Abstract
New classes of antibacterial compounds are urgently needed to respond to the high frequency of occurrence of resistances to all major classes of known antibiotics. Microbial natural products have been for decades one of the most successful sources of drugs to treat infectious diseases but today, the emerging unmet clinical need poses completely new challenges to the discovery of novel candidates with the desired properties to be developed as antibiotics. While natural products discovery programs have been gradually abandoned by the big pharma, smaller biotechnology companies and research organizations are taking over the lead in the discovery of novel antibacterials. Recent years have seen new approaches and technologies being developed and integrated in a multidisciplinary effort to further exploit microbial resources and their biosynthetic potential as an untapped source of novel molecules. New strategies to isolate novel species thought to be uncultivable, and synthetic biology approaches ranging from genome mining of microbial strains for cryptic biosynthetic pathways to their heterologous expression have been emerging in combination with high throughput sequencing platforms, integrated bioinformatic analysis, and on-site analytical detection and dereplication tools for novel compounds. These different innovative approaches are defining a completely new framework that is setting the bases for the future discovery of novel chemical scaffolds that should foster a renewed interest in the identification of novel classes of natural product antibiotics from the microbial world.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: the lack of new antibiotics and the role of natural products in modern antibiotic discovery
Microbial natural products (NPs) have been one of the most important sources for the discovery of novel antibiotics in the past decades, with an important number of drugs and analogs successfully introduced in the market and still used today in clinical practice. All classes of antibiotics have seen the emergence of resistance compromising their use, with antibiotics of the same class exhibiting rapid cross resistance to that class of drugs. The emergence and dissemination of multi-drug-resistant (MDR) bacteria and the lack of new antibiotics with new modes of action are today one of the major challenges to treat infectious diseases, one of the leading causes of death worldwide (Livermore 2009). The increased prevalence of MDR pathogens and the rapid development of cross resistances with new antibiotics, both in community and hospital-acquired infections, are limiting the management of bacterial infections. One of the major concerns is the emergence of multi-drug resistances in the so-called ESKAPE pathogens (E nterococcus faecium, S taphylococcus aureus, K lebsiella pneumoniae, A cinetobacter baumannii, P seudomonas aeruginosa and E nterobacter spp.), allowing escape from the action of most antibiotics, and the lack of novel antimicrobial compounds in development to combat these infections (Boucher et al. 2009; Livermore 2009; Payne 2007; Silver 2011). Most antibiotic classes in use were discovered before 1970 and since then a large majority of the new approved compounds have been based on chemical modifications of existing old scaffolds (Table 1). Whereas most research efforts have been focused on Gram-positive bacteria and especially S. aureus MRSA, very limited options have been developed against MDR Gram-negative bacteria, which are frequently associated with high mortality rates and few treatment options in hospital-acquired infections when even last resort antibiotics such as colistin and polymixin B are facing resistances (Silver 2011). This problem can be also extended to foodborne diseases caused by Escherichia coli, Salmonella spp. and Clostridium difficile, or pathogens such as Mycobacterium tuberculosis and Neisseria gonorrhea (Wright 2012).
The intensive antibacterial discovery effort that generated the large number of highly potent broad-spectrum antibiotics, has seen a dramatic decline in the large pharma industry in the last two decades, with very few companies still active in the area, resulting in a lack of new classes of antibiotics with novel mechanisms of action reaching the clinic (Silver 2011).
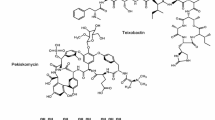
The decline in the number of new chemical scaffolds and the rediscovery problem of old known molecules became a hurdle for NPs discovery programs in most large pharma companies. The limited availability or structural complexity of the molecules frequently not amenable to medicinal chemistry improvement programs were major reasons for discontinuing these activities despite the earlier success in delivering new antibiotics. This decline in microbial NPs discovery programs was paralleled by shrinking antibiotic pipelines and a loss of decades of industrial experience and resources. However, synthetic molecules did not replace microbial NPs as sources of new antibiotics despite the massive efforts used in the search for novel classes of antibiotics from large synthetic chemical collections (Payne et al. 2007). Different reports have highlighted the unique and biologically relevant chemical space represented by NPs when compared to bioactive synthetic molecules. This theoretical analysis in terms of compound lipophilicity, size, rigidity, and aromaticity, has shown that NPs expand into a broader and more diverse chemical space not covered by combinatorial libraries and overlaps with validated drugs (Dobson 2004; Rosén et al. 2009). It is generally accepted that NPs are the result of an extended evolutionary selection process by gain, loss, or modification of constituents of their biosynthetic pathways that generate biologically active molecules with the potency and selectivity to bind to biological targets. In fact with the exception of the synthetic antibiotic linezolid, three of the four new classes of antibacterials that have been introduced in clinical use in the last decade are NPs derived antibiotics: the lipopeptide daptomycin, the diterpene retapamulin and the macrocyclic polyketide fidaxomicin (Fischbach and Walsh 2009; Monneret 2013; Novak 2012; Silver 2011; Venugopal and Johnson 2012) (Fig. 1). Other clinical candidates that were developed more recently are semi-synthetic derivatives or are inspired by well known NPs after revisiting old scaffolds in an attempt to overcome specific resistance mechanisms (Butler and Cooper 2011; Kirst 2012; Genilloud and Vicente 2013) Table 1). The launch of the first semi-synthetic glycopeptide telavancin (Corey et al. 2009) and clinical trials with the glycopeptide dalbavancin, the tetracyclines tigecycline and omadacycline, or the aminoglycoside ACHN-490 among others are examples of the potential of expanding the antimicrobial chemical space by semi-synthesis (Genilloud 2012; Marinelli and Genilloud 2013). Therefore NPs, their semi-synthetic derivatives and NPs inspired compounds represent one of the most important sources of chemical diversity and a tremendous reservoir to mine for bioactive novel structures to fight the challenge of antibiotic resistance (Butler and Buss 2006; Ganesan 2008; Kirst 2013; Koehn and Carter 2005; Newman and Cragg 2012). More recently new genomic data derived from massive whole-genome sequencing projects have shown the untapped metabolic capabilities of microbial sources and are fostering a new interest in the exploitation of these secondary metabolite producers (Walsh and Fishbach 2010; Corre and Challis 2009; Winter et al. 2011). Whereas the advances in the field that have revolutionized NPs research have been numerous and encompass diverse disciplines, this review will focus on some of the recent contributions ranging from more traditional approaches to in silico biosynthetic predictions and novel engineered heterologous expression, that have inspired the isolation of new NPs and shown their potential application in the discovery of novel antibiotics.
