Abstract
Samples of produced water and oil obtained from the Enermark field (near Medicine Hat, Alberta, Canada) were separated into oil and aqueous phases first gravitationally and then through centrifugation at 20°C in an atmosphere of 90% N2 and 10% CO2. Biomass that remained associated with oil after gravitational separation (1×g) was dislodged by centrifugation at 25,000×g. DNA was isolated from the aqueous and oil-associated biomass fractions and subjected to polymerase chain reaction amplification with primers targeting bacterial and archaeal 16S rRNA genes. DNA pyrosequencing and bioinformatics tools were used to characterize the resulting 16S rRNA gene amplicons. The oil-associated microbial community was less diverse than that of the aqueous phase and had consistently higher representation of hydrogenotrophs (methanogens of the genera Methanolobus and Methanobacterium and acetogens of the genus Acetobacterium), indicating the oil phase to be a primary source of hydrogen. Many known hydrocarbon degraders were also found to be oil-attached, e.g. representatives of the gammaproteobacterial genus Thalassolituus, the actinobacterial genus Rhodococcus and the alphaproteobacterial genera Sphingomonas, Brevundimonas and Stappia. In contrast, all eight representatives of genera of the Deltaproteobacteria identified were found to be associated with the aqueous phase, likely because their preferred growth substrates are mostly water-soluble. Hence, oil attachment was seen for genera acting on substrates found primarily in the oil phase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oil is frequently produced by water injection. Wells in many mature on-land reservoirs typically produce 5–10% oil and 90–95% water, with the water cut increasing over time (Planckaert 2005; Youssef et al. 2007). Microbially enhanced oil recovery (MEOR) is one of the technologies that may help increase the fraction of the recovered oil (Bryant and Burchfield 1989; Banat 1995; Li et al. 2002; McInerney et al. 2005). Microorganisms attached to oil are particularly interesting for MEOR applications. They may be present in core matrix pores, where oil is trapped in micro-droplets and where microbial activity may lead to oil degradation and methane production (Zengler et al. 1999; Head et al. 2003).
It has been shown that microorganisms may need to produce alcohols and/or biosurfactants to get attached to an oil–water interface (Abbasnezhad et al. 2008). On the other hand, surfactants, especially when combined with alcohols, are known to reduce interfacial tension between the hydrocarbon and aqueous phases and may in this way enhance oil recovery (Bryant and Burchfield 1989; Banat 1995; Li et al. 2002; McInerney et al. 2005). Cells attached to the oil–water interface are also known to behave like adsorbed colloidal particles. Thus, they may allow the application of an enhanced oil recovery mechanism, which does not depend on interfacial tension reduction, but instead depends on plugging of high permeability zones (Kang et al. 2008). It is also important to note that in some MEOR applications transportability of the microbes throughout the oil reservoir may be an important parameter, and in such cases reliance on the microbes associated with the aqueous phase may be required.
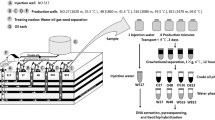
A number of methods have been used to study hydrophobic interactions of cells, for example those based on defining the degree of adhesion of cells to liquid hydrocarbons following a brief period of mixing; contact angle measurements of dried cell layers; and partitioning of bacteria in aqueous polymer two-phase systems (Rosenberg et al. 1980). A method known as MATH (microbial adhesion to hydrocarbons) has gained popularity due to its simplicity and acceptable level of accuracy, despite some disadvantages (Rosenberg 2006; van der Mei et al. 1995; Zoueki et al. 2010). It is based on defining the degree of adhesion of cells to liquid hydrocarbons following mixing (Rosenberg et al. 1980; Rosenberg 2006). In this study, we employed an idea similar to that of the MATH method in order to determine the compositions of the OC (microbial community associated with an oil phase) and the AC (microbial community associated with an aqueous phase). In our method, oil-attached cells firstly rise to the surface with the oil during gravitational separation (1×g), and secondly they are dislodged from the oil and pelleted by centrifugation at 104×g. To our knowledge, the use of this method has not been reported previously.
