the degeneration of the mycelium of the ant fungus infected with Escovopsis spp. Coincidentally, the coiling of Escovopsis hyphae on a gongylidium observed in our experiments occured only when the Escovopsis isolate was isolated from the same ant species than the mutualistic fungus used in the tests. However, the levels of virulence of different Escovopsis isolates do not seem to be related to the specific nature of this relationship, as shown in our experiments where strains with enhanced virulence have been isolated from different ant species. Both chemical and physical mechanisms seem to act together in the parasitism by Escovopsis, as death of the host was observed only in dual-culture assays.
The study by Currie et al. (2003) showed that Escovopsis shares an ancient evolutionary history with the ants and their mutualistic fungi, probably dating from 50 million years. During the course of this interaction, at the parasite’s point of view it is not beneficial to kill its host. In fact, in nature, there are few records of dead colonies due to the action of Escovopsis (Currie et al. 1999). If the parasite apparently does not cause severe damage to colonies in natural conditions, why did Escovopsis evolve into a dead-end parasitism? Perhaps Escovopsis remained aggressive during evolution because the ants exhibit different prophylaxis strategies for protecting the mutualistic fungus. For example, the infected parts of the fungus garden by Escovopsis are quickly eliminated by ant workers (Currie and Stuart 2001), which makes it necessary for Escovopsis sp. to rapidly grow and sporulate to spread in the colony (Currie et al. 1999). The ants also harbor on their cuticles bacteria (Pseudonocardia and other actinomycetes) that secrete antimicrobial compounds that inhibit the parasite (Currie et al. 1999; Sen et al. 2009). In this way, due to barriers imposed by the ants, it is likely that this destructive lifestyle observed in Escovopsis was maintained during evolution.
Concerning the antagonism bioassays with the strains of E. nivea only physical contact between the hyphae of both fungi was observed. Due to the difficulty of distinguishing the hyphae of both fungi under SEM, we cannot afirm that E. nivea does not form specialised structures for parasitism. Considering the results for the two isolates we noted that E. nivea is an antagonist of the fungus L. gongylophorus, as shown in both dual-culture and crude extract assays. However, the observed interactions suggest that E. nivea is not as aggressive when compared to Escovopsis spp. Further analysis using live ant colonies are essential to determine whether E. nivea causes negative impacts to the colonies of these insects as those observed for Escovopsis. Collectively, the data from the present study shows that Escovopsis and most likely E. nivea use a combination of chemical and physical mechanisms to interact with the fungus cultivated by the leaf-cutter ants.
References
Augustin JO, Groenewald JZ, Nascimento RJ, Mizubuti ESG, Barreto RW, Elliot SL, Evans HC (2013) Yet more ‘‘weeds’’ in the garden: fungal novelties from nests of leaf-cutting ants. PLoS One 8:e82265. doi:10.1371/journal.pone.0082265
Barnett HL (1964) Mycoparasitism. Mycologia 56:1–19
Birnbaum SL, Gerardo NL (2016) Patterns of specificity of the pathogen Escovopsis across the fungus-growing ant symbiosis. Am Nat 188:52–65. doi:10.1086/686911
Carreiro SC, Pagnocca FC, Bueno OC, Bacci M Jr, Hebling MJA, Silva OA (1997) Yeast associated with nests of the leaf-cutting ant Atta sexdens rubropilosa Forel, 1908. Anton Leeuw Int J G 71:243–248. doi:10.1023/A:1000182108648