Whole cell target based approaches to maximize the probabilities of discovery of new NPs antibiotics
Most classes of NP-derived antibiotics in the clinic were discovered by empirical screening. Integrated genomic and proteomics analyses of the last decades identified orphan and conditional essential genes that were used as targets in new antibiotic screening strategies as part of a new genomics-based approach to drug discovery (Payne et al. 2000). Whereas the success rate of the concerted genomic and high throughput target-based screening was extremely low, the use of these targets were combined then with whole cell assays to ensure the identification of antibiotic compounds that could overcome cellular barriers and interfere with biological cell systems as revealed by the expression of different reporters. The antisense technology is one of the best examples of whole cell target based screening assays designed to permit the use of strains with increased sensitivity for interfering molecules under the control of the differential expression of the modulating antisense RNA (Singh et al. 2007; Donald et al. 2009) (Fig. 2). These assays were applied intensively at Merck to interrogate upfront large NPs libraries and were useful in finding potent but otherwise overlooked activities among NPs. Alternatively a mode of action hypothesis for hits derived from traditional antibacterial empirical screens was proposed with the anti-sense fitness test platform, a screening technology for mechanism-based profiling of antibacterial compounds based on 245 S. aureus antisense RNA strains engineered for reduced expression of target genes essential for S. aureus growth (Donald et al. 2009). This integrated effort resulted in the identification of novel molecules with novel modes of action, of which the most relevant are the novel class of fabF/H inhibitors platensimycin and platencin (Wang et al. 2006, 2007) and the new DNA gyrase inhibitor kibdelomycin (Phillips et al. 2011) (Fig. 3). A long list of novel RNA translation and protein secretion inhibitors such as okilactomycins, lucensimycins, pleosporone, phaeosphenone, coelomycin, coniothyorione, glabramycins and pannomycin were identified from this approach as being produced by a wide diversity of bacterial and fungal strains and showing the potential of the technology to uncover the presence of novel compounds (Goetz et al. 2010; Ondeyka et al. 2007; Parish et al. 2009; Singh et al. 2006, 2011; Zhang et al. 2008, 2009).
Antisense technology. Highly sensitive target-based whole-cell antibacterial discovery strategy by antisense RNA silencing a induction of antisense RNA leads to differential growth sensitivities between target-depleted cells and control cells without antisense induction. b Differential sensitivity assay on Staphylococcus aureus strain expressing anti-sense RNA (adapted from Singh et al. 2007)
Expanding the diversity of cultured microbial sources
The discovery of a novel antibiotic class is a rare event and the success of any NPs screening approach is completely dependent on the chemical diversity of the NPs library used (Fig. 4). The quality of these NPs libraries is determined by the diversity of the microbial sources; the tools used to exploit their biosynthetic potential translated, in most of the cases, in the appropriate production or expression conditions; and the application of effective early dereplication methods to avoid the rediscovery of well known old drugs (Butler 2004; Genilloud et al. 2011). Chemical diversity in NPs libraries has been traditionally based on ensuring the widest diversity of promising strains and the use of appropriate conditions for the production of the compounds (Donadio et al. 2010; Genilloud et al. 2011). Recent reports have shown that the extraordinary richness, as biosynthetic potential, of bacterial and fungal strains supports the need to invest in novel approaches targeted at enriching collections in these novel species, with special emphasis on actinomycetes and endophytic fungi from a large diversity of specific and unique microbial communities (Brakhage and Schroeckh 2011; Nett et al. 2009). These studies also emphasize the important contribution to the production of novel molecules of other bacterial groups such as myxobacteria, pseudomonads or cyanobacteria, and many of these taxa are being actively explored by research groups as potential sources of new antibiotics (Gross and Loper 2009; Kalaizis et al. 2009; Wenzel and Muller 2009). In addition, the continued access to a wide diversity of terrestrial and marine habitats including endophytic plant species and symbiotic assemblages of bacteria and fungi, has shown that the capacity to produce novel compounds by secondary metabolism is broadly distributed across many bacterial and fungal taxa, emphasizing the need to apply key selection criteria to ensure that the strains of major interest and biosynthetic potential are efficiently selected (Bills et al. 2009, 2013; Bode 2009; Crawford and Clardy 2011; Jensen et al. 2007; Singh et al. 2010; Zotchev 2012). Whereas the production of many ubiquitous NPs such as the actinomycins, rifamycins, azalomycins, echinomycins or novobiocins, just to name some frequently occurring compounds, have been observed as produced by a broad diversity of extensively screened taxonomic groups within the actinomycetes, many novel compounds have been shown to be species-specific (Genilloud et al. 2011). Recent diversity analyses focused at understanding the distribution of biosynthetic gene clusters have shown that the production of many compounds is limited to certain species. This is the case of the production of salinosporamide associated to the species Salinispora tropica or the synthesis of platensimycin with members of the species Streptomyces platensis from quite diverse environments (Ziemert et al. 2014; Genilloud et al. 2011). Despite the trends observed in the production of many of these compounds, most NPs have been only reported once by unique strains isolated from very specific habitats. The limited characterization of the microbial metabolic diversity that remains to be exploited and the richness in microbial biosynthetic pathways observed from genetic studies with many of the newly explored taxa provide a measure of the need to expand the search for novel compounds and interrogate poorly explored taxa, including not-yet cultivated bacterial and fungal species.
Culture-based approaches to express underexploited NPs biosynthetic genes
The exploitation of the extraordinary richness in biosynthetic genes of different microbial groups has been addressed from quite diverse perspectives, including culture-based attempts to express silent genes as revealed from genomic studies. One of the first challenges when dealing with wild-type strains with little or no knowledge of their physiological characteristics has been to address how to manipulate the cultivation conditions by modulating culture growth parameters and introducing stress factors that might influence drastically their secondary metabolism. The effect of many of these factors have been well documented in previous works and they involve, among others, modifications of media components, nutrient depletions, incubation conditions affecting O2 and CO2 concentrations, temperature and pH variations, as well as changes in osmolarity, iron homeostasis or redox stress (Hanke 2001; Hesketh et al. 2009; Rigali et al., 2008; Ruiz et al. 2010). The integrated manipulation of the production conditions to further exploit their microbial biosynthetic potential is represented by the OSMAC approach (one strain-many compounds) that supports the capacity of any given strain to produce many active compounds (Bode et al. 2002; Scherlach and Hertweck 2009). The introduction of multiple nutritional conditions with miniaturized parallel fermentation devices has been intensively exploited in our laboratory to expand the possibilities of triggering the production of new secondary metabolites from a larger number of strains of actinomycetes and fungi. The fermentation of a large collection of new isolates using up to 8–12 production media in a 96-well plate format, well integrated with the automated extraction and high throughput screening platforms, was translated into an increase in the number of antibiotic activities identified against a broad panel of human bacterial pathogens (S. aureus, A. baumannii, P. aeruginosa and E. coli) by all the microbial groups tested (Bills et al. 2008; Genilloud et al. 2011). This screening approach combined with the early low-resolution LC–MS dereplication of known compounds against a proprietary database of known antibiotics and the application of the previously described anti-sense Fitness test mode of action profiling of antibacterial activities (Donald et al. 2009), has represented one of the most important shifts in NPs antimicrobial discovery and has delivered many of the most important novel antibacterial scaffolds recently described (Genilloud 2012).
