Abstract
Recombinant acetate kinase (AcK) was obtained from the aerobic haloalkalitolerant methanotroph Methylomicrobium alcaliphilum 20Z by heterologous expression in Escherichia coli and purification by affinity chromatography. The substrate specificity, the kinetics and oligomeric state of the His6-tagged AcK were determined. The M. alcaliphilum AcK (2 × 45 kDa) catalyzed the reversible phosphorylation of acetate into acetyl phosphate and exhibited a dependence on Mg2+ or Mn2+ ions and strong specificity to ATP/ADP. The enzyme showed the maximal activity and high stability at 70 °C. AcK was 20-fold more active in the reaction of acetate synthesis compared to acetate phosphorylation and had a higher affinity to acetyl phosphate (K m 0.11 mM) than to acetate (K m 5.6 mM). The k cat /K m ratios indicated that the enzyme had a remarkably high catalytic efficiency for acetate and ATP formation (k cat/K m = 1.7 × 106) compared to acetate phosphorylation (k cat/K m = 2.5 × 103). The ack gene of M. alcaliphilum 20Z was shown to be co-transcribed with the xfp gene encoding putative phosphoketolase. The Blast analysis revealed the ack and xfp genes in most genomes of the sequenced aerobic methanotrophs, as well as methylotrophic bacteria not growing on methane. The distribution and metabolic role of the postulated phosphoketolase shunted glycolytic pathway in aerobic C1-utilizing bacteria is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methanotrophs are a highly specialized group of aerobic bacteria utilizing methane as a carbon and energy source (Hanson and Hanson 1996; Murrell and Jetten 2009). Methanotrophs are ubiquitous in nature, thus playing an essential role in the global carbon and nitrogen cycles and being involved in the biodegradation of hazardous organic materials (Lontoh and Semrau 1998; Lontoh et al. 2000; Jiang et al. 2010; Semrau 2011). Methanotrophs are promising agents for future technologies aimed at obtaining various useful compounds such as single cell protein, biofuel, bioprotectants, etc. (Fei et al. 2014; Kalyuzhnaya 2015). The study of specific biochemical pathways and enzymes in methanotrophs is a prerequisite for efficient implementation of their metabolic potential. The haloalkalitolerant methanotrophs belonging to the Gammaproteobacteria class are very promising for practical applications due to their fast and stable growth in a wide pH and salinity range and the ability to synthesize the cyclic imino acid ectoine for balancing external osmolarity (Khmelenina et al. 1999, 2015). The haloalkalitolerant methanotroph Methylomicrobium alcaliphilum 20Z is a model organism for studying osmoadaptation mechanisms and biosynthetic capacity.
In M. alcaliphilum 20Z and in most obligate methanotrophs, the first stage of methane oxidation into methanol is catalyzed by the methane monooxygenase bound with the well developed intracytoplasmic membranes (ICM). M. alcaliphilum 20Z assimilates methane carbon via the ribulose monophosphate (RuMP) cycle, where the condensation of formaldehyde and ribulose-5-phosphate leads to C6-phosphosugars as the primary intermediates, and triose phosphates are formed in the simultaneously operating Embden-Meyerhoff-Parnas and Entner–Doudoroff (ED) pathways (Trotsenko and Murrell 2008). In this bacterium, the common precursor for ICM and ectoine synthesis is acetyl-CoA thought to be generated through oxidative decarboxylation of pyruvate catalyzed by pyruvate dehydrogenase. The draft analysis of the M. alcaliphilum genome revealed the open reading frames (ORFs) that encode a putative d-xylulose 5-phosphate/d-fructose 6-phosphate phosphoketolase (XFP, EC 4.1.2.9/EC 4.1.2.22) and acetate kinase (AcK, EC 2.7.2.1). The co-location of the xfp- and ack-like genes in the chromosome indicates that the phosphoketolase pathway in M. alcaliphilum 20Z could represent a pathway for phosphosugars breakdown and an additional source of C2 compounds for acetyl-CoA synthesis. The functionality, distribution and metabolic role of this pathway in aerobic methanotrophs need to be clarified.
In this paper, the recombinant AcK from the aerobic haloalkalitolerant methanotroph M. alcaliphilum 20Z has been purified and characterized for the first time. The properties of the enzyme demonstrate that it is well adapted to its role in acetate synthesis from acetylphosphate being formed from phosphosugars cleaved by phosphoketolase. Also, the distribution of AcK and phosphoketolase in methanotrophs and other C1-utilizing bacteria was studied by genomic analysis.