Materials and methods
Isolation of biomass
Samples of oil-containing produced water were collected in 1l Nalgene bottles from production wells in the Enermark field (near Medicine Hat, Alberta, Canada) on 12/01/2010 and 02/02/2010. The bottles were filled to the top to prevent oxygenation (Voordouw et al. 2009), delivered to the laboratory within five hours after filling and stored in an anaerobic hood (90% N2 and 10% CO2) at 20°C. The samples were separated into oil and aqueous phases in the anaerobic hood in a separatory funnel (gravitational separation, 1×g). Following standing overnight, the aqueous phase was drained off and biomass associated with it was collected through centrifugation at 25,000×g for 20 min in 250 ml centrifuge bottles. The pellets were re-suspended in 1 ml of sterile synthetic brine (90 mM NaCl; 1 mM KCl; 1 mM MgCl2; 2 mM CaCl2; 10 mM NaHCO3; pH 7.5) by agitating for 50–60 s with a Vortex mixer, transferred to 1.5 ml microcentrifuge tubes and centrifuged at 17,000×g for 10 min. The supernatant was removed and collected biomass was subjected to DNA extraction. The separatory funnel containing the remaining oil phase was replenished with 1–1.5 l of sterile synthetic brine, manually shaken for 2 min and allowed to stand for at least 24 h. Following this, the synthetic brine was drained off and the process was repeated at least twice to remove microorganisms associated with the aqueous phase. Oil-associated biomass was dislodged from the oil by centrifuging at 25,000×g for 20 min in 250 ml centrifuge bottles containing 50–100 ml of sterile synthetic brine (depending on the amount of oil). The oil-containing supernatant was removed, 25–30 ml of sterile synthetic brine was added to the pellet and the tube was agitated for 60 s with a Vortex mixer to suspend the cells. The cell suspension was transferred to 50 ml centrifuge tubes and re-centrifuged. Sterile synthetic brine (1 ml) was added and the samples were processed by microcentrifugation as indicated above. The entire process was replicated five times, so that totally ten samples of biomass were collected (five samples of oil-associated biomass and five samples of water-associated biomass) to allow analysis of the composition of five OCs and five ACs (Table 1).
DNA isolation
DNA was isolated according to Marmur (1961), with a bead beating step modification. Oil- and water-associated biomass was re-suspended in 280 μl 0.15 M NaCl and 0.1 M EDTA (pH 8) and subjected to digestion with lysozyme (20 μl of 5 mg/ml, at 37°C for 10 min or longer). Samples were then transferred into 2 ml bead-containing lysing matrix tubes (MP Biomedicals) and subjected to bead-beating in a FastPrep-24 homogenizer (MP Biomedicals) for 1 min; subsequently, they were centrifuged at 17,000×g for 1 min and transferred to microcentrifuge tubes; 72 μl 5 M NaClO4 and 420 μl chloroform-isoamyl alcohol (24:1) were added and the mix was placed on a rotating wheel for a minimum of 1 h. The mix was centrifuged for 3.5 min at 17,000×g in the same tube at 20°C; 350 μl of the top layer was transferred to another microcentrifuge tube and 700 μl of 95% ethanol was added. Following centrifugation (15 min, 17,000×g, 4°C) the pellet was dissolved in 200 μl of TE (10 mM Tris, 0.1 mM EDTA pH 8) and incubated at 20°C with DNAse-free RNAse (25 μl, 50 μg/ml) and proteinase K (10 μl, 20 mg/ml) sequentially, with each for 1 h. Following phenol extraction and ethanol precipitation, the air-dried DNA pellets were dissolved in 30–50 μl of TE-buffer, depending on the size of the pellet.
DNA pyrosequencing
DNA samples were amplified through a two-step PCR amplification. The first PCR (25 cycles) was performed with 16S primers 926Fw16S (AAACTYAAAKGAATTGRCGG) and 1392R16S (ACGGGCGGTGTGTRC). Once the presence of a 500 bp PCR product was confirmed by agarose gel electrophoresis, a second PCR (ten cycles) was performed using FLX titanium primers 454T_RA_X and 454T_FwB with the sequences for 926Fw16S and 1392R16S as their 3′-ends. Primer 454T_RA_X had a 25 nucleotide A-adaptor (CGTATCGCCTCCCTCGCGCCATCAG) and a ten-nucleotide multiplex identifier barcode sequence. Primer 454T_FwB had a 25 nucleotide B-adaptor sequence (CTATGCGCCTTGCCAGCCCGCTCAG). The PCR product was checked by agarose gel electrophoresis, purified with QIAquick PCR Purification Kit (Qiagen), and the concentration of the purified product was determined with Qubit fluorometer (Invitrogen) using Quant-iT™ dsDNA HS Assay Kit (Invitrogen). PCR products (25 μl of 5 ng/μl) were pyrosequenced at the Genome Quebec and McGill University Innovation Centre (Montreal, Quebec) using a Genome Sequencer FLX Instrument with a GS FLX Titanium Series Kit XLR70 (Roche Diagnostics Corporation).