The production of novel secondary metabolites in bacteria has been stimulated frequently with different classes of known chemical inducers (e.g. siderophores, rare earths, metabolism intermediates) (Kawai et al. 2007; van Wezel and McDowall 2011; Yamanaka et al. 2005). In actinomycetes, small diffusible bacterial hormone-like molecules such as the γ-butyrolactones, and other widely distributed butenolides have enhanced antibiotic production in many species (Corre et al. 2008; Nishida et al. 2007). Avenolide, a butenolide-type autoregulator from Streptomyces avermitilis, is one of the most recent examples of this class of signaling molecules and it has been shown to be critical to elicit avermectin production (Kitani et al. 2011). Recent approaches developed for the activation of cryptic pathways have involved the discovery and application of new elicitors of secondary metabolism. These have included, among others, the addition of N-acetyl-glucosamine to production media to modulate the N-acetyl-glucosamine-responsive protein DasR as an alternative to introducing dasR deletion mutants (Rigali et al. 2008); the generation of ribosomal mutations resulting in altered ppGpp biosynthesis and catabolite repression; and favouring the enhancement of biosynthesis (Hosaka et al. 2009), or the overexpression of the pathway regulator LAL leading to the specific expression of previously unknown stambomycin (Laureti et al. 2011). The introduction of an absA1 mutant of the S.coelicolor two component pleiotropic regulator AbsA1/2 was also described to lead in heterologous Streptomyces species to the activation of the production of compounds such as pulvomycin (McKenzie et al. 2010). More recently the modulation of precursors has been shown as another efficient way to elicit the synthesis of previously undetected compounds. Nodwell and his group observed that triclosan-related compounds inhibiting FabI enoyl reductase and the consequent partial inhibition of the biosynthesis of fatty acid synthesis increased the availability of acetyl-CoA to be used as substrate supply for polyketide biosynthesis, not only enhancing secondary metabolism, but revealing previously unreported compounds (Craney et al. 2012).
In the case of fungi, epigenetic modulation by histone acetylation and methylation represent another gene expression regulatory level with deep influence on antibiotic production (Brakhage and Schroeckh 2011). The first demonstration of the effect of histone acetylation on the transcriptional activation of gene clusters was obtained with the production of sterigmatocystin and penicillin in Aspergillus nidulans upon disruption of the histone deacetylase gene (Shwab et al. 2007). Small-molecule epigenetic modifiers that are currently used to inhibit histone deacetylase (HDAC) or DNA methyltransferase (DMAT) can be used to activate silent natural product pathways in different fungal species. The HDAC inhibitor suberoylanilide hydoxamic acid (SAHA) has been shown to potentiate the production of secondary metabolites such as cladochromes and calphostin B in Cladosporium cladosporioides, nygerone A in Aspergillus niger or in Emericella species (De la Cruz et al. 2012; Henrikson et al. 2009; Williams et al. 2008). The methyltransferase inhibition with 5-azacytidine has also been shown to induce the de novo production of oxylipins and lunalides by C. cladosporioides and Diatrype sp., respectively (Williams et al. 2008). Despite the unpredicted effects resulting from the treatments with epigenetic modifiers, this approach is considered more efficient than other gene-dependent approaches for the screening of large numbers of filamentous fungi (Schmitt et al. 2011). Similarly to fungi in their control of biosynthetic pathways, HDAC inhibitors such as sodium butyrate or splitomycin have been described to activate cryptic pathways in S. coelicolor. HDAC orthologues have been identified to be broadly distributed not only among Streptomyces but in members of Pseudonocardia, Saccharopolyspora, and Amycolatopsis, prolific taxa for production of secondary metabolites (Moore et al. 2012), and offer new avenues to induce cryptic or poorly expressed secondary metabolites in these taxa.
Interspecies signalling and antibiotic production in co-cultures
Microbial interactions play an essential role in maintaining cohabitation of bacteria and fungi in a large diversity of environments, as well as in the case of mutualistic, pathogenic and symbiotic relationships (Brakhage and Schroeckh 2011). The regulation of secondary metabolism and interspecies signalling are driving forces leading to the activation of silent cryptic biosynthetic pathways and represent a key factor in determining the success of the approach. Co-cultivation cross-feeding experiments with Streptomyces species clearly showed the enhancement of antimicrobial activity with almost 20 % of strains responding to the metabolites excreted by the other strains (Ueda et al. 2000). The stimulatory effects of these interactions on the production of novel antimicrobial compounds from microbial co-cultivation have been reported between different species, and in some situations a close bacteria–fungal interaction is required for the production of novel compounds as is the case of the production of polyketides by A. nidulans upon interaction with different actinomycetes (Brakhage and Schroeckh 2011; Schroeckh et al. 2009). Exposing seaweed-associated bacteria to other marine epibionts or human pathogens also resulted in the production of different antibiotic compounds (Burgess et al. 1999), similarly to what was observed when confronting Streptomyces species with mycolic acid containing bacteria or Myxobacteria (Onaka et al. 2011; Pérez et al. 2011; Schroeckh et al. 2009). Pestalone is another potent antibiotic against methicillin-resistant S. (MRSA), and an example of an antibiotic produced from a co-culture of the marine fungus Pestalotia sp. in response to a bacterial challenge from an alpha-proteobacterium (Cueto et al. 2001). Other examples include the production of diterpenone libertellenones by marine microbial competition, or the 100-fold enhancement of the production of emericellamides A and B from the marine-derived fungus Emericella sp. in competing co-culture with the marine actinomycete Salinispora arenicola (Oh et al. 2007). Another case of interspecies cross-talk and synergistic metabolite production was observed from a mixed bacterial culture from marine sediments producing a bioactive blue pigment identified as pyocyanin. The compound, normally produced by Pseudomonas species in response to quorum sensing signalling, was only obtained in a co-culture of a strain of P. aeruginosa unable to induce the production of this molecule, with a strain of Enterobacter sp. producing the inducing signal (Angell et al. 2006). Despite the diverse examples reported by different groups, most of these signals are produced in small amounts, frequently below the detection limits, and therefore the molecular and chemical nature behind this interspecies cross-talk remains largely poorly described and difficult to detect (Brakhage and Schroeckh 2011).
Access to uncultivated microbial sources
Despite the efforts to diversify the microbial sources, only a small fraction of microbial species have been investigated, and the majority have never been tested for bioactivity (Berdy 2012). Therefore a large diversity of microbial sources remains largely underexplored, opening new avenues for the isolation of new strains. Standard cultivation-based methods often fail to isolate the dominant members of microbial communities (Rappé et al. 2003) and the lack of cultivability of the majority of bacterial species in laboratory conditions is limiting the characterization of many potential active compounds not previously described. Progress has been made from the perspective of microbial and chemical ecology to understand the natural interactions between species that can lead to culturability. Isolation efforts targeted at unusual bacterial niches have succeeded in the isolation of novel compounds such as the novel lysine-rich cyclic peptides from the entomopathogenic bacteria Photorhabdus luminescens and Xenorhabdus nematophila (Fuchs et al. 2011), or the new compound closthioamide from the strict anaerobe Clostridium spp. (Lincke et al. 2010). Nevertheless culturing species previously considered as non-culturable continues to represent one of the major challenges in the search for potential producers of novel molecules. Many of these species exist as part of complex microbial communities that cannot be cultured in standard laboratory conditions or require the interaction with other species for survival or the production of secondary metabolites (Piel 2004). The use of specific chambers exposed to environment signals, and the presence of other community members that are required for growth in artificial media has also opened new avenues in the exploitation of these resources and their metabolic potential (Kaeberlein et al. 2002; Lewis et al. 2010; Lewis 2013; Stewart 2012). The novel glycosylated macrolactams novolactamycin and novobiomycin, discovered by NovoBiotic Pharmaceuticals, are examples of new compounds identified using a screening method to search for antibiotics among microorganisms that cannot be cultured and developing methods to activate silent pathways observed from genome sequences (Lewis 2013). As limited number of examples of the cultivation of previously uncultured groups exist, the limitations to get access to these new species represent a serious challenge to evaluate the potential of these groups to produce novel compounds.