Materials and methods
Bacteria and growth conditions
M. alcaliphilum 20Z (VKM B-2133T = NCIMB 14124T) was used as a source of genomic DNA and RNA. The culture was grown at 30 °C in methane:air atmosphere (1:1) on a mineral 2P medium supplemented with 0.1 M NaHCO3 and 0.3 M NaCl (Khmelenina et al. 1999). The Escherichia coli Rosetta (DE3) obtained from Stratagene (La Jolla, Calif.) was routinely grown at 37 °C in Luria–Bertani (LB) medium (Sambrook and Russell 2001 ). If required, 100 μg ml−1 ampicilin and 25 μg ml−1chloramphenicol were added to the medium.
Cloning, expression of the ack gene, and purification of the recombinant AcK
The chromosomal DNA from M. alcaliphilum 20Z was prepared as described previously (Kalyuzhnaya et al. 1999). The ack gene (CCE24519) was amplified by PCR using the primers designed from the sequence available in GenBank (accession number NC_016112): forward (5′-ATCATATGACAATACCAAACGGCAACATTCTCG) and reverse (5′-ACCTCGAGTGCGTGATCTTTATCAAGCACAGCC), containing the recognition sites for the NdeI and XhoI restriction endonucleases, respectively. The pET22b:ack vector was transformed into E. coli Rosetta (DE3) and ack expression was induced by adding 0.5 mM isopropyl-1-thio-β-d -galactopyranoside (IPTG, Sigma-Aldrich) in the logarithmic phase of culture growth (OD600 of 0.6–0.8). After 18-h growth at 18 °C, the cells were harvested by centrifugation for 30 min at 5000 g and 8 °C and stored at −20 °C. AcK-His6-tag was purified by affinity chromatography on a Ni2+-nitrilotriacetic acid (Ni–NTA) column as described earlier (Reshetnikov et al. 2008). The enzyme purity was verified by 12 % SDS-PAGE. The pure enzyme was stored in 40 % glycerol at −20 °C.
Determination of AcK molecular mass
The quaternary form of the enzyme was analyzed by non-denaturating gel electrophoresis using pore-limiting gradient polyacrylamide (4–30 %) (Slater 1969). Ferritin (440 kDa), amylase (200 kDa), alcohol dehydrogenase (150 kDa), and bovine serum albumin (66 kDa) obtained from Sigma-Aldrich Group were used as reference proteins.
DNA manipulations
Plasmid isolation, digestion by restriction enzymes, agarose gel electrophoresis, ligation and transformation of E. coli cells were performed according to Sambrook and Russell (2001). Restriction enzymes, T4 DNA-ligase, Taq- and Pfu DNA-polymerases, restriction endonucleases, dNTP mixture, RNase inhibitor, HMinus M-MuLV reverse transcriptase, Gene Ruler™ DNA Ladder Mix and Page Ruler Prestained Protein Ladder for SDS-PAGE were purchased from Thermo Scientific (Lithuania).
RNA isolation and RT-PCR analysis
Total RNA was isolated from 10 ml of exponential culture (OD600 = 0.8) as described earlier (Reshetnikov et al. 2006). For each RT-PCR reaction, a mixture of 5 μg total RNA and 20 pmol primer RT-ack-xfp (5′-CGGGAAATGAGCCAAGCAACT) was heated for 5 min at 70 °C and immediately placed on ice. 4 μl of reverse transcriptase buffer (5×), 1 mM of each dNTP, and 10 units of RNase inhibitor were added to the mixture. For cDNA synthesis, 10 units of HMinus M-MuLV reverse transcriptase were added; the sample was incubated at 42 °C for 1 h and then heated at 70 °C for 15 min to terminate the reaction. For amplification, 2 μl of this reverse transcriptase reaction mixture containing cDNA was added to 30 μl of the amplification reaction mix containing 3 μl of Taq buffer (10 ×), 1 unit of Taq DNA polymerase, 2 mM of MgCl2, 0.2 mM of each dNTP, 10 pmol of primers xfp-5′ (5′-TTTTCAATTTCCACGGCTATCCCTG) and RT-ack-xfp. In each case, PCR without the RT stage was used as a control for DNA contamination in the RNA preparations. After 1-min incubation at 94 °C, the samples were subjected to 30 amplification cycles (30 s at 94 °C, 20 s at 55 °C, and 1 min at 72 °C) followed by the final incubation at 72 °C for 1 min.
