Abstract
‘Candidatus Magnetoglobus multicellularis’ is a magnetotactic microorganism composed of several bacterial cells. Presently, it is the best known multicellular magnetotactic prokaryote (MMP). Recently, it has been observed that MMPs present a negative photoresponse to high intensity ultraviolet and violet-blue light. In this work, we studied the movement of ‘Candidatus Magnetoglobus multicellularis’ under low intensity light of different wavelengths, measuring the average velocity and the time to reorient its trajectory when the external magnetic field changes its direction (U-turn time). Our results show that the mean average velocity is higher for red light (628 nm) and lower for green light (517 nm) as compared to yellow (596 nm) and blue (469 nm) light, and the U-turn time decreased for green light illumination. The light wavelength velocity dependence can be understood as variation in flagella rotation speed, being increased by the red light and decreased by the green light relative to yellow and blue light. It is suggested that the dependence of the U-turn time on light wavelength can be considered a form of light-dependent magnetotaxis, because this time represents the magnetic sensibility of the magnetotactic microorganisms. The cellular and molecular mechanisms for this light-dependent velocity and magnetotaxis are unknown and deserve further studies to understand the biochemical interactions and the ecological roles of the different mechanisms of taxis in MMPs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnetotactic bacteria are flagellated microorganisms capable of orienting to magnetic fields. This is possible due to the presence of intracellular arrangements of magnetosomes, which are magnetic nanoparticles enveloped by a membrane (Bazylinski and Frankel 2004). Several different species of magnetotactic bacteria show phototactic behavior. Magnetococcus marinus strain MC-1 responds to short-wavelength light (λ ≤ 500 nm) but not to red light (Frankel et al. 1997). Magnetospirillum magnetotacticum MS-1 does not show any wavelength-dependent effect of illumination (Frankel et al. 1997) whereas M. magneticum AMB-1 exhibits positive thermotaxis and phototaxis independent of wavelength and magnetic field (Chen et al. 2011). Despite some speculation (e.g. Shapiro et al. 2011), the ecological function of phototaxis in magnetotactic bacteria is still not known. Multicellular magnetotactic prokaryotes (MMPs) are spherical or elliptical ensembles of bacterial cells that are magnetotactic as a whole, but not as individual cells (Keim et al. 2006; Zhou et al. 2012). ‘Candidatus Magnetoglobus multicellularis’ (CMm) is the best described MMP. This microorganism is spherical and composed of 10–40 gram-negative cells arranged radially around an acellular internal compartment at the center of the microorganism (Abreu et al. 2007). CMm has a net magnetic moment as it aligns in the geomagnetic field in the same way as unicellular magnetotactic bacteria. This magnetic moment is the vector summation of a distribution of individual magnetic moments for each constituent cell (Winklhofer et al. 2007; Acosta-Avalos et al. 2012). CMm microorganisms are very motile and reach velocities of 50–150 μm s−1 in forward motion (Perantoni et al. 2009; Almeida et al. 2013). Shapiro et al. (2011) observed that a brief (less than 2 s) exposure to UV light (365 nm) at high intensity during observations in a fluorescence microscope led to a reverse of the swimming direction (RSD) of the MMPs. Two to five seconds exposure to UV light appeared to be lethal. A similar response was observed when exposure of 30 s to approximately 80 W m−2 of violet-blue light (395–440 nm filter) or 200 W m−2 of blue light (450–490 nm filter). No photo response was observed for wavelengths longer than 490 nm, but after 5–10 min exposure to green light MMPs lost their motility, eventually undergoing disaggregation. Elliptical MMPs also show RSD under light of wavelengths 450–480, 400–410 or 330–385 nm (Zhou et al. 2012). CMm present a similar behaviour to UV light (Almeida et al. 2013). As described above, light affects MMPs behaviour, but there is no report describing any photokinetic effect. In the present work, we report the effect of light of different wavelengths on the motion of CMm, measuring its translational velocity and on its magnetic sensibility measuring the U-turn time.