Genome mining, engineering and heterologous expression of biosynthetic pathways
The increasing number of available sequences of microbial genomes in public databases have confirmed the broad diversity of gene clusters involved in the biosynthesis of NPs. Genome mining approaches have untapped many cases of silent or ‘cryptic’ biosynthetic gene clusters, potentially involved in the production of novel NPs that have never been detected in microbial extracts (Challis 2008). The tremendous progress in understanding the mechanisms that underlie the biosynthesis and regulation of NPs, supported by the development of new genomic and analytical tools, and the emergence of synthetic biology approaches have become critical disciplines required not only to identify new silent biosynthetic pathways, but to predict the synthesis of novel compounds and engineer their production by heterologous expression (Gross 2009; Scherlach and Hertweck 2009; Winter et al. 2011; Zerikly and Challis 2009).
The massive genome sequencing of a large number of actinomycetes and filamentous fungi, well known as excellent producers of secondary metabolites, have completely changed the perception of these strains as unparalleled proficient secondary metabolite producers with genomes harbouring large numbers of biosynthetic gene clusters (Nett et al. 2009; Khaldi et al. 2010). Since the first publication of the S. coelicolor and A. nidulans genome sequences (Bentley et al. 2002; Bok et al. 2006), >1,204 fungal and >2,200 actinobacterial full genome sequences are available in 2014 (http://www.ncbi.nlm.nih.gov/genome/browse/). Comparative genomic analysis of the growing number of sequenced bacterial and fungal genomes has revealed that the gene numbers involved in the production and regulation of secondary metabolite biosynthesis are much higher than those anticipated even in well known producers. The availability of automated genome mining tools, such as the recently developed antiSMASH (Medema et al. 2011), permit the identification of genes of unknown function but proposed for secondary metabolite production. Homology searches of genes encoding known proteins motifs involved in secondary metabolism have revealed that the genomes of actinomycetes can contain up to 30 of diverse secondary biosynthesis gene clusters and in Aspergilli these can reach 30–40 metabolite gene clusters (Brakhage and Schroeckh 2011). This metabolite richness is clearly under expressed in microbial NPs collections and the existence of silent genes has set the genomic basis for a new NP discovery paradigm based on genome mining and exploitation of these resources (Brakhage and Schroeckh 2011; Corre and Challis 2009; Zerikly and Challis 2009). The ‘genomisotopic approach’ proposed by Gross et al. (2007) for genome mining using an isotope-guided fractionation should permit the isolation of novel metabolites related to cryptic gene clusters, as was the case in the discovery of orphamide A from Pseudomonas fluorescens Pf-5.
Culture-independent metagenomic approaches have been applied to exploit the uncultivated majority avoiding the difficulty associated with cultivating environmental microbial strains (Fig. 5). These metagenomic technologies can enable the characterization of complete biosynthetic pathways of microorganisms that remain incapable of being cultured in the laboratory from environmental DNA. Functional analysis of metagenomic libraries selecting for recombinant clones with biological activity might capture complete biosynthetic genes clusters from the environment and therefore the production of novel compounds (Schofield and Sherman 2013; Piel 2011; Wilson and Piel 2013; Banik and Brady 2010). The isolation of metabolites from bioactive clones led to the characterization of several bioactive compounds, including new N-acylated amines, the pigments violacein, indigo, or turbomycin, and the cyclic peptides patellamides. The possibility to use metagenomics to identify groups of related gene clusters allowed the identification of clones containing genes associated with the biosynthesis of teicoplanin and vancomycin glycopeptides (Banik and Brady 2010). Despite the reduced number of compounds identified so far by culture-independent methods, uncultured bacteria represent a rich source of bioactive molecules and genomics-guided functional screening and heterologous expression will be important steps in future NP screening. Host selection and engineering based on comparative genomic approaches should support the development of the most appropriate universal hosts that can express genes from a wide range of sources. The recent development of single-cell and metagenomics-based discovery tools by the group of Piel has represented a breakthrough in exploring secondary metabolism from uncultured bacteria. The identification of the polytheonamides from uncultured cells of the sponge symbionts ‘Entotheonella’, one of the largest post-transcriptionally modified ribosomal peptides reported to date, and later the description of the secondary metabolite richness and distribution of the uncultured candidate phylum represent new opportunities for drug discovery (Freeman et al. 2012; Wilson et al. 2014).
The systematic analysis of microbial genomes and their cryptic biosynthetic gene clusters has revealed that new enzymology is involved in the assembly of novel metabolic products with new selectivities in polyketide synthases (PKS) and nonribosomal peptide synthetases (NRPS), and different module skipping for non-linear peptide synthesis, different starter and extender units, novel terpenes synthases, or novel macrocyclic biosynthesis. Despite our current capacity to identify a given biosynthetic gene cluster in the genome sequence data from the structure of a known compound, predicting the exact structure of a NP from sequence data is often not possible given the lack information about post-assembly modifications and cyclization patterns, and some biosynthetic processes skip domains or do not respond to a co-linearity pattern. Current genome mining methods rely on homology-based queries, and the identification of truly novel chemistry will require new strategies for bioinformatic genome mining.
This challenge has greatly contributed to study the mechanisms involved in NPs synthesis and the origin of their structural diversity, as well as to the establishment of new initiatives to obtain new derivatives via metabolic engineering (Corre and Challis 2009; Scharf and Brakhage 2013). Recent developments in genetic engineering have been focused at developing new ways to increase chemical diversity, activate silent biosynthetic pathways, generate derivatives of known compounds or create tailor-made/artificial new biosynthesis pathways from previously unknown metabolites. In addition it has been shown that overexpression of pathway specific regulators is a valuable tool to activate silent biosynthesis gene clusters leading to novel compounds, where cross-talk with fungal species can activate other unrelated genes (Scharf and Brakhage 2013).
In this context existing microbial collections appear as unique and largely underexploited sources for the production of novel antibiotics or other classes of compounds. Microbial collections clearly standout not only as extremely rich reservoirs of genes and clusters associated with new biosynthetic pathways as revealed by whole genome sequencing (but overlooked by traditional empiric screening programs) in well studies families of bacteria (i.e. Streptomycetaceae, Micromonosporaceae, and Pseudonocardiaceae) and fungi (Aspergillus and Penicillium spp.), but also in other underexplored microbial taxa.
NP microbial collections generated by pharma NPs discovery programs hold over hundreds of thousands of microbial strains distributed among fungi, actinomycetes and other bacteria selected from a great diversity of environments after decades of intensive environmental mining. These collections have been shown to be extremely prolific in delivering novel compounds and important antibiotic discoveries. Today they represent an extraordinary untapped source of metabolic diversity from which novel gene sequences and novel pathways can be identified by genome mining. The exploitation of these unique resources to express these new pathways in laboratory conditions will require the identification of regulatory signals ensuring the activation of those silent biosynthetic systems normally undergoing low efficiency expression rates, inappropriate culture conditions, a largely undesirable metabolic background or simply the lack of the inducing control signals from other members of the original microbial community.