Currie CR (2001) A community of ants, fungi, and bacteria: a multilateral approach to studying symbiosis. Annu Rev Microbiol 55:357–380. doi:10.1146/annurev.micro.55.1.357
Currie CR, Stuart AE (2001) Weeding and grooming of pathogens in agriculture by ants. Proc R Soc Lond B 268:1033–1039. doi:10.1098/rspb.2001.1605
Currie CR, Mueller UG, Malloch D (1999) The agricultural pathology of ant fungus gardens. Proc Natl Acad Sci USA 96:7998–8002. doi:10.1073/pnas.96.14.7998
Currie CR, Wong B, Stuart AE, Schultz TR, Rehner SA, Muller UG, Sung GH, Spatafora JW, Straus NA (2003) Ancient tripartite coevolution in the attine ant-microbe symbiosis. Science 299:386–388. doi:10.1126/science.1078155
Elizondo-Wallace DE, Vargas-Ansesio JG, Pinto-Tomás AA (2014) Correlation between virulence and genetic structure of Escovopsis strains from leaf-cutting ant colonies in Costa Rica. Microbiology 160:1727–1736. doi:10.1099/mic.0.073593-0
Folgarait P, Gorosito N, Poulsen M, Currie CR (2011a) Preliminary in vitro insights into the use of natural fungal patoghens of leaf-cutting as biocontrol agents. Curr Microbiol 63:250–258. doi:10.1007/s00284-011-9944-y
Folgarait PJ, Marfetán JA, Cafaro MJ (2011b) Growth and conidiation response of Escovopsis weberi (Ascomycota:Hypocreales) against the fungal cultivar of Acromyrmex lundii (Hymenoptera:Formicidae). Environ Entomol 40:342–349. doi:10.1603/EN10111
Gerardo NM, Mueller UG, Price SL, Currie CR (2004) Exploiting a mutualism: parasite specialization on cultivars within the fungus-growing ant symbiosis. Proc R Soc Lond B 1550:1791–1798. doi:10.1098/rspb.2004.2792
Gerardo NM, Jacobs SR, Currie CR, Mueller UG (2006) Ancient host-pathogen associations maintained by specificity of chemotaxis and antibiosis. PLoS Biol 4:e235. doi:10.1371/journal.pbio.0040235
Jeffries P (1995) Biology and ecology of mycoparasitism. Can J Bot 73:1284–1290
Man TJB, Stajich JE, Kubicek CP, Teiling C, Chenthamara K, Atanasova L, Druzhinina IS, Levenkova N, Birnbaum SSL, Barribeau SM, Bozick BA, Suen G, Currie CR, Gerardo NM (2016) Small genome of the fungus Escovopsis weberi, a specialized disease agent of ant agriculture. Proc Natl Acad Sci USA 113:3567–3572. doi:10.1073/pnas.1518501113
Marfetán JA, Romero AI, Folgarait PJ (2015) Pathogenic interaction between Escovopsis weberi and Leucoagaricus sp.: mechanisms involved and virulence levels. Fungal Ecol 17:52–61. doi:10.1016/j.funeco.2015.04.002
Meirelles LA, Solomon SE, Jr Bacci, Wright AM, Mueller UG, Rodrigues A (2015) Shared Escovopsis parasites between leaf-cutting and non-leaf-cutting ants in the higher attine fungus-growing ant symbiosis. R Soc Open Sci 2:150257. doi:10.1098/rsos.150257
Möller A (1893) Die Pilzgärten einiger südamerikanischer Ameisen. Bot Mitt Tropen 6:1–127
Pagnocca FC, Silva OA, Hebling-Beraldo MJ, Bueno OC, Fernandes JB, Vieira PC (1990) Toxicity of sesame extracts to the symbiotic fungus of leaf-cutting ants. Bull Entomol Res 80:349–352
Pagnocca FC, Masiulionis VE, Rodrigues A (2012) Specialized fungal parasites and opportunistic fungi in gardens of attine ants. Psyche 12:1–9. doi:10.1155/2012/905109
R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reis BMS, Silva A, Alvarez MR, Oliveira TB, Rodrigues A (2015) Fungal communities in gardens of the leafcutter ant Atta cephalotes in forest and cabruca agrosystems of southern Bahia State (Brazil). Fungal Biol 119:1170–1178. doi:10.1016/j.funbio.2015.09.001
Reynolds HT, Currie CR (2004) Pathogenicity of Escovopsis weberi: the parasite of the attine ant-microbe symbiosis directly consumes the ant-cultivated fungus. Mycologia 96:955–959