Experimental procedures
Motion velocity
Samples of water and sediment containing the microorganism CMm were collected at Araruama lagoon (22º50′S; 42º13′W), a hypersaline (~55 ‰) coastal lagoon of Rio de Janeiro State, Brazil. They were collected at a depth of 1 m and maintained in the laboratory into an aquarium of 12 × 14 × 20 cm with approximately 1/3 of sediment and 2/3 of water, near to a window. Several days after collection, a portion of the same sample, containing water and sediments, was collected somewhat below the sediment surface and magnetically concentrated as described by Lins et al. (2003). A permanent magnet was used to isolate the south-seeking magnetotactic microorganisms. After 15 min a drop of water was collected, put on a coverslip and inverted on an O-ring located over a microscope slide (the hanging droplet method) (Abreu et al. 2006). A digital microscope (Celestron No. 33340) was used to record the CMm movement. A pair of coils was adapted to the sides of the microscope and connected to a DC power supply providing a homogeneous magnetic field of 1.44 G in the sample region. The coils axis was oriented perpendicular to the geomagnetic field vector (magnitude of 0.13 G in laboratory), so that the magnetic field intensity in the sample area was about 1.45 G. A magnet was used to concentrate many CMms at the drop edge. After that, the magnet was taken out, the power supply turned on and the movement of the prokaryotes recorded. Four different recordings were done, using different drops and LED lamps (Xelux PAR 20 with 30 individual LEDs; colors blue, green, yellow and red. The wavelengths were measured with a spectrophotometer HP 8453. The monochromatic light from the LED lamp was directed to the hanging drop using a glass slide above the condenser lens to split the light. Approximately 1 min of CMm movement was recorded for each lamp.
The light intensity reaching the drop in the microscope was determined measuring the optical power (Newport Optical Power Meter Model 1916-C) and divided by the condenser lens output area (0.11 × 10−3 m2). The light intensities determined for blue (469 nm), green (517 nm), yellow (596 nm) and red (628 nm) lamps were 0.8, 0.46, 0.05 and 0.16 W m−2, respectively.
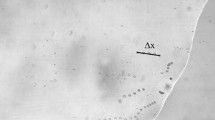
Each video was imported from the digital microscope to a computer. The AVS Video Converter 6.0 software was used to obtain the frames from the videos. The trajectories were reconstructed by the superposition of video frames using the Adobe Photoshop software (Fig. 1). The average speed was calculated by V = ∆x/∆t (∆x represents the distance and ∆t, the time interval). ∆t was calculated by ∆t = (N-1) ∆T, where ∆T is the time between frames and N is the number of frames. As the frame rate was 25 fps, then ∆T = 0.04 s. To calculate the distance ∆x, a ruler of 100 μm was recorded at the same microscope and same conditions. The Graphpad Instat software was used for the statistics of the 4 groups of speeds (one for each wavelength). The Microcal Origin 6.0 software was used to construct the box-plots.
U-turn motion
When the magnetic field changes its direction, magnetotactic microorganisms respond following a trajectory with U form, known as U-turn movement. Studies of the U-turn curve parameters have been done to estimate the magnetic moment of magnetotactic bacteria (Esquivel and Lins de Barros 1986; Bahaj and James 1993) and of CMms (Perantoni et al. 2009). These parameters are the U-turn time τ and the diameter L. To determine the diameter L a necessary condition is that the plane of the bacterial movement be the microscope focal plane. As this condition is not fulfilled in all the observed magnetotactic bacteria trajectories, the measurement of L from video-microscopy is not reliable (Perantoni et al. 2009). The U-turn time τ depends only on the size of the microorganism (through its radius R) and on the magnetic energy mB, where m is the magnetic moment and B is the external magnetic field. The parameter τ measures the time to reorient the magnetic moment of the microorganism parallel to the external magnetic field and does not depend on the motility of the microorganism (Lins de Barros and Esquivel 1985; Perantoni et al. 2009). As the U-turn time τ depends on the magnetic energy it reflects the magnetic sensibility of a magnetotactic microorganism. In principle, it is expected that the time τ does not change when the microorganisms are illuminated with light from different wavelengths.