Analysis of pyrosequencing data
Phoenix 2, an in-house developed SSU rRNA gene data analysis pipeline, was used to conduct the analysis. Raw pyrosequence reads were subjected to stringent systematic checks in order to remove low quality reads and minimize sequencing errors that could be introduced during the pyrosequencing process (Huse et al. 2007). Eliminated sequences included ones that: (1) did not perfectly match the adaptor and primer sequences, (2) had ambiguous bases, (3) had an average quality score below 27, (4) contained homopolymer lengths greater than eight, and (5) were shorter than 200 bp after clipping off the primers. The remaining high-quality sequences were compared against the non-redundant SSURef data set of SILVA102 (Pruesse et al. 2007) using the Tera-Blast algorithm on a 16-board TimeLogic Decypher system (Active Motif, Inc.). Sequences that had the best alignment covering less than 90% of the trimmed read length, with greater than 90% sequence identity to the best BLAST match within the matched region and no match to the ends of the sequence, were labeled as potential chimeras and excluded from further analysis. While this might remove some true high-quality BLAST matches, in most cases a low percentage (less than 19.14%) of the sequences were eliminated, indicating that most of the real BLAST hits were included in the final study.
Sequences that passed the quality control and chimerical sequence removal were clustered into OTUs (Operational Taxonomic Units) at 3% distance by using the average linkage algorithm (Schloss and Westcott 2011). After grouping sequences into OTUs, several alpha diversity indices (Table S1), as well as the total numbers of OTUs were calculated for each sample. A taxonomic consensus for each representative sequence from each OTU was derived by using the classifier.otu function implemented in the Mothur software package (Schloss et al. 2009). The entire set of the raw reads is available at the NCBI Sequence Read Archive (SRA) under accession numbers SRR088842, SRR088844, SRR088845, SRR088846, SRR088847, SRR088848, SRR088850, SRR088852, SRR088854 and SRR088855. Table 1 provides the information on the numbers of reads identified at the phylum, class and genus levels for the ten samples that were analyzed.
To explore potential relationships between microbial communities from different environments, samples were clustered into Newick-formatted trees using the UPGMA algorithm with the distance between communities calculated with the thetaYC coefficient as a measurement of similarity between community structures (Yue and Clayton 2005) and the Jaccard index as a measurement of similarity between community memberships in the Mothur software package. The trees were visualized using Dendroscope (Huson et al. 2007b). In addition, parsimony testing (Fitch 1971), as well as weighted unifrac and unweighted unifrac testing (Hamady et al. 2010; Lozupone and Knight 2005) were used to determine the statistical significance of clustering within the trees. Rank abundance plots were built with MEGAN software (Huson et al. 2007a).
Results
Alpha- and beta-diversity
Two distinct OC and AC branch clusters were formed in the J-tree (Fig. 1A). The clustering pattern within the YC-tree (Fig. 1B) was very similar to that in the J-tree. The robustness of the inferred sample relation trees was confirmed using parsimony, weighted unifrac, unweighted unifrac and AMOVA (Analysis of Molecular Variance) hypothesis testing (Table 2). This suggested that the differences between OCs and ACs were statistically significant.