Enzyme assays
The AcK activity was determined at 30° using different assay systems: (i) by measuring the rate of ADP formation from ATP with the coupling enzymes pyruvate kinase (PK; Sigma-Aldrich) and lactate dehydrogenase (LDH; Sigma-Aldrich) or (ii) by monitoring the formation of acetyl phosphate, which was registered as acetyl hydroxamate at 540 nm (Rose et al. 1954). The reaction mixture (i) contained (1 ml): Tris–HCl buffer (pH 8.0), 100 mM; MgCl2, 10 mM; acetate K, 100 mM; ATP, 10 mM; phosphoenolpyruvate, 5 mM; NADH, 0.25 mM; PK, 12 U; LDH 24 U; and M. alcaliphilum AcK, 2.4 ng. The reaction was initiated by adding acetate or ATP. This method was used to determine the apparent K m for acetate (the concentration range 0.781–100 mM).
The reaction mixture (ii) contained (0.5 ml): Tris–HCl buffer (pH 8.0), 150 mM; MgCl2, 5 mM; acetate K, 100 mM; ATP, 10 mM; neutralized hydroxylamine, 705 mM; and the recombinant AcK, 2.4 ng. After 10-min incubation, the reaction was stopped by adding an equal volume of 10 % trichloroacetic acid and then 2.5 % FeCl3 in 2 N HCl. A colored complex was registered at λ = 540 nm. This method was used to determine the apparent K m values for ATP, MgCl2 and MnCl2 and to test various effectors, thermostability of the enzyme, the specificities to nucleotides and divalent cations, and the pH and temperature optima.
The apparent K m value for acetyl phosphate was determined by measuring the rate of ATP formation in the presence of various concentrations of acetyl phosphate using the coupling enzymes hexokinase (HK) and glucose-6-phosphate dehydrogenase (G6PD). The reaction mixture (1 ml) contained: Tris–HCL buffer (pH 8.0), 100 mM; acetyl phosphate, 0.156–5 mM; ADP, 10 mM; MgCl2, 10 mM; glucose, 5 mM; NADP, 0.3 mM; 10 units of each HK and G6PD; and 0.24 ng of the recombinant AcK. The apparent Km for ADP was determined by measuring the decrease in acetyl phosphate from the reaction mixture (ii) containing 0.0078–10 mM ADP. The reaction mixture containing the initial concentration of acetyl phosphate without AcK was used as a control.
The pH dependence of AcK was studied using the following buffers (100 mM): K–phosphate (pH 6.0–8.0), Tris–HCl (pH 7.6–8.9), and Glycine–NaOH (pH 9.0–10.0). Pyruvate, phosphoenolpyruvate, AMP, fructose 1,6-bisphosphate, fructose 6-phosphate, ribose 5-phosphate, erythrose 4-phosphate (5 mM), glycerate, lactate, malate, oxaloacetate, α-ketoglutarate, citrate, serine, pyrophosphate (1 mM), or КH2PO4 (10 mM) were tested as potential effectors. The effects of divalent metals were examined by adding the aquatic stock solutions of CuCl2, MgCl2, MnCl2, CoCl2, BaCl2, ZnCl2 or CaCl2 to a final concentration of 1 mM. The thermal stability of AcK was determined by incubating the aliquots of the enzyme in Eppendorf tubes for 5 min to 3 h at 30, 40, 50, 60 and 70 °C. The tubes were rapidly cooled in an ice bath, and residual AcK activity was determined at 30 °C. The percentage of residual activity was calculated by comparison with the activity of the non-incubated enzyme. The optimal temperature for AcK activity was tested by measuring the reaction at 25–90 °C. Protein concentrations were assayed by the Lowry method using BSA as a standard.
K m and V max values for acetyl phosphate, acetate, Mg2+ and Mn2+ were determined through nonlinear data analysis fit to the Michaelis–Menten Eq. (1) uzing the program SigmaPlot (version 12.5):
K 1/2 values for ADP and ATP were determined through nonlinear data analysis fit to the Hill Eq. (2) uzing the program SigmaPlot (version 12.5):
The values of standard deviation for replicate reactions were calculated using SigmaPlot (version 12.5).