The U-turn is easily observed in the trajectory image obtained during a magnetic field reversal (Fig. 2). The U-turn tracks were analyzed in the same way as the motion analysis. The movements were recorded in the digital microscope, each video was imported from the digital microscope to a computer and the U-turn tracks were reconstructed by the superposition of video frames using the Adobe Photoshop software.
Example of composed image used for measure the U-turn time. In this case, CMms were illuminated with green light. The numbers on the curve represent the frame numbers starting from one chosen arbitrarily as number 1. The number does not represent the true sequence, because the superposition was done picking the frames in sequence of two: after choosing the frame n the next one was the n + 2. Insert: The same curve numbered is represented with the tangent trajectories that demarcate the beginning (B) and the end (E) of its U-turn. In this example, the measured τ is 0.88 s
To measure the time τ, it is necessary to determine the beginning and the end of the U-turn curve. One way to do that is to synchronize the image recording to a square wave magnetic field generated by a microcomputer (Perantoni et al. 2009). However in the absence of synchronization, it is not obvious how to determine the beginning and the end of the U-turn. Observing Fig. 1b in Perantoni et al. (2009) and the Fig. 7 in (Nogueira and de Lins Barros 1995), it can be seen that the microorganism trajectories before the beginning and after the end of the U-turn are tangents to the curved U-turn. The insert to Fig. 2 shows an example of the tangent trajectories to the U-turn. That characteristic was used as a criterion to determine the U-turn time τ, through the number N′ of frames from the beginning and the end of the U-turn track and through the relation τ = (N′−1) (0.04 s). Again, the Graphpad Instat software was used for the statistics of the 4 groups of times (one for each wavelength) and the Microcal Origin 6.0 software was used to construct the box-plots.
Results and discussion
Table 1 shows the statistics for the average velocities measured in CMm groups illuminated with monochromatic light of different wavelengths and Fig. 3 shows the box-plots for the average velocity distributions as a function of the light wavelength. The distribution of average velocities for each wavelength is not Gaussian (Kolmogorov–Smirnov test, p > 0.05) and consequently the statistical tests used to compare the groups were non-parametrical. The Kruskal–Wallis test (Zar 1999) shows that the average velocity groups are statistically different (p < 0.0001). A non-parametric multiple comparison (Zar 1999) shows that average velocities for blue and yellow light are statistically similar and different from green and red light. Table 1 shows that red light average velocities were greater and green light average velocities were lower than blue and yellow light average velocities. The average velocities for white light illumination were also measured (Mean velocity = 85.8 μm s−1; SE = 1.23 μm s−1; N = 444). The blue, yellow and white light average velocity groups are all statistically similar (p < 0.0001, Kruskal–Wallis test). The white light corresponds to a standard microscope illumination lamp.
Table 1 shows the statistics for the groups of time (τ) as function of the wavelength and Fig. 4 shows the box-plot for τ as a function of the light wavelength. The sampling number of analyzed U-turn curves was lower than the sampling number of velocities because of the application of the restrictive criteria explained in “Experimental procedures” above. The ANOVA test (Zar 1999) shows that the groups are different (p < 0.05) and the Tukey–Kramer test (Zar 1999) shows that green light times are statistically different compared with the other groups. Times for the blue, yellow and red light groups are statistically similar. It was observed that green light decreases the U-turn time.
Changes in swimming velocity under light of different wavelength and/or intensity are known as photokinesis (Diehn et al. 1977). Photokinesis is found in many phototrophic microorganisms, either prokaryotes or eukaryotes (Thar and Kühl 2001; Streif et al. 2008) and also in non-phototrophic eukaryotic microorganisms (Iwatsuki 1992).