Clustering analysis of pyrosequencing data. A Jaccard sample relation tree built using the UPGMA clustering algorithm based on the classical Jaccard values. B ThetaYC tree built using the UPGMA algorithm based on Yue and Clayton (2005) theta values. Both trees were calculated in Mothur and visualized with Dendroscope software
To eliminate possible differences introduced by sampling bias, data for all OC amplicon libraries were pooled together to form the OCP (pooled microbial community associated with the oil phase) and the data for all AC amplicon libraries were pooled together to form the ACP (pooled microbial community associated with the aqueous phase). The alpha-diversity indices (Table S1) showed that the ACP was more diverse than the OCP (e.g. the Shannon-Wiener indices were 4.38 and 3.89, and the Simpson indices were 0.039 and 0.054, respectively). The richness shown by rarefaction curves (Fig. 2) was also higher in the ACP than in the OCP. The non-parametric Kendall tau rank correlation coefficient (Kendall 1938) was calculated by using the R statistical software package (Gentleman 2008). The P-value (0.0022) and tau (0.0551) suggested that the OCP and the ACP were highly independent from each other.
Characterization of the OCP and the ACP
Phylum and class levels
Proteobacteria and Euryarchaeota were found to be the most abundant phyla represented in both the OCP and the ACP (Table 3). The phyla Actinobacteria, Firmicutes and Tenericutes (Table 3: #2, #3 and #1) were more numerous in the OCP, whereas the phyla Spirochaetes and Synergistetes had higher representation in the ACP (Table 3: #10 and #11). At the class level, the Alphaproteobacteria and Methanomicrobia, being the most numerous in both phases, had almost equal representation in the OCP and the ACP (Table 4: #7 and #8). Deltaproteobacteria, Epsilonproteobacteria and Synergistia had consistently higher representation in the ACP, whereas Methanobacteria, Gammaproteobacteria and Clostridia were more numerous in the OCP (Table 4; Figs. S1, S2, S4 and S5).
Order and genus levels
The most abundant genera identified in the OCP and the ACP are presented in the Fig. S3 and S6. Three genera with the highest combined representation in the OCP and the ACP were all methanogens (Table 5: #27 Methanosaeta, #12 Methanobacterium and #8 Methanolobus). Of these, Methanosaeta was equally represented in two communities, whereas Methanobacterium and Methanolobus had higher representation in the OCP. Two other methanogens of the order Methanosarcinales, #1 Methanosarcina and #6 Methanomethylovorans, were more abundant in the OCP by 77- and 11-fold respectively. In contrast, methanogens of the order Methanomicrobiales, namely genera Methanofollis, Methanocalculus and Methanoculleus, had higher representation in the ACP (Table 5: #30, #44 and #45). Methanospirillum belonging to the order Methanomicrobiales was equally represented in the OCP and the ACP (Table 5: #23). Hence, there appeared to be a distinct distribution of methanogens with the order Methanosarcinales being predominantly oil-attached, except for the acetotrophic methanogen Methanosaeta, and the order Methanomicrobiales being more abundant in the aqueous phase.
Although the class Alphaproteobacteria was equally represented in the OCP and ACP (Table 4), large differences were seen in distributions at the genus level. Representatives of four genera were more abundant in the OCP (Table 5: #2 Sphingomonas, #11 Rhodovulum, #14 Brevundimonas and #15 Stappia), whilst seven genera had higher representation in the ACP (Table 5: #50 Sphingobium, #46 Tistrella, #43 Donghicola, #42 Oceanibaculum, #37 Mesorhizobium, #33 Hyphomonas and #32 Ensifer), and four genera were equally represented in the OCP and the ACP (Table 5: #16 Kaistia, #19 Hyphomicrobium, #22 Rhizobium, and #26 Rhodobacter). Likewise, Betaproteobacteria phylotypes were either oil-associated (Table 5: #4 Azovibrio) or mostly associated with the aqueous phase (#41 Azonexus). Thalassolituus (Table 5: #3) was the most prominent oil-associated representative of the class Gammaproteobacteria. The genera Pseudoxanthomonas and Azomonas (Table 5: #17 and #25) were equally represented in the two communities. Remarkably, all of eight genera identified within the Deltaproteobacteria preferred the aqueous phase (#51 Geobacter, #49 Desulfocapsa, #48 Desulfomicrobium, #47 Desulfobulbus, #38 Syntrophus, #36 Desulfuromonas, #35 Pelobacter and #34 Desulfovibrio). Of these, Geobacter had the least affinity to oil, being 80-fold more abundant in the ACP than in the OCP. The epsilonproteobacterium Arcobacter was also predominantly associated with the aqueous phase (Table 5: #39).