Rodrigues A, Pagnocca FC, Bacci M Jr, Hebling MJA, Bueno OC, Pfenning LH (2005) Variability of non-mutualist fungi associated with Atta sexdens rubropilosa nests. Folia Microbiol 50:421–425
Rodrigues A, Bacci M Jr, Mueller UG, Ortiz A, Pagnocca FC (2008) Microfungal “weeds” in the leafcutter ant symbiosis. Microb Ecol 56:604–614. doi:10.1007/s00248-008-9380-0
Rodrigues A, Cable RN, Mueller UG, Bacci M Jr, Pagnocca FC (2009) Antagonistic interactions between garden yeast and microfungal garden pathogens of leaf-cutting ants. Anton Leeuw Int J G 96:331–342. doi:10.1007/s10482-009-9350-7
Santos AV, Dillon RJ, Dillon VM, Reynolds SE, Samuels RJ (2004) Occurrence of the antibiotic producing bacterium Burkholderia sp. in colonies of the leaf-cutting ant Atta sexdens rubropilosa. FEMS Microbiol Lett 239:319–323. doi:10.1016/j.femsle.2004.09.005
Savoie JM, Mata G, Billette C (1998) Extracellular laccase production during hyphal interactions between Trichoderma sp. and shiitake, Lentinula edodes. Appl Microbiol Biotechnol 49:589–593. doi:10.1007/s002530051218
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to imageJ: 25 years of image analysis. Nat Methods 9:671–675. doi:10.1038/nmeth.2089
Schultz TR, Brady SG (2008) Major evolutionary transitions in ant agriculture. Proc Natl Acad Sci USA 105:5435–5440. doi:10.1073/pnas.0711024105
Sen R, Ishak HD, Estrada D, Dowd SE, Hong E, Mueller UG (2009) Generalized antifungal activity and 454-screening of Pseudonocardia and Amycolatopsis bacteria in nests of fungus-growing ants. Proc Natl Acad Sci USA 106:17805–17810. doi:10.1073/pnas.0904827106
Silva A, Rodrigues A, Bacci M Jr, Pagnocca FC, Bueno OC (2006) Susceptibility of the ant-cultivated fungus Leucoagaricus gongylophorus (Agaricales: Basidiomycota) towards microfungi. Mycopathologia 162:115–119. doi:10.1007/s11046-006-0037-6
Suen G, Scott JJ, Aylward FO, Adams SM, Tringe SG, Pinto-Tomás AA, Foster CE, Pauly M, Weimer PJ, Barry KW, Goodwin LA, Bouffard P, Li L, Osterberger J, Harkins TT, Slater SC, Donohue TJ, Currie CR (2010) An insect herbivore microbiome with high plant biomass-degrading capacity. PLoS Genet 6:e1001129. doi:10.1371/journal.pgen.1001129
Tsujiyama S, Minami M (2005) Production of phenol-oxidizing enzymes in the interaction between white-rot fungi. Mycoscience 46:268–271. doi:10.1007/s10267-005-0243-y
Weber NA (1972) Gardening ants, the Attines. American Philosophical Society, Philadelphia
Acknowledgements
The authors are grateful to “FAPESP - Fundação de Amparo a Pesquisa do Estado de São Paulo” for financial support (Grant 2011/16765-0) and “CAPES - Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” for providing a scholarship to SSVH and the PNPD Grant 1455/2008 to TRA. We also thank Dr. Fernando C. Pagnocca (CEIS/UNESP, Rio Claro) for providing strain FF2006 of the mutualistic fungus used in this study, Dr. Christian Jost (University of Toulouse, France) for helping with statistical analysis, and Quimi Vidaurre Montoya (UNESP, Rio Claro) and Antônio Teruyoshi Yabuki (UNESP, Rio Claro) for technical assistance. We are in debt to three anonymous reviewers that provided constructive comments on this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Varanda-Haifig, S.S., Albarici, T.R., Nunes, P.H. et al. Nature of the interactions between hypocrealean fungi and the mutualistic fungus of leaf-cutter ants. Antonie van Leeuwenhoek 110, 593–605 (2017). https://doi.org/10.1007/s10482-016-0826-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-016-0826-y