The photoresponse of CMm shown in this work differs markedly from those published before on both spherical and ellipsoidal MMPs, including CMm. We found differences in translational velocity (photokinesis) under green and red light relative to blue, yellow and white light, whereas previous studies found RSD under blue, violet and UV light (Shapiro et al. 2011; Zhou et al. 2012; Almeida et al. 2013). The main experimental difference with previous studies is that the light intensities used here were much lower. This could explain the fact that we observed similar velocities under blue, yellow and white light, whereas other studies observed RSD to short wavelength light. For example, the blue light wavelength used in our study (469 nm) is in the region reported by Shapiro et al. (2011) to induce RSD, but our light intensity (0.8 W m−2) is about 250 times lower (200 W m−2 in Shapiro et al. (2011)). That is most likely the reason we did not observe RSD using blue light. Within the range of intensities used in this study, we did not observe any effect of light intensity on the swimming velocity, nor on the U-turn time. With our system we are able to reach about 80 W m−2 of blue light and CMM cells do not change their behaviour when illuminated by about 1 min with that high intensity. On the other hand, Shapiro et al. (2011) reported photo-magnetotaxis using a light intensity of about 60–645 W m−2 in the spectral region of 450–490 nm, and with response times from 2 s (for higher intensities) to 400 s (for lower intensities). Perhaps, the 1 min exposure used in the present study is not sufficient to elicit the photo-magnetotactic response using 80 W m−2. In addition, Shapiro et al. (2011) did not observe any photoresponse to green light, although long time exposures were found to be lethal, whereas we observed a decrease in velocity. Previous studies did not investigate longer wavelengths such as yellow and red, nor the effects of wavelength on translational velocity at any wavelength. As reported for other bacteria, the variation in velocity under light of different wavelengths is most likely due to changes in the flagellar rotation. Our results and previous reports suggest that CMm has atleast two types of photoreceptors leading to two different signaling pathways and two qualitatively different behavioural responses: one for green/red light, which is related to the photokinesis reported here, and the other for short wavelength light at relatively high intensities (higher than about 60 W m−2), which triggers the reversal of swimming direction as reported previously (Shapiro et al. 2011; Zhou et al. 2012; Almeida et al. 2013).
Surprisingly, the U-turn time for green light was found to be lower than for the other wavelengths analyzed in this study. As mentioned above, it is expected that the time τ does not depend on the wavelength, but our results show a decrease in τ with green light. Changes in τ are difficult to explain. Firstly, it can be observed that τ is a function of R and m. So, considering m to be constant, τ can decrease if the microorganism radius R also decreases; but considering R to be constant τ would decrease if m increases, because the τ function monotonically decreases with the value of m. It has been observed that Escherichia coli size decreases when irradiated with UV light (366 nm) for some hours because of a mismatch between cell growth and division (Caldeira de Araujo and Favre 1985) but, as far as we know, green or red light illumination does not change bacterial size. In addition, the time of exposure of CMm to light was much shorter than that necessary to produce an effect in the cell size of E. coli. As τ depends directly on R 3, the effect of green light in decreasing τ must be related to a microorganism radius decrease of about 6 %. So, if the original radius R is 3 μm the reduced value must be 2.8 μm. We were not able to observe that size reduction in vivo with our experimental setup. Whatever the cause of the green light τ reduction, it implies that the CMm magnetic sensibility changes with green light, responding in a different way to the external magnetic fields.
The dependence of the CMm magnetotactic behaviour on the light wavelength (through the reorientation time) reveals that magnetotaxis is more complex than is believed. Greenberg et al. (2005) analyzed the MMP escape motility (or “ping-pong” motility) and concluded that it cannot be explained assuming only a magnetotactic behaviour, suggesting a magnetoreceptive capability in these organisms. As the light wavelength changes the response of CMm to the inversion in magnetic field direction, we suggest a “light-dependent” magnetotaxis for CMm, different from the “photo-magnetotaxis” suggested by Shapiro et al. (2011) which is related to an inversion in the MMP swimming direction.