The oil-association of the phyla Firmicutes and Tenericutes (Table 3) was largely because of members of the genus Acetobacterium (Table 5: #7) and Acholeplasma (#9), respectively. Likewise, the preference of the phylum Synergistetes for the aqueous phase was mostly due to members of the genus Thermovirga (Table 5: #40). Among Actinobacteria, Propionicicella was one of the most highly represented genera in the OCP (Table 5: #5); Rhodococcus (Table 5: #10) was also oil-associated; other Actinobacteria identified at the genus level (e.g. #29 Georgenia, Table 5) had about equal representation in the two communities.
Discussion
Why attach to oil?
Oil in the Enermark field, from which the samples used in this study were taken, is produced by water injection. The injected water contains a low concentration of sulfate (1 mM) and nitrate (2 mM). The latter is added to prevent sulfide production by sulfate reducing bacteria or oxidize sulfide that is already present. As indicated elsewhere (Voordouw et al. 2009), both injected electron acceptors are reduced completely along the flow path. As a result, produced waters contain no nitrate or nitrite, no sulfate and on average 0.5 mM sulfide (Voordouw et al. 2009). The predominance of methanogens in the microbial community of produced waters (Table 5) is in agreement with the previously noted depletion of electron acceptors and suggests that oil degradation coupled to methanogenesis is a primary degradative mechanism in the vicinity of producing wells. Enermark oil is heavy, meaning that it is depleted of low molecular weight components such as toluene and enriched in higher molecular weight components such as asphaltenes. One may expect that microorganisms, which act primarily on substrates in the oil phase have an incentive to be oil-attached through hydrophobic cell surfaces. These may be primary oil degraders or utilizers of H2, which has a higher solubility in oil than in water.
Methanogenic oil degradation depends on the activities of: (1) syntrophic bacteria hydrolyzing hydrocarbons to H2, CO2 and acetate, (2) hydrogenotrophic methanogens converting H2 and CO2 to methane, and (3) acetotrophic methanogens converting acetate to methane and CO2 (Zengler et al. 1999; Head et al. 2003; Gieg et al. 2008). Syntrophic hydrocarbon degradation requires effective removal of hydrogen and acetate to drive this thermodynamically uphill reaction (Zengler et al. 1999). The deltaproteobacterium Syntrophus was identified as a primary degrader of a methanogenic hexadecane-degrading consortium (Zengler et al. 1999). However, oil has a wide variety of hydrocarbons including aromatic and polyaromatic hydrocarbons making it likely that other genera are involved as well. For example, Gieg et al. (2008) reported that an oil-utilizing methanogenic consortium consisted of Deltaproteobacteria, Firmicutes, Actinobacteria, Chloroflexi and Bacterioidetes. These phyla were also found in the current study (Table 3).
Oil attachment of H2-utilizers
H2 generated during anaerobic, methanogenic oil degradation or other anaerobic processes will distribute over both oil and aqueous phases, however it is more soluble in oil than in water at 30°C (Cai et al. 2001). An association of obligate hydrogenotrophs, such as hydrogenotrophic methanogens with the oil phase may therefore be expected. Indeed, two of the three most abundant genera identified here were oil-associated hydrogenotrophic methanogens (Table 5: #8 Methanolobus and #12 Methanobacterium). The acetotrophic methanogen Methanosaeta, one of the most abundant genera identified, does not need to be oil-associated, because at the resident pH of 7–8 acetate is more water- than oil-soluble. Methanosarcina species consume both acetate and H2 (Beckmann et al. 2011). However, the strong association of this species with oil (Table 5) indicates that hydrogen may be its preferred substrate in the Enermark field. Species of Methanomethylovorans produce methane from dimethylsulfide, methanethiol and methylamine (Lomans et al. 1999), compounds that dissolve in oil rather than water, explaining the association of this genus with oil (Table 5). The hydrogenotrophic methanogens Methanocalculus and Methanoculleus require acetate as a carbon source but not as a substrate for methanogenesis (Lai et al. 2002, 2004; Mikucki et al. 2003), which may explain their preferential association with the aqueous phase. The hydrogenotrophic acetogen Acetobacterium was also strongly oil-associated (Table 5).