Sequence analysis
The full-length amino acid sequences from the NCBI protein databases (http://www.ncbi.nlm.nih.gov) were obtained by BLAST searches. The alignment of amino acid sequences of different AcKs was generated with multiple alignment mode of ClustalX2 (Thomson et al. 1997). The Gonnet series of protein weight matrix was used, the gap opening was 10 and the gap extension was 0.2. 420 informative positions in the final dataset were analyzed. Phylogenetic analysis was performed by MEGA 6 and the Neighbor-Joining model (Tamura et al. 2007). 1000 bootstrap replicates were used.
Results
Expression of the ack gene and AcK-His6 purification
The ORF annotated as a gene encoding putative AcK was successfully expressed in E. coli Rosetta (DE3). The recombinant AcK–His6 protein was purified from the crude extract of E. coli cells by a single-step metal-chelating affinity chromatography. The purified enzyme catalyzed the reversible reaction of acetate phosphorylation by ATP to acetyl phosphate. SDS-PAGE of AcK-His6 revealed a single band of about 45 kDa (Fig. 1), which was in good agreement with the theoretically calculated subunit size (43.6 kDa). According to the native gradient electrophoresis, the M r of the M. alcaliphilum AcK is 80 kDa, corresponding to its homodimeric form. The homodimeric structure is typical for most AcKs, with the exception of the monomeric enzyme from Clostridium thermoaceticum and the homotetrameric enzyme from Bacillus stearothermophilus (Schaupp and Ljungdahl 1974; Nakijima et al. 1978).
Catalytic properties of the recombinant acetate kinase
The AcK from M. alcaliphilum 20Z was active in a wide pH range (pH 6.0–9.5) and exhibited the maximal activity at pH 8.0-8.5 (Figure 1S). The enzyme activity was fivefold higher at 70 °C compared to that at 30 °C (Fig. 2). 50–70 % activity was retained after a 3-h exposure at 30–70 °C. Rather similar thermal stability was displayed by AcK from mesophilic Lactobacillus sanfranciscensis showing the maximal activity at 50 °C and stability at 60 °C for 30 min (Knorr et al. 2001). The highest thermal stability was shown for AcKs from thermophilic microorganisms (Methanosarcina thermophila, Thermotoga maritima). Since M. alcaliphilum 20Z is a mesophilic methanotroph, the properties of AcK were studied at 30 °C.
Like most other AcKs, the M. alcaliphilum enzyme is strongly dependent on divalent metal ions (Table 1): Mg2+ (100 %), Mn2+ (100 %), or Co2+ (68 %); the activity was not maintained by Ba2+, Ca2+, Zn2+ or Cu2+. The reaction rate was 12-fold higher in the direction of acetate and ATP synthesis than in the direction of acetate phosphorylation (1288 vs. 90 U mg−1, respectively). The dependence of the activity on acetate and acetyl phosphate concentrations obeyed the Michaelis–Menten kinetics, whereas the dependence on ATP and ADP concentrations was sigmoidal and the Hill coefficients n were 1.67 ± 0.18 (ATP) and 2.05 ± 0.26 (ADP) (Figure 2S). At 30 °C and the optimal pH, the following apparent K m values were found: 0.28 ± 0.03 mM (for Mg2+), 0.47 ± 0.06 mM (Mn2+) 0.11 ± 0.01 mM (acetyl phosphate), and 5.6 mM ± 0.7 mM (acetate). The K 1/2 values for ADP and ATP were 1.28 ± 0.09 mM and 2.36 ± 0.23 mM respectively. The sigmoid responses to ATP for AcKs from B. stearothermophilus, Veillonella alcalescens and C. thermoaceticum were earlier shown (Nakijima et al. 1978; Bowmann et al. 1976; 1974). Unlike AcKs from Salmonella typhimurium (Chittori et al. 2012), Methanosarcina thermophila (Aceti and Ferry 1988), Thermotoga maritima (Bock et al. 1999), the enzyme from M. alcaliphilum 20Z was strongly specific for ATP as phosphoryl donor and did not use CTP, GTP, or UTP. The calculated k cat/K m ratios indicated that the enzyme had an about 700-fold preference for acetyl phosphate (k cat/K m = 1.7 × 106) over acetate (k cat/K m = 2.5 × 103). Therefore, AcK from M. alcaliphilum 20Z had a markedly higher catalytic efficiency toward acetate and ATP synthesis than acetate phosphorylation. Although M. alcaliphilum 20Z is a halotolerant bacterium, Na+ ions had no influence on AcK activity. Pyruvate, phosphoenolpyruvate, citrate, fructose 1,6-bisphosphate, fructose 6-phosphate, ribose 5-phosphate, glycerate, lactate, malate, oxaloacetate, serine, AMP, pyrophosphate, or KH2PO4 did not serve as effectors.