CMm organisms are found mainly in the upper 1–5 cm of the sediment of Araruama lagoon. They seem to migrate upwards and downwards in the sediment, although no specific reason was found for this migration (Sobrinho et al. 2011). The measurement of the light intensity of the sun in the Araruama lagoon just below the water surface, at the top of the sediments (below about 60 cm of water) and below the sediments (about 5 cm deep plus 60 cm of water column) was done using a light meter (cal-light 400L, the Cooke Corp.) The results, in lx, are for sun light. Light intensities were: 92 klx (below the water surface), 15.8 lx (top of the sediments) and 0.86 lx (below the sediments). An approximate correspondence of these sun light intensities with the light intensity of the green and blue LED lamps used in this work was done by measuring the light intensity in lx and the power in W for the same lamp, at the same distance in the laboratory. The estimation for the Araruama lagoon is: below the water surface 751 W m−2 (blue) and 103 W m−2 (green), top of the sediments 0.13 W m−2 (blue) and 0.016 W m−2 (green) and below the sediments 0.007 W m−2 (blue) and 0.0009 W m−2 (green). These values are overestimated but show that the light intensities used in previous works on photo-responses of MMPs (Shapiro et al. 2011) are much higher than the intensities found in the sediments of Araruama lagoon. Consequently the results of Shapiro et al. (2011) probably explain well the behaviour of MMPs when they approach the water surface, whereas the results shown here would describe the behaviour of MMPs in the top of the sediments.
Table 1 shows that green light produced the lowest and red light the highest CMm mean velocity. The water from Araruama lagoon is commonly greenish, because of the presence of cyanobacteria and microalgae (personal observation). It is known that red wavelengths are absorbed in the first few meters of water. Thus, blue and green wavelengths are often the most common light in deep water (Johnsen and Sosik 2004).
Light in the microenvironments where MMPs thrive is filtered by the water column and superficial sediment. The red light found near the surface of the water column could increase the velocity of the microorganisms while magnetotaxis would drive them downwards to the sediments, where they would find their preferred microenvironments. With depth, the intensity of larger wavelength light (such as red) would decrease faster, resulting in a predominance of blue and green light, leading the CMm microorganisms to decrease their velocity. Because in most bacteria chemotaxis is based on measurements of concentrations over time, smaller velocities lead to concentration measurements within shorter distances and thus detection of weaker concentration gradients. Thus, a smaller speed would increase the chemotaxis sensibility. The integration of photo-, chemo- and magnetotaxis could result in finely tuned and faster finding of the CMm preferred microenvironment.
In conclusion, for the first time a photokinetic behaviour in a magnetotactic microorganism was demonstrated through the analysis of the velocity as function of the light wavelength. Because MMPs probably have a chemotrophic sulfate-reducing energetic metabolism (Wenter et al. 2009), CMms could be the first chemotrophic microorganisms known to perform photokinesis. Also, it was shown for the first time that the magnetic sensibility of MMPs changes with green light illumination. This “light-dependent” magnetotaxis deserves more study. Presently, we are analyzing the CMm velocity as function of the external magnetic field and of the wavelength to calculate the magnetic moment (Kalmijn 1981) as function of the wavelength. The cellular and molecular mechanisms for this light-dependent velocity and magnetotaxis are unknown and deserve further studies to understand the biochemical interactions and the ecological roles of the different mechanisms of taxis in MMPs.
References
Abreu F, Silva KA, Martins JL, Lins U (2006) Cell viability in magnetotactic multicellular prokaryotes. Int Microbiol 9:267–272
Abreu F, Martins JL, Silveira TS, Keim CN, de Lins Barros HGP, Gueiros Filho FJ, Lins U (2007) ‘Candidatus Magnetoglobus multicellularis’, a multicellular, magnetotactic prokaryote from a hypersaline environment. Int J System Evol Microbiol 57:1318–1322