Primary hydrocarbon degraders attached to oil
Gammaproteobacteria
Thalassolituus, the major gammaproteobacterium identified in the Enermark field, was clearly oil-associated. Thalassolituus oleivorans is an obligate oil-degrading bacterium capable of utilizing water-insoluble alkanes (Yakimov et al. 2010). This species was previously found in crude oil-containing marine environments and characterized as an aerobic chemoorganoheterotrophic organism unable to grow by fermentation or nitrate reduction (Yakimov et al. 2010). Oil-attachment of Thalassolituus in the Enermark field indicates that some species of this genus metabolize oil anaerobically.
Alphaproteobacteria
Members of the four oil-associated genera Sphingomonas, Rhodovulum, Brevundimonas, and Stappia have all been shown to degrade hydrocarbons. Sphingomonas spp. are well-known degraders of polyaromatic hydrocarbons. Although generally described as aerobic, it has been previously identified in anaerobic oil-degrading consortia (Zhang et al. 2011). Oil-degrading species of Rhodovulum were isolated recently by Teramoto et al. (2010), whereas species of Brevundimonas and Stappia have also been characterized as oil-degraders (Al-Awadhi et al. 2007; Chaîneau et al. 1999).
Betaproteobacteria
Azovibrio, found in this study to be associated with the oil phase, is closely related to Azoarcus (and also related to Thauera); some species of Azoarcus are known to degrade aromatic hydrocarbons under denitrifying conditions (Reinhold-Hurek and Hurek 2006), suggesting that Azovibrio may possibly also be able to degrade oil.
Actinobacteria
Some representatives of this phylum/class (for example, some species of Rhodococcus) have been characterized as degraders of poorly soluble in water organic compounds (Andreoni et al. 2000) and our finding that most Actinobacteria present in the Enermark field are oil-associated corresponds well to this information. Propionicicella was the most numerous genus of Actinobacteria in the OCP, but it was not abundant in the ACP. Interestingly, a close relative of this genus, Propionicimonas, was found in the production water from another Canadian oil reservoir (Grabowski et al. 2005a); its known species, Propionicimonas paludicola, has been described as a fermenting bacterium (Akasaka et al. 2003), whose presence in the oil reservoir production water/oil mixture was not easy to explain (Grabowski et al. 2005a). Propionicicella superfundia was found to be a chlorosolvent-tolerant, propionate-forming, facultative anaerobic bacterium (Bae et al. 2006). Unable to directly biotransform chlorinated solvents, it nevertheless has been thought to play a role in their biodegradation (Bae et al. 2006). It is not likely that chlorosolvents have been present in the produced water–oil mixture. However the apparent hydrophobicity of the cells of this bacterium may assist it in consuming hydrocarbons that are insoluble in water.
Tenericutes
Acholeplasma was one of the ten most abundant genera represented in the OCP. Representatives of this genus are known to be carbohydrate fermenters (Green et al. 2008). Somewhat unexpected in an oil field, Acholeplasma was previously found in a similar environment, a coal-bed methane well (Green et al. 2008). Its association with the oil phase suggests that species inhabiting this oil field may be able to metabolize hydrocarbons.
Microorganisms associated with the aqueous phase
Deltaproteobacteria
All eight identified genera of the Deltaproteobacteria (Table 5) were associated with the aqueous phase. This is understandable for those using organic acids such as acetate as electron donor for sulfate or Fe(III) reduction (Geobacter, Pelobacter, Desulfobulbus, Desulfomicrobium, Desulfuromonas, Desulfovibrio and Desulfocapsa). One might have expected the genus Syntrophus, implicated as the primary degrader in anaerobic hexane degradation (Zengler et al. 1999), to be oil-associated; however this hydrophilic bacterium may be able to access water-insoluble substrates through a biosurfactant-mediated oil pseudosolubilization (2008).