The use of fungal and bacterial heterologous hosts to activate the expression of cryptic secondary metabolite gene clusters is an emerging field. This can help to redesign metabolites, delete or over-express putative negative or positive transcriptional regulators, and potentially solve the limitations of the expression of gene clusters which are not amenable to genetic intervention. Most biosynthetic pathways consist of PKS, NRPS or hybrid PKS-NRPS elements and their modularity is prone to the recombination of protein domains to generate novel analogues. Engineered strains of Aspergillus oryzae and A. nidulans have been frequently used for the heterologous production of different compounds, the genetic engineering of modular PKS and NRPS permitting the exchange domains of multimodular synthases, and therefore generating novel analogues (Scharf and Brakhage 2013). Heterologous expression of silent biosynthetic pathways offers another strategy for de-silencing biosynthetic pathways. The emergence of phage protein based recombineering approaches has significantly aided in manipulating large DNA fragments and has made heterologous expression of natural product pathways a viable option (Thomason et al. 2007). Several heterologous hosts have been developed successfully to express natural products including E. coli, Pseudomonas putida, Myxococcus xanthus, and various Streptomyces strains (Schmidt et al. 2011). Similarly different strains of S. coelicolor, Streptomyces lividans and S. avermitilis, among others, have been developed for the heterologous production of structurally diverse actinomycete NPs, but limitations still exist to efficiently express pathways from certain Streptosporangiaceae and Micromonosporaceae species that require special attention (Gómez-Escribano and Bibb 2014; Komatsu et al. 2013). A transformation-associated recombination (TAR) cloning platform using the homologous recombination of Saccharomyces cerevisiae has been recently designed to capture large genome clusters and ensure heterologous expression of silent clusters. This new tool has efficiently allowed the heterologous expression of Taromycin A from a cryptic pathway in the marine Saccharomonospora sp (Yamanaka et al. 2014).
Synthetic biology represents an attractive and powerful platform to generate unnatural compounds with enhanced biological features and to develop new potential drug candidates (Nguyen et al. 2012). Reprogramming NP assembly lines and manipulating the biosynthetic machinery responsible for the production of unnatural metabolites will deliver unnatural products with improved biological features (Winter and Tang 2012).
This progress needs to be aligned with current mass spectrometry developments in metabolomics that permit the application of the capacity for analytical detection of NPs directly from bacterial cultures growing on a plate. Recent reports by the Dorrestein’s group propose diverse mass spectrometry technologies to overcome the current limitations of analysis (Esquenazi et al. 2009; Kersten et al. 2011; Watrous et al. 2010). These techniques are complementing the new improvements in chromatographic and analytical methods, particularly NMR and MS techniques applied for the identification of novel compounds. Using a similar approach, novel products of cryptic biosynthetic gene clusters including secondary metabolites, new isomers, as well as novel derivatives of known structures, have been detected and some of these compounds are promising valuable sources.
Future trends
The information derived from the rapid access to the genomes of many microbial pathogens has been providing new routes to antibiotic discovery, and made bioassay-based screening efforts more effective. Recent discoveries related to the structural basis of many of these molecular targets and resistance mechanisms are opening the door to new strategies amenable to be applied in coordination with more classical approaches in the field of new NPs antibiotic discovery in order to identify the new classes of antibacterial drugs to fight infections.
The continued increase in the number of available bacterial and fungal genome sequences has clearly opened new avenues to discover novel molecules by genome mining. Whereas genomics and bioinformatic tools are required for the prediction of novel compounds and their physicochemical properties, the automated identification of gene clusters greatly depends on the prediction tools used. The different strategies covering these multiple approaches have been reviewed extensively by other authors and multiple disciplines have emerged as critical for the consolidation of the field.
The main conditions under which a given gene cluster is expressed in a microbial community is still largely unknown, but research advances in NPs and in synthetic biology are providing fundamental insights into microbial communication and chemical ecology. Many of these innovative approaches have already demonstrated the potential of their exploitation in the development of novel compounds combined with new plug-and-play platforms for heterologous expression. These disciplines are becoming more necessary than ever, not only to understand the trends of the NPs production and its influence on other organisms in the same niche, but as well as to stimulate and simulate the ecosystems of the NP producing microorganisms. Future research in this field will continue to develop innovative strategies to stimulate NP biosynthesis and to simulate natural microbial habitats. These efforts will require to be always complemented by the extraordinary advances in analytical chemistry, with the semi-automated chemical de-convolution and the chemical isolation and structure elucidation of potential novel NPs leads. Undoubtedly, these advances are establishing new trends in the discovery and development of future novel NP-based antibiotic candidates. Nevertheless this new knowledge should also be applied to revisit well established cultures collections that have been completely overlooked by these recent approaches and still remain untapped resources that need to be mined for the biosynthesis of novel bioactive molecules under these new scenarios.
In summary, there is still good reason to believe that the integrated strategy of exploring the chemical diversity of microbial NPs in all its dimensions with the development of new synthetic biology platforms permitting the heterologous exploitation of cultivated and un-cultivated resources should result in the discovery of new antibacterial classes urgently needed to cope with bacterial resistance. Within this new paradigm, it may also be expected that NPs, dramatically abandoned by most discovery programs in the last decade, will see a come-back to become part of most screening initiatives as continued unique inspiration sources of new chemical diversity, and new sources for the urgently needed future antibiotic leads.
References