Analysis of transcriptional organization of the xfp and ack genes
In the genome of M. alcaliphilum 20Z, the ack gene is preceded by the xfp gene coding for the putative phosphoketolase (XFP) with an intergenic region of 459 bp. XFP is known as a bi-functional enzyme that converts fructose 6-phosphate/xylulose 5-phosphate into erythrose 4-phosphate/glyceraldehyde 3-phosphate and acetyl phosphate. RT-PCR was used to test whether the cluster could be an operon. The RT-PCR product of the correct size and sequence was obtained across the xfp and ack pair of genes (Fig. 3). The direct PCR without the RT step was a negative control for DNA contamination of the RNA preparation. The data demonstrated the cotranscription of the xfp and ack genes.
RT-PCR analysis of the region of the xfp-ack genes of Methylomicrobium alcaliphilum 20Z. (a) Organization of the xfp-ack gene cluster and positions of the oligonucleotide primers used for RT-PCR assays. (b) Gel electrophoresis of the RT-PCR products obtained with primers xfp-5′ and RT-ack-xfp (lane 1); negative controls for PCR: the presence of total RNA, primers and Taq-polymerase, but not reverse transcriptase in the reaction mixture (lane 2); positive controls for PCR: the presence of genomic DNA and same primers (lane 3). M, molecular mass markers GeneRuler™ DNA Ladder Mix (Thermo Scientific). The primer positions relative to the start codon of the xfp gene are given in brackets
Distribution of acetate kinase and phosphoketolase in C1-utilizing bacteria
Blast searches revealed ack-like genes in almost all sequenced genomes of aerobic methanotrops including methanotrophic representatives from the Verrucomicrobia phylum (Table 2). Generally, in the gammaproteobacterial methanotrophs, the ack genes are co-located with the XFP encoding genes. Alternatively, in the alphaproteobacterial methanotrophs, the ack gene is usually co-located with the pat gene coding for the putative phosphate acetyltransferase (EC 2.3.1.8) which reversibly converts acetyl phosphate into acetyl-CoA, but the xfp gene occurs less frequently and is located separately in their chromosomes (Table 2). In addition to methanotrophs, AcK and XFP occurred in most methylobacteria not growing under methane, with the exception of betaproteobacterial representatives of the genera Methylotenera, Methylibium (M. petroleiphilum) and Methylovorus (M. glucosetrophus) lacking either ack or xfp. Among the gammaproteobacterial methylobacteria, only Methylophaga thiooxydans has neither AcK nor XFP. Interestingly, the putative ack and xfp genes were found in almost all cyanobacteria and in 25 out of 46 representatives of the bacteria belonging to the genus Burkholderia (Betaproteobacteria class). These Burkholderia species are potentially autotrophic methylotrophs, since their genomes code for the putative methanol dehydrogenase and ribulose bisphosphate carboxylase (Rubisco). The genomic analysis therefore implies that the variety of aerobic methylotrophs, chemoautotrophs and cyanobacteria possess genetic determinants for the phosphoketolase pathway. Remarkably, the duplication of the phosphoketolase gene was revealed in salt-dependent methanotrophs. Thus, two xfp homologs (CCE24247 and CCE24518) are present in the M. alcaliphilum 20Z chromosome and one more is in the plasmid (CCE25819).
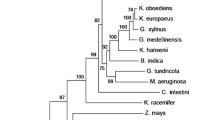
Phylogenetically, methanotrophic AcK sequences could be divided into two large groups showing low similarity (<35 % identity). AcKs from the alphaproteobacterial methylotrophs form a monophyletic group, whereas AcKs from the gammaproteobacterial methylotrophs are found at two distinct locations within the phylogenetic tree of AcK (Figure 3S). AcKs from verrucomicrobial methanotrophs Methylacidiphilum infernorum and M. fumariolicum are positioned separately from other methanotrophic sequences. Though AcK from M. alcaliphilum 20Z has only distant sequence similarities with those from the organotroph Salmonella typhimurium and the archeon Methanosarcina thermophila (43 and 42 % identities of amino acid sequences), it possesses the same residues, which are important for binding of acetate, NTP and metals, except for position 181, where Phe is replaced by Ala (Ingram-Smith et al. 2005; Gorrell et al. 2005).