Alphaproteobacteria
Of the seven genera with representatives which preferred the aqueous phase (Sphingobium, Tistrella, Donghicola, Oceanibaculum, Mesorhizobium, Hyphomonas and Ensifer), some have been described as hydrocarbon degraders. Sphingobium species were reported to consume both water-insoluble aromatic hydrocarbons and such water-soluble substances as benzoate (Liang and Lloyd-Jones 2010). Being associated with the aqueous phase suggests that Sphingobium strains may preferentially consume water-soluble organics in the Enermark oil field. Tistrella increased the speed of phenanthrene degradation by another bacterium, Sphingomonas; however by itself it could not degrade this compound (Zhao et al. 2008) and the results of our study suggest that its primary metabolic substrate(s) must be water-soluble; alternatively, it may be able to produce biosurfactants for accessing water-insoluble organics. We came to the same conclusion regarding Oceanibaculum, some species of which have been characterized by Lai et al. (2009) and Dong et al. (2010). Donghicola has been thought to play a role in oil degradation, however it could also use water-soluble acetate (Tan et al. 2009). To our knowledge, Mesorhizobium, Hyphomonas and Ensifer have not been reported to degrade hydrocarbons.
Bacterioidetes and Synergistetes
Petrimonas was found in our study to have representation in the ACP about twice as large as in the OCP (Table 5: #31). The species Petrimonas sulfuriphila was shown by Grabowski et al. (2005b) to consume acetate and other organic acids. The phylum Bacteroidetes, to which Petrimonas belongs, was about one and half times more abundant in the ACP than the OCP (Table 3), showing that it is represented in the Enermark oil field largely by hydrophilic bacteria whose major metabolic substrates are, most likely, organic acids.
Thermovirga lienii was isolated by Dahle and Birkeland (2006) from a North Sea oil well and shown to consume some organic acids, and this fact corresponds well to our finding that Thermovirga is a bacterium associated with the aqueous phase.
Role of diazotrophs
Members of the order Rhizobiales, generally known to be diazotrophs, were found either to be nearly equally represented in the OC and the AC (Table 5: #16 Kaistia #19 Hyphomicrobium and #22 Rhizobium, the latter genus being one of the most represented bacteria in both the OC and the AC) or associated with the aqueous phase (Table 5: #32 Ensifer and #37 Mesorhizobium). Although ammonium was found in the Enermark field in submillimolar concentrations, preferential denitrification of the injected nitrate combined with anammox (Cornish-Shartau et al. 2010) can lead to a lack of NH3 and abundance of N2 in some zones of the oil reservoir. Therefore, Rhizobiales, together with some other bacteria (e.g. Azovibrio and Azonexus) may indeed convert N2 produced by nitrate-reducing bacteria to NH3 for biomass formation.
Concluding remarks
We have shown that a distinct microbial community is attached to oil. Many primary hydrocarbon degraders, especially among the Gamma- and Alphaproteobacteria, were found to be biased towards oil attachment. Hydrogenotrophs also appeared to attach to oil, suggesting that the oil phase is a source of hydrogen. Although oil degradation in the Enermark field is anaerobic, we would like to note with respect to aerobic hydrocarbon degradation that O2, like hydrogen, is more soluble in oil than in water, providing an additional incentive for some aerobic hydrocarbon degraders to associate with oil.
Concerning future research, it is important to note that the composition of oil has been reported to influence microbial adhesion to it. In particular, the presence of asphaltenes and resins may decrease affinity of microbial cells to hydrocarbons, whereas the presence of toluene may lead to its increase (Zoueki et al. 2010). Therefore, analysis of the oil composition in future experiments may help explain possible differences in the compositions of the OC and the AC from different oil fields.