Acosta-Avalos D, Santos Azevedo LM, Andrade TS, Lins de Barros H (2012) Magnetic configuration model for the multicellular magnetotactic prokaryore Candidatus Magnetoglobus multicellularis. Eur Biophys J 41:405–413
Almeida FP, Viana NB, Lins U, Farina M, Keim CN (2013) Swimming behaviour of the multicellular magnetotactic prokaryote ‘Candidatus Magnetoglobus multicellularis’ under applied magnetic fields and ultraviolet light. Ant Leeuw 103:845–857
Bahaj AS, James PAB (1993) Characterisation of magnetotactic bacteria using image processing techniques. IEEE Trans Mag 29:3358–3360
Bazylinski DA, Frankel RB (2004) Magnetosome formation in prokaryotes. Nat Rev Microbiol 2:217–230
Caldeira de Araujo A, Favre A (1985) Induction of size reduction in Escherichia coli by near-ultraviolet light. Eur J Biochem 146:605–610
Chen C, Ma Q, Jiang W, Song T (2011) Phototaxis in the magnetotactic bacterium Magnetospirillum magneticum strain AMB-1 is independent of magnetic fields. Appl Microbiol Biotechnol 90:269–275
Diehn B, Feinleib M, Haupt W, Hildebrand E, Lenci F, Nultsch W (1977) Terminology of behavioral responses of motile microorganisms. Photochem Photobiol 26:559–560
Esquivel DMS, de Lins Barros HGP (1986) Motion of magnetotactic microorganisms. J Exp Biol 121:153–163
Frankel RB, Bazylinski DA, Johnson MS, Taylor BL (1997) Magneto-aerotaxis in marine coccoid bacteria. Biophys J 73:994–1000
Iwatsuki K (1992) Stentor coeruleus shows positive photokinesis. Photochem Photobiol 55:469–471
Johnsen S, Sosik H (2004) Shedding light on light in the ocean. Oceanus Mag 43:1–5
Kalmijn AJ (1981) Biophysics of geomagnetic field detection. IEEE Trans Mag 17:1113–1124
Keim CN, Martins JL, de Lins Barros H, Lins U (2006) Structure, behavior, ecology and diversity of multicellular magnetotactic prokaryotes. In: Schuler D (ed) Magnetoreception and magnetosomes in bacteria. Springer Verlag, Heidelberg, pp 104–132
Lins U, Freitas F, Keim CN, de Lins Barros HGP, Esquivel DMS, Farina M (2003) Simple homemade apparatus for harvesting uncultured magnetotactic microorganisms. Braz J Microbiol 34:111–116
Nogueira FS, de Lins Barros HGP (1995) Study of the motion of magnetotactic bateria. Eur Biophys J 24:13–22
Perantoni M, Esquivel DMS, Wajnberg E, Acosta-Avalos D, Cernicchiaro G, de Lins Barros H (2009) Magnetic properties of the microorganism Candidatus Magnetoglobus multicellularis. Naturwissenschaften 96:685–690
Shapiro OH, Hatzenpichler R, Buckley DH, Zinder SH, Orphan VJ (2011) Multicellular photo-magnetotactic bacteria. Environ Microbiol Rep 3:233–238
Sobrinho RL, Lins U, Bernardes MC (2011) Geochemical characteristics related to the gregite-producing multicellular magnetotactic prokaryote Candidatus Magnetoglobus multicellularis in a hypersaline lagoon. Geomicrobiol J 28:705–713
Streif S, Staudinger WF, Marwan W, Oesterhelt D (2008) Flagellar rotation in the archaeon Halobacterium salinarum depends on ATP. J Mol Biol 384:1–8
Thar R, Kühl M (2001) Motility of Marichromatium gracile in response to light, oxygen, and sulfide. Appl Environ Microbiol 67:5410–5419
Wenter R, Wanner G, Schuler D, Overmann J (2009) Ultrastructure, tactic behavior and potential for sulfate reduction of a novel multicellular magnetotactic prokaryote from North sea sediments. Environ Microbiol 11:1493–1505
Winklhofer M, Abraçado LG, Davila AF, Keim CN, de Lins Barros HGP (2007) Magnetic optimization in a multicellular magnetotactic organism. Biophys J 92:661–670
Zar JH (1999) Biostatistical analysis, 4th edn. Pearson Education Inc, Boston
Zhou K, Zhang WY, Yu-Zhang K, Pan HM, Zhang SD, Zhang WJ, Yue HD, Li Y, Xiao T, Wu LF (2012) A novel genus of multicellular magnetotactic prokaryotes from the Yellow Sea. Environ Microbiol 14:405–413
Acknowledgments
We acknowledge financial support from the Brazilian agencies CNPq, FAPERJ and CAPES.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Azevedo, L.V., de Barros, H.L., Keim, C.N. et al. Effect of light wavelength on motility and magnetic sensibility of the magnetotactic multicellular prokaryote ‘Candidatus Magnetoglobus multicellularis’. Antonie van Leeuwenhoek 104, 405–412 (2013). https://doi.org/10.1007/s10482-013-9964-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-013-9964-7