Discussion
In this work, the recombinant AcK from the aerobic methanotroph M. alcaliphilum 20Z has been purified and characterized for the first time. Similarly to some other microbial AcK, the M. alcaliphilum AcK is a homodimeric enzyme displaying the pH optimum near neutrality and the dependence on Mg2+ or Mn2+. Unlike many AcK from bacteria and archaea, M. alcaliphilum enzyme has a strong specificity to ATP. It is a highly thermostable enzyme retaining 50 % activity after heating at 70 °C for at least 3 h.
The M. alcaliphilum AcK has a higher activity in the reaction of acetate synthesis compared to acetate phosphorylation and higher affinity to acetyl phosphate than to acetate. These kinetic properties imply that the enzyme prefers to catalyze acetate and ATP synthesis from acetyl phosphate and ADP. Along with the co-transcription of the xfp and ack genes, these features indicate that AcK of M. alcaliphilum 20Z is a member of the phosphoketolase pathway described as a bifid shunt, which is a potential alternative carbon route to the enhancement of acetyl-CoA production in lactic acid bacteria, bifidobacteria, and xylose fermenting yeasts (Ratledge and Holdsworth 1985; Sánchez et al. 2010). XFPs are known to be bi-functional enzymes converting hexoses into acetic and lactic acids or directly splitting xylulose-5-phosphate into glyceraldehyde-3-phosphate and acetyl-phosphate bypassing the non-oxidative branch of the pentose phosphate pathway (Sánchez et al. 2010). In any case, the phosphoketolase pathway is known to be more efficient in anaerobic ATP generation compared to the classical glycolysis or the pentose phosphate pathway.
For the aerobic obligate methanotrophs, the dual function of this pathway could be predicted. It might play a role in energy generation and acetyl-CoA formation from the primary metabolites of C1 assimilation, fructose-6-phosphate and/or xylulose-5-phosphate. The respective enzymes, phosphate acetyltransferase and acetyl-CoA synthetase utilizing acetyl phosphate and acetate formed in this pathway has been detected in M. alcaliphilum 20Z (CCE24023 and CCE24530, respectively) and all sequenced gammaproteobacterial methanotrophs. While another route for acetyl-CoA synthesis via oxidative decarboxylation of pyruvate is functional in the RuMP pathway methanotrophs, this mechanism is less efficient as it is accompanied by the breakdown of the C–C bond formed during C1-assimilation. Duplication of the xfp gene in the halotolerant methanotrophs that require additional amount of acetyl-CoA for the biosynthesis of the osmoprotective compound ectoine confirms this supposition (Table 2). Moreover, the presence in M. alcaliphilum 20Z of the third xfp copy of plasmid location is consistent with the highest production of intracellular ectoine by this bacterium, compared to other salt-dependent methanotrophs (Khmelenina et al. 2015). The only exception is the halophilic methanotroph Methylohalobius crimeensis 10Ki possessing a single copy of the xfp gene. Although M. crimeensis 10Ki has the highest salt tolerance among any cultured methanotrophs, its genome encodes, besides the ectoine biosynthesis pathway, additional mechanisms for osmoadaptation such as the synthesis and transport of another compatible solute betaine (Sharp et al. 2015).
The wide distribution of XFP and AcK in various C1-utilizers indicates the importance of the XFP-AcK system in autotrophic bacteria. Thus, the putative AcK and XFP encoding genes could be identified in many genomes of the bacteria assimilating CO2 via the Calvin cycle: in the verrucomicrobial methanotrophs of the Methylacidiphilum genus, in the Burkholderia species harboring both methanol dehydrogenase and Rubisco genes (Table 2), in chemoautotrophic Proteobacteria and variety of the sequenced cyanobacterial strains (data not shown). Thus, it could be predicted that the phosphoketolase route as a mechanism of acetyl-CoA synthesis provides an advantage to microbes whose carbon assimilation proceeds via de novo formation of the C–C bond.