Angell S, Bench BJ, Williams H, Watanabe CM (2006) Pyocyanin isolated from a marine microbial population: synergistic production between two distinct bacterial species and mode of action. Chem Biol 13:1349–1359
Banik JJ, Brady SF (2010) Recent application of metagenomic approaches toward the discovery of antimicrobials and other bioactive small molecules. Curr Opin Microbiol 13:603–609
Bentley SD, Chater KF, Cerdeño-Tárraga AM, Challis GL, Thomson NR, James KD et al (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147
Berdy J (2012) Thoughts and facts about antibiotics: where we are now and where we are heading. J Antibiot (Tokyo) 65:385–395
Bills GF, Platas G, Fillola A, Jimenez MR, Collado J, Vicente F, Martin J, Gonzalez A, Bur-Zimmermann J, Tormo JR, Peláez F (2008) Enhancement of antibiotic and secondary metabolite detection from filamentous fungi by growth on nutritional arrays. J Appl Microbiol 104:1644–1658
Bills G, Overy D, Genilloud O, Peláez F (2009) Contributions of pharmaceutical antibiotic and secondary metabolite discovery to the understanding of microbial defense and antagonism. In: White J, Torres MS (eds) Defensive mutualism in microbial symbiosis. Dekker, New York
Bills GF, Gloer JB, An Z (2013) Coprophilous fungi: antibiotic discovery and functions in an underexplored arena of microbial defensive mutualism. Curr Opin Microbiol 16:549–565
Bode HB (2009) Entomopathogenic bacteria as a source of secondary metabolites. Curr Opin Chem Biol 13:224–230
Bode HB, Bethe B, Höfs R, Zeeck A (2002) Big effects from small changes: possible ways to explore nature’s chemical diversity. ChemBioChem 3:619–627
Bok JW, Noordermeer D, Kale SP, Keller NP (2006) Secondary metabolic gene cluster silencing in Aspergillus nidulans. Mol Microbiol 61:1636–1645
Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J (2009) Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12
Brakhage AA, Schroeckh V (2011) Fungal secondary metabolites. Strategies to activate silent gene clusters. Fungal Genet Biol 48:15–22
Burgess JG, Jordan EM, Bregu M, Mearns-Spragg A, Boyd KG (1999) Microbial antagonism: a neglected avenue of natural products research. J Biotechnol 70:27–32
Bush K (2012) Improving known classes of antibiotics: an optimistic approach for the future. Curr Opin Pharmacol 12:527–534
Butler MS (2004) The role of natural product chemistry in drug discovery. J Nat Prod 67:2141–2153
Butler MS, Buss AD (2006) Natural products—the future scaffolds for novel antibiotics? Biochem Pharmacol 71:919–929
Butler MS, Cooper MA (2011) Antibiotics in the clinical pipeline in 2011. J Antibiot (Tokyo) 64:413–425
Challis GL (2008) Mining microbial genomes for new natural products and biosynthetic pathways. Microbiology 154:1555–1569
Corey GR, Stryjewski ME, Weyenberg W, Yasothan U, Kirkpatrick P (2009) Telavancin. Nat Rev Drug Discov 8:929–993
Corre C, Challis GL (2009) New natural product biosynthetic chemistry discovered by genome mining. Nat Prod Rep 26:977–986
Corre C, Song L, O’Rourke S, Chater KF, Challis GL (2008) 2-Alkyl-4-hydroxymethylfuran-3-carboxylic acids, antibiotic production inducers discovered by Streptomyces coelicolor genome mining. Proc Natl Acad Sci USA 105:17510–17515
Craney A, Ozimok C, Pimentel-Elardo SM, Capretta A, Nodwell JR (2012) Chemical perturbation of secondary metabolism demonstrates important links to primary metabolism. Chem Biol 19:1020–1027
Crawford JM, Clardy J (2011) Bacterial symbionts and natural products. Chem Commun 47:7559–7566
Cueto M, Jensen PR, Kauffman C, Fenical W, Lobkovsky E, Clardy J (2001) Pestalone, a new antibiotic produced by a marine fungus in response to bacterial challenge. J Nat Prod 64:1444–1446
De La Cruz M, Martín J, González-Menéndez V, Pérez-Victoria I, Moreno C, Tormo JR, El Aouad N, Guarro J, Vicente F, Reyes F et al (2012) Chemical and physical modulation of antibiotic activity in Emericella species. Chem Biodivers 9:1095–1113
Dobson D (2004) Chemical space and biology. Nature 432:824–828
Donadio S, Maffioli S, Monciardini P, Sosio M, Jabes D (2010) Antibiotic discovery in the twenty-first century: current trends and future perspectives. J Antibiot (Tokyo) 63:423–430
Donald RG, Skwish S, Forsyth RA, Anderson JW, Zhong T, Burns C, Lee S, Meng X, LoCastro L, Jarantow LW, Martin J, Lee SH, Taylor I, Robbins D, Malone C, Wang L, Zamudio CS, Youngman PJ, Phillips JW (2009) A Staphylococcus aureus fitness test platform for mechanism-based profiling of antibacterial compounds. Chem Biol 16:826–836
Esquenazi E, Yang YL, Watrous J, Gerwick WH, Dorrestein PC (2009) Imaging mass spectrometry of natural products. Nat Prod Rep 26:1521–1534
Fischbach MA, Walsh CT (2009) Antibiotics for emerging pathogens. Science 325:1089–1093
Freeman MF, Gurgui C, Helf MJ, Morinaka BI, Uria AR, Oldham NJ, Sahl H-G, Matsunaga S, Piel J (2012) Metagenome mining reveals polytheonamides as posttranslationally modified ribosomal peptides. Science 338:387–390
Fuchs SW, Proschak A, Jaskolla TW, Karas M, Bode HB (2011) Structure elucidation and biosynthesis of lysine-rich cyclic peptides in Xenorhabdus nematophila. Org Biomol Chem 9:3130–3132
Ganesan A (2008) The impact of natural products upon modern drug discovery. Curr Opin Chem Biol 12:306
Genilloud O (2012) Current challenges in the discovery of novel antibacterials from microbial natural products. Recent Pat Antiinfect Drug Discov 7:189–204
Genilloud O, Vicente F (2013) Tetracycline antibiotics and novel analogs. In: Marinelli F, Genilloud O (eds) Antimicrobials. New and old molecules in the fight against multi-resistant bacteria. Springer, New York, pp 231–245
Genilloud O, González I, Salazar O, Martín J, Tormo JR, Vicente F (2011) Current approaches to exploit actinomycetes as a source of novel natural products. J Ind Microbiol Biotechnol 38:375–389
Goetz MA, Zhang C, Zink DL, Arocho M, Vicente F, Bills GF, Polishook J, Dorso K, Onishi R, Gill C, Hickey E, Lee S, Ball R, Skwish S, Donald RG, Phillips JW, Singh SB (2010) Coelomycin, a highly substituted 2,6-dioxo-pyrazine fungal metabolite antibacterial agent discovered by Staphylococcus aureus fitness test profiling. J Antibiot (Tokyo) 63:512–518
Gomez-Escribano JP, Bibb MJ (2014) Heterologous expression of natural product biosynthetic gene clusters in Streptomyces coelicolor: from genome mining to manipulation of biosynthetic pathways. J Ind Microbiol Biotechnol 41:425–431
Gross H (2009) Genomic mining—a concept for the discovery of new bioactive natural products. Curr Opin Drug Discov Devel 12:207–219
Gross H, Loper JE (2009) Genomics of secondary metabolite production by Pseudomonas spp. Nat Prod Rep 26:1408–1446
Gross H, Stockwell VO, Henkels MD, Nowak-Thompson B, Loper JE, Gerwick WH (2007) The genomisotopic approach: asystematic method to isolate products of orphan biosyntheticgene clusters. Chem Biol 14:53–63
Hantke K (2001) Iron and metal regulation in bacteria. Curr Opin Microbiol 4:172–177