The Blast searches showed that alphaproteobacterial methanotrophs with the serine cycle of C1-assimilation also possess the ack-like genes, but they are frequently clustered with the pat genes (Table 2). Obviously, the facultative representatives of alphaproteobacterial methanotrophs realize the AcK-PAT pathway to activate acetate for use as a carbon and energy source. However, the role of this pathway in obligate serine pathway methanotrophs not growing on any polycarbon compounds still needs to be elucidated. It is not improbable that they are able to utilize acetate as a co-substrate under certain environmental conditions.
The contribution of phosphoketolase pathway to C2-compound production and its role in C1 assimilation and/or acetate production in methanotrophs from the Gammaproteobacteria class have been recently predicted (Kalyuzhnaya 2015; Kalyuzhnaya et al. 2015). In oxygen limiting growth conditions they perform formaldehyde fermentation accompanied by the excretion of several organic acids including acetate into the medium (Kalyuzhnaya et al. 2013). The presence of the phosphoketolase pathway, along with the pyrophosphate-dependent glycolysis and the Entner–Doudoroff pathway, suggests the metabolic flexibility of the gammaproteobacterial methanotrophs that has an important eco-physiological consequence. Such ability seems to be an important part of their bioenergetics explaining surviving in microaerobic ecosystems. The potential role of the phosphoketolase shunted glycolytic pathway in ATP synthesis bypass of the respiratory chain is not improbable but needs further investigations.
References
Aceti DJ, Ferry JG (1988) Purification and characterization of acetate kinase from acetate grown Methanosarcina thermophila. J Bacteriol 263:15444–15448
Bock A-K, Glasemacher J, Schmidt R, Schönheit P (1999) Purification and characterization of two extremely thermostable enzymes, phosphate acetyltransferase and acetate kinase, from the hyperthermophilic eubacterium Thermotoga maritima. J Bacteriol 181:1861–1867
Bowmann CM, Valdez RO, Nishimura JS (1976) Acetate kinase from Veillonella alcalescens. Regulations of enzyme activity by succinate and substrates. J Biol Chem 251:3117–3121
Chittori S, Savithri HS, Murthy MRN (2012) Structural and mechanistic investigations on Salmonella typhimurium acetate kinase (AckA): identification of a putative ligand binding pocket at the dimeric interface. BMC Struct Biol 12:24
Fei Q, Guarnieri MT, Tao L, Laurens LM, Dowe N, Pienkos PT (2014) Bioconversion of natural gas to liquid fuel: opportunities and challenges. Biotechnol Adv 32:596–614
Gorrell A, Lawrence SH, Ferry JG (2005) Structural and kinetic analyses of arginine residues in the active site of the acetate kinase from Methanosarcina thermophila. J Biol Chem 280:10731–10742
Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiol Rev 60:439–471
Ingram-Smith C, Gorrell A, Lawrence SH, Iyer P, Smith K, Ferry JG (2005) Characterization of the acetate binding pocket in the Methanosarcina thermophila acetate kinase. J Bacteriol 187:2386–2394
Jiang H, Chen Y, Pa Jiang, Zhanga C, Smith TJ, Murrell JC, Xinga X-H (2010) Methanotrophs: multifunctional bacteria with promising applications in environmental bioengineering. Biochem Eng J 49:277–288
Kalyuzhnaya MG (2015) Methane biocatalysis: selecting the right microbe. Biotechnology for biofuel production and optimization. doi: 10.1016/B978-0-444-63475-7.00013-3
Kalyuzhnaya M, Khmelenina VN, Kotelnikova S, Holmquist L, Pedersen K, Trotsenko YA (1999) Methylomonas scandinavica sp. nov., a new methanotrophic psychrotrophic bacterium isolated from deep igneous rock ground water of Sweden. Syst Appl Microbiol 22:565–572
Kalyuzhnaya MG, Puri AW, Lidstrom ME (2015) Metabolic engineering in methanotrophic bacteria. Metab Eng 29:142–152
Kalyuzhnaya MG, Yang S, Rozova ON, Smalley NE, Clubb J, Lamb A, Gowda GA, Raftery D, Fu Y, Bringel F, Vuilleumier S, Beck D, Trotsenko YA, Khmelenina VN, Lidstrom ME (2013) Highly efficient methane biocatalysis revealed in methanotrophic bacterium. Nat Commun 4:2785. doi:10.1038/ncomms3785
Khmelenina VN, Kalyuzhnaya MG, Sakharovsky VG, Suzina NE, Trotsenko YA, Gottschalk G (1999) Osmoadaptation in halophilic and alkaliphilic methanotrophs. Arch Microbiol 172:321–329
Khmelenina VN, Rozova ON, But YS, Mustakhimov II, Reshetnikov AS, Beschastnyi AP, Trotsenko YA (2015a) Biosynthesis of secondary metabolites in methanotrophs: biochemical and genetic aspects (Review). Appl Biochem Microbiol (Moscow) 51:150–158
Khmelenina VN, Rozova ON, But CY, Mustakhimov II, Reshetnikov AS, Beschastnyi AP, Trotsenko YA (2015b) Prikl Biokhim Mikrobiol 51:140–150
Knorr R, Ehrmann MA, Vogel RF (2001) Cloning, expression, and characterization of acetate kinase from Lactobacillus sanfranciscensis. Microbiol Res 156:267–277
Lontoh S, Semrau JD (1998) Methane and trichloroethylene oxidation by the particulate methane monooxygenase of Methylosinus trichosporium OB3b. Appl Environ Microbiol 64:1106–1114
Lontoh S, Zahn JA, DiSpirito AA, Semrau JD (2000) Identification of intermediates of in vivo trichloroethylene oxidation by the membrane-associated methane monooxygenase. FEMS Microbiol Lett 186:109–113
Murrell JC, Jetten MSM (2009) The microbial methane cycle. Environ Microbiol Rep 1:279–284
Nakijima H, Suzuki K, Imahori K (1978) Purification and properties of acetate kinase from Bacillus stearothermophilus. J Biochem 84:193–203
Ratledge C, Holdsworth JE (1985) Properties of a pentulose-5-phosphate phosphoketolase from yeast grown on xylose. Appl Microbiol Biotechnol 22:217–221
Reshetnikov AS, Khmelenina VN, Trotsenko YA (2006) Characterization of the ectoine biosynthesis genes of haloalkalitolerant obligate methanotroph “Methylomicrobium alcaliphilum 20Z”. Arch Microbiol 184:286–296
Reshetnikov AS, Rozova ON, Khmelenina VN, Mustakhimov II, Beschastny AP, Murrell JC, Trotsenko YA (2008) Characterization of the pyrophosphate-dependent 6-phosphofructokinase from Methylococcus capsulatus Bath. FEMS Microbiol Lett 288:202–210
Rose IA, Grunberg-Manago M, Korey SF, Ochoa S (1954) Enzymatic phosphorylation of acetate. J Biol Chem 211:737–756
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory, New York
Sánchez B, Zúniga Manuel F, González-Candelas Reyes-Gavilán CG, Margollez A (2010) Bacterial and eukaryotic phosphoketolases: phylogeny, distribution and evolution. J Mol Microbiol Biotechnol 18:37–51
Schaupp A, Ljungdahl LG (1974) Purification and properties of acetate kinase from Clostridium thermoaceticum. Arch Microbiol 100:121–129
Sharp CE, Smirnova AV, Kalyuzhnaya MG, Bringel F, Hirayama H, Jetten MSM, Reshetnikov AS, Klotz MG, Knief C, Kyrpides N, Op den Camp HJM, Sakai Y, Shapiro N, Trotsenko YA, Vuilleumier S, Woyke T, Dunfield PF (2015) Draft genome sequence of the moderately halophilic methanotroph, Methylohalobius crimeensis strain 10Ki. Genome Announc 3(3):e00644
Semrau J (2011) Bioremediation via methanotrophy: overview of recent findings and suggestions for future research. Front Microbiol. doi:10.3389/fmicb.2011.00209
Slater GG (1969) Stable pattern formation and determination of molecular size by pore-limit electrophoresis. Anal Chem 41:1039–1041
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol doi: 10.1093/molbev/msm092
Thomson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res 24:4876–4882
Trotsenko YA, Murrell JC (2008) Metabolic aspects of obligate aerobic methanotrophy. Adv Appl Microbiol 63:183–229
Acknowledgments
This work was supported by the Russian Science Foundation, Project No 14-14-01045.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rozova, O.N., Khmelenina, V.N., Gavletdinova, J.Z. et al. Acetate kinase-an enzyme of the postulated phosphoketolase pathway in Methylomicrobium alcaliphilum 20Z. Antonie van Leeuwenhoek 108, 965–974 (2015). https://doi.org/10.1007/s10482-015-0549-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-015-0549-5