Henrikson JC, Hoover AR, Joyner PM, Cichewicz RH (2009) A chemical epigenetics approach for engineering the in situ biosynthesis of a cryptic natural product from Aspergillus niger. Org Biomol Chem 7:435–438
Hesketh A, Kock H, Mootien S, Bibb M (2009) The role of absC, a novel regulatory gene for secondary metabolism, in zinc-dependent antibiotic production in Streptomyces coelicolor A3(2). Mol Microbiol 74:1427–1444
Hosaka T, Ohnishi-Kameyama M, Muramatsu H, Murakami K, Tsurumi Y, Kodani S, Yoshida M, Fujie A, Ochi K (2009) New strategies for drug discovery: activation of silent or weakly expressed microbial gene clusters. Nat Biotechnol 27:462–464
Jensen PR, Williams PG, Oh D-C, Zeigler L, Fenical W (2007) Species specific secondary metabolite production in marine actinomycetes of the genus Salinispora. Appl Environ Microbiol 73:1146–1152
Kaeberlein T, Lewis K, Epstein SS (2002) Isolating uncultivable microorganisms in pure culture in a simulated natural environment. Science 296:1127–1129
Kalaitzis JA, Lauro FM, Neilan BA (2009) Mining cyanobacterial genomes for genes encoding complex biosynthetic pathways. Nat Prod Rep 26:1447–1465
Kawai K, Wang G, Okamoto S, Ochi K (2007) The rare earth, scandium, causes antibiotic overproduction in Streptomyces spp. FEMS Microbiol Lett 274:311–315
Kersten RD, Yang YL, Xu Y, Cimermancic P, Nam SJ, Fenical W, Fischbach MA, Moore BS, Dorrestein PC (2011) A mass spectrometryguided genome mining approach for natural product peptidogenomics. Nat Chem Biol 7:794–802
Khaldi N, Seifuddin FT, Turner G, Haft D, Nierman WC, Wolfe KH, Fedorova ND (2010) SMURF: genomic mapping of fungal secondary metabolite clusters. Fungal Genet Biol 47:736–741
Kirst HA (2012) Recent derivatives from smaller classes of fermentation-derived antibacterials. Expert Opin Ther Pat 22:15–35
Kirst HA (2013) Developing new antibacterials through natural product research. Expert Opin Drug Discov 8:479–493
Kitani S, Miyamoto KT, Takamatsu S, Herawati E, Iguchi H, Nishitomi K, Uchida M, Nagamitsu T, Omura S, Ikeda H, Nihira T (2011) Avenolide, a Streptomyces hormone controlling antibiotic production in Streptomyces avermitilis. Proc Natl Acad Sci USA 108:16410–16415
Koehn FE, Carter GT (2005) The evolving role of natural products in drug discovery. Nat Rev Drug Discov 4:206–220
Komatsu M, Komatsu K, Koiwai H, Yamada Y, Kozone I, Izumikawa M, Hashimoto J, Takagi M, Omura S, Shin-ya K, Cane DE, Haruo Ikeda H (2013) Engineered Streptomyces avermitilis host for heterologous expression of biosynthetic gene cluster for secondary metabolites. ACS Synth Biol 2:384–396
Laureti L, Song L, Huang S, Corre C, Leblond P, Challis GL, Aigle B (2011) Identification of a bioactive 51-membered macrolide complex by activation of a silent polyketide synthase in Streptomyces ambofaciens. Proc Natl Acad Sci USA 108:6258–6263
Lewis K (2013) Platforms for antibiotic discovery. Nat Rev Drug Discov 12:371–387
Lewis K, Epstein S, D’Onofrio A, Losee L, Ling LL (2010) Uncultured microorganisms as a source of secondary metabolites. J Antibiot (Tokyo) 63:468–476
Lincke T, Behnken S, Ishida K, Roth M, Hertweck C (2010) Closthioamide: an unprecedented polythioamide antibiotic from the strictly anaerobic bacterium Clostridium cellulolyticum. Angew Chem Int Ed Engl 49:2011–2013
Livermore DM (2009) Has the era of untreatable infections arrived? J Antimicrob Chemother 64:i29–i36
Marinelli F, Genilloud O (2013) Antimicrobials. New and old molecules in the fight against multi-resistant bacteria. Springer, Berlin
McKenzie NL, Thaker M, Koteva K, Hughes DW, Wright GD, Nodwell JR (2010) Induction of antimicrobial activities in heterologous streptomycetes using alleles of the Streptomyces coelicolor gene absA1. J Antibiot (Tokyo) 63:177–182
Medema MH, Blin K, Cimermancic P, de Jager V, Zakrzewski P, Fischbach MA, Weber T, Takano E, Breitling R (2011) antiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res 39:W339–W346
Monneret C (2013) Four new drugs on the market: abiraterone, belatacept, vandetanib and fidaxomicin. Ann Pharm 71:95–103
Moore JM, Bradshaw E, Seipke RF, Hutchings MI, McArthur M (2012) Methods in enzymology, natural product biosynthesis by microorganisms and plants, Part C. In: Hopwood D (ed) Use and discovery of chemical elicitors that stimulate biosynthetic gene clusters in streptomyces bacteria, vol 517. Academic Press, Oxford, pp 368–385
Nett M, Ikeda H, Moore BS (2009) Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat Prod Rep 26:1362–1384
Newman DJ, Cragg GM (2012) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75:311–335
Nguyen Q-T, Merlo ME, Medema MH, Jankevics A, Breitling R, Takano E (2012) Metabolomics methods for the synthetic biology of secondary metabolism. FEBS Lett 586(2012):2177–2183
Nishida H, Ohnishi Y, Beppu T, Horinouchi D (2007) Evolution of γ-butyrolactone synthases and receptors in Streptomyces. Environ Microbiol 9:1986–1994
Novak R (2012) Retapamulin: a first-in-class pleuromutilin antibiotic. In: Genilloud O, Vicente F (eds) Drug discovery from natural products. RSC Publishing, London
Oh DC, Kauffman CA, Jensen PR, Fenical W (2007) Induced production of emericellamides A and B from the marine-derived fungus Emericella sp. in competing co-culture. J Nat Prod 70:515–520
Onaka H, Mori Y, Igarashi Y, Furumai T (2011) Mycolic acid-containing bacteria induce natural-product biosynthesis in Streptomyces species. Appl Environ Microbiol 77:400–406
Ondeyka JG, Zink D, Basilio A, Vicente F, Bills G, Diez MT, Motyl M, Dezeny G, Byrne K, Singh SB (2007) Coniothyrione, a chlorocyclopentandienyl benzopyrone as a bacterial protein synthesis inhibitor discovered by antisense technology. J Nat Prod 70:668–670
Parish CA, de la Cruz M, Smith SK, Zink D, Baxter J, Tucker-Samaras S, Collado J, Platas G, Bills G, Díez MT, Vicente F, Peláez F, Wilson K (2009) Antisense-guided isolation and structure elucidation of pannomycin, a substituted cis-decalin from Geomyces pannorum. J Nat Prod 72:59–62
Payne DJ, Wallis NG, Gentry DR, Rosenberg M (2000) The impact of genomics on novel antibacterial targets. Curr Opin Drug Discov Devel 3:177–190
Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL (2007) Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 6:29–40
Pérez J, Muñoz-Dorado J, Braña AF, Shimkets LJ, Sevillano L, Santamaría RI (2011) Myxococcus xanthus induces actinorhodin overproduction and aerial mycelium formation by Streptomyces coelicolor. Microb Biotechnol 4:175–183
Phillips JW, Goetz MA, Smith SK, Zink DL, Polishook J, Onishi R, Salowe S, Wiltsie J, Allocco J, Sigmund J, Dorso K, Lee S, Skwish S, de la Cruz M, Martín J, Vicente F, Genilloud O, Lu J, Painter RE, Young K, Overbye K, Donald RG, Singh SB (2011) Discovery of kibdelomycin, a potent new class of bacterial type II topoisomerase inhibitor by chemical-genetic profiling in Staphylococcus aureus. Chem Biol 18:955–965
Piel J (2004) Metabolites from symbiotic bacteria. Nat Prod Rep 21:519–538
Piel J (2011) Approaches to capturing and designing biologically active small molecules produced by uncultured microbes. Annu Rev Microbiol 65:431–453
Rappé MS, Giovannoni SJ (2003) The uncultured microbial majority. Annu Rev Microbiol 57:369–394
Rigali S, Titgemeyer F, Barends S, Mulder S, Thomae AW, Hopwood DA, van Wezel GP (2008) Feast or famine: the global regulator DasR links nutrient stress to antibiotic production by Streptomyces. EMBO Rep 9:670–675
Rosén J, Gottfries J, Muresan S, Backlund A, Oprea TI (2009) Novel chemical space exploration via natural products. J Med Chem 52:1953–1962
Ruiz B, Chavez A, Forero A, Garcia-Huante Y, Romero A, Sanchez M, Rocha D, Sanchez B, Rodriguez-Sanoja R, Sanchez S, Langley E (2010) Production of microbial secondary metabolites: regulation by the carbon source. Crit Rev Microbiol 36:146–167
Scharf DH, Brakhage AA (2013) Engineering fungal secondary metabolism: a roadmap to novel compounds. J Biotechnol 163:179–183
Scherlach K, Hertweck C (2009) Triggering cryptic natural product biosynthesis in microorganisms. Org Biomol Chem 7:1753–1760
Schmitt EK, Moore CM, Krastel P, Petersen F (2011) Natural products as catalysts for innovation: a pharmaceutical industry perspective. Curr Opin Chem Biol 15:497–504
Schofield MM, Sherman DH (2013) Meta-omic characterization of prokaryotic gene clusters for natural product biosynthesis. Curr Opin Biotechnol 24:1151–1158
Schroeckh V, Scherlach K, Nützmann HW, Shelest E, Schmidt-Heck W, Schuemann J, Martin K, Hertweck C, Brakhage AA (2009) Intimate bacterial–fungal interaction triggers biosynthetic of archetypal polyketides in Aspergillus nidulans. Proc Nat Acad Sci USA 106:14558–14563
Shwab EK, Bok JW, Tribus M, Galehr J, Graessle S, Keller NP (2007) Histone deacetylase activity regulates chemical diversity in Aspergillus. Eukaryot Cell 6:1656–1664
Silver LL (2011) Challenges of antibacterial discovery. Clin Microbiol Rev 24:71–109
Singh SB, Zink DL, Huber J, Genilloud O, Salazar O, Diez MT, Basilio A, Vicente F, Byrne KM (2006) Discovery of lucensimycins A and B from Streptomyces lucensis MA7349 using an antisense strategy. Org Lett 8:5449–5452
Singh SB, Phillips JW, Wang J (2007) Highly sensitive target-based whole-cell antibacterial discovery strategy by antisense RNA silencing. Curr Opin Drug Discov Devel 10:160–166
Singh SB, Genilloud O, Pelaez F (2010) NP structural diversity II—secondary metabolite sources, evolution and selected molecular structures: terrestrial micro-organisms—bacteria. In: Moore B, Crews P (eds) Comprehensive natural products chemistry II. Elsevier, Oxford
Singh SB, Young K, Miesel L (2011) Screening strategies for discovery of antibacterial natural products. Expert Rev Anti Infect Ther 9:589–613
Stewart EJ (2012) Growing unculturable bacteria. J Bacteriol 194:4151–4160
Thomason L, Court DL, Bubunenko M, Costantino N, Wilson H, Datta S, Oppenheim A (2007) Recombineering: genetic engineering in bacteria using homologous recombination. Curr Protoc Mol Biol Chapter 1: Unit 1.16
Ueda K, Kawai S, Ogawa HO, Kiyama A, Kubota T, Kawanobe H, Beppu T (2000) Wide distribution of interspecific stimulatory events on antibiotic production and sporulation among Streptomyces species. J Antibiot (Tokyo) 53:979–982
van Wezel GP, McDowall KJ (2011) The regulation of the secondary metabolism of Streptomyces: new links and experimental advances. Nat Prod Rep 28:1311–1333
Venugopal AA, Johnson S (2012) Fidaxomicin: a novel macrocyclic antibiotic approved for treatment of Clostridium difficile infection. Clin Infect Dis 54:568–574
Walsh CT, Fischbach MA (2010) Natural products version 2.0: connecting genes to molecules. J Am Chem Soc 132:2469–2493
Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, Painter R, Parthasarathy G et al (2006) Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature 441:358–361
Wang J, Kodali S, Lee SH, Galgoci A, Painter R, Dorso K, Racine F, Motyl M, Hernandez L et al (2007) Discovery of platencin, a dual FabF and FabH inhibitor with in vivo antibiotic properties. Proc Natl Acad Sci USA 104:7612–7616
Watrous J, Hendricks N, Meehan M, Dorrestein PC (2010) Capturing bacterial metabolic exchange using thin film desorption electrospray ionization-imaging mass spectrometry. Anal Chem 82:1598–1600
Wenzel SC, Muller R (2009) The impact of genomics on the exploitation of the myxobacterial secondary metabolome. Nat Prod Rep 26:1385–1407
Williams RB, Henrikson JC, Hoover AR, Lee AE, Cichewicz RH (2008) Epigenetic remodeling of the fungal secondary metabolome. Org Biomol Chem 6:1895–1897
Wilson MC, Piel J (2013) Metagenomic approaches for exploiting uncultivated bacteria as a resource for novel biosynthetic enzymology. Chem Biol 20:636–647
Wilson MC, Mori T, Ruckert C, Uria AR, Helf MJ, Takada K, Gernert C, Steffens UAE, Heycke N, Schmitt S, Rinke C, Helfrich EJN, Brachmann AO, Gurgui C, Wakimoto T, Kracht M, Crusemann M, Hentschel U, Abe I, Matsunaga S, Kalinowski J, Takeyama H, Piel J (2014) An environmental bacterial taxon with a large and distinct metabolic repertoire. Nature 506:58–62
Winter JM, Tang Y (2012) Synthetic biological approaches to natural product biosynthesis. Curr Opin Biotechnol 23:736–743
Winter JM, Behnken S, Hertweck C (2011) Genomics-inspired discovery of natural products. Curr Opin Chem Biol 15:22–31
Wright GD (2012) Antibiotics: a new hope. Chem Biol 19:3–10
Yamanaka K, Oikawa H, HO Ogawa, Hosono K, Shinmachi F, Takano H, Sakuda S, Beppu T, Ueda K (2005) Desferrioxamine E produced by Streptomyces griseus stimulates growth and development of Streptomyces tanashiensis. Microbiology 151:2899–2905
Yamanaka K, Reynolds KA, Kersten RD, Ryan KS, Goonzalez DJ, Nizet V, Dorrestein PC, Moore BS (2014) Direct cloning and refactoring of a silent lipopeptide biosynthetic gene cluster yields the antibiotic taromycin A. Proc Natl Acad Sci USA. doi:10.1073/pnas.1319584111
Zerikly M, Challis GL (2009) Strategies for the discovery of new natural products by genome mining. ChemBioChem 10:625–633
Zhang C et al (2008) Isolation, structure, and antibacterial activity of phaeosphenone from a Phaeosphaeria sp. discovered by antisense strategy. J Nat Prod 71:1304–1307
Zhang C, Ondeyka JG, Zink DL, Basilio A, Vicente F, Collado J, Platas G, Huber J, Dorso K, Motyl M, Byrne K, Singh SB (2009) Isolation, structure and antibacterial activity of pleosporone from a pleosporalean ascomycete discovered by using antisense strategy. Bioorg Med Chem 17:2162–2166
Ziemert N, Lechner A, Wietz M, Millán-Aguiñaga N, Chavarria KL, Jensen PR (2014) Diversity and evolution of secondary metabolism in the marine actinomycete genus Salinispora. Proc Natl Acad Sci USA 111:E1130–E1139. doi:10.1073/pnas.1324161111
Zotchev S (2012) Marine actinomycetes as an emerging resource for the drug development. J Biotechnol 159:168–175
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Genilloud, O. The re-emerging role of microbial natural products in antibiotic discovery. Antonie van Leeuwenhoek 106, 173–188 (2014). https://doi.org/10.1007/s10482-014-0204-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-014-0204-6