References
Abbasnezhad H, Gray MR, Foght JM (2008) Two different mechanisms for adhesion of Gram-negative bacterium, Pseudomonas fluorescens LP6a, to an oil–water interface. Colloid Surface B 62:36–41
Akasaka H, Ueki A, Hanada S, Kamagata Y, Ueki K (2003) Propionicimonas paludicola gen. nov., sp. nov., a novel facultatively anaerobic Gram-positive, propionate-producing bacterium isolated from plant residue in irrigated rice field soil. Int J Syst Evol Microbiol 53:1991–1998
Al-Awadhi H, Sulaiman RH, Mahmoud HM, Radwan SS (2007) Alkaliphilic and halophilic hydrocarbon-utilizing bacteria from Kuwaiti coasts of the Arabian Gulf. Appl Microbiol Biot 77:183–186
Andreoni V, Bernasconi S, Colombo M, van Beilen JB, Cavalca L (2000) Detection of genes for alkane and naphthalene catabolism in Rhodococcus sp. strain 1BN. Environ Microbiol 2:572–577
Bae H-S, Moe WM, Yan J, Tiago I, da Costa MS, Rainey FA (2006) Propionicicella superfundia gen. nov., sp. nov., a chlorosolvent-tolerant propionate-forming, facultative anaerobic bacterium isolated from contaminated groundwater. Syst Appl Microbiol 29:404–413
Banat IM (1995) Biosurfactants production and possible uses in microbial enhanced oil recovery and oil pollution remediation: a review. Bioresource Technol 51:1–12
Beckmann S, Lueders T, Krüger M, von Netzer F, Engelen B, Cypionka H (2011) Acetogens and acetoclastic Methanosarcinales govern methane formation in abandoned coal mines. Appl Environ Microb 77(11):3749–3756
Bryant RS, Burchfield TE (1989) Review of microbial technology for improving oil recovery. SPE Reservoir Eng 4:151–154
Cai H-Y, Shaw JM, Chung KH (2001) Hydrogen solubility measurements in heavy oil and bitumen cuts. Fuel 80:1055–1063
Chaîneau CH, Morel J, Dupont J, Bury E, Oudot J (1999) Comparison of the fuel oil biodegradation potential of hydrocarbon-assimilating microorganisms isolated from a temperate agricultural soil. Sci Total Environ 227(2–3):237–247
Cornish-Shartau SL, Yurkiw M, Lin S et al (2010) Ammonium concentrations in produced waters from a mesothermic oil field subjected to nitrate injection decrease through formation of denitrifying biomass and anammox activity. Appl Environ Microbiol 76:4977–4987
Dahle H, Birkeland N-K (2006) Thermovirga lienii gen. nov., sp. nov., a novel moderately thermophilic, anaerobic, amino-acid-degrading bacterium isolated from a North Sea oil well. Int J Syst Evol Micr 56:1539–1545
Dong C, Lai Q, Chen L, Sun F, Shao Z, Yu Z (2010) Oceanibaculum pacificum sp. nov., isolated from hydrothermal field sediment of the south-west Pacific Ocean. Int J Syst Evol Micr 60:219–222
Fitch WM (1971) Toward defining the course of evolution: Minimum change for a specific tree topology. Syst Zool 20:406–416
Gentleman R (2008) Bioinformatics with R. Chapman & Hall/CRC, Boca Raton
Gieg LM, Duncan KE, Suflita JM (2008) Bioenergy production via microbial conversion of residual oil to natural gas. Appl Environ Microbiol 74:3022–3029
Grabowski A, Nercessian O, Fayolle F, Blanchet D, Jeanthon C (2005a) Microbial diversity in production waters of a low-temperature biodegraded oil reservoir. FEMS Microbiol Ecol 54:427–443
Grabowski A, Tindall BJ, Bardin V, Blanchet D, Jeanthon C (2005b) Petrimonas sulfuriphila gen. nov., sp. nov., a mesophilic fermentative bacterium isolated from a biodegraded oil reservoir. Int J Syst Evol Micr 55:1113–1121
Green MS, Flanegan KC, Gilcrease PC (2008) Characterization of a methanogenic consortium enriched from a coalbed methane well in the Powder River Basin, USA. Int J Coal Geol 76:34–45
Hamady M, Lozupone C, Knight R (2010) Fast UniFrac: facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J 4:17–27
Head IM, Jones DM, Larter SR (2003) Biological activity in the deep subsurface and the origin of heavy oil. Nature 426:344–352
Huse S, Huber J, Morrison H, Sogin M, Welch D (2007) Accuracy and quality of massively parallel DNA pyrosequencing. Genome Biol 8(7):R143
Huson DH, Auch AF, Qi J, Schuster SC (2007a) MEGAN analysis of metagenomic data. Genome Res 17:377–386
Huson DH, Richter DC, Rausch C, Dezulian T, Franz M, Rupp R (2007b) Dendroscope: An interactive viewer for large phylogenetic trees. BMC Bioinformatics 8(1):460
Kang Z, Yeung A, Foght JM, Gray MR (2008) Hydrophobic bacteria at the hexadecane–water interface: examination of micrometre-scale interfacial properties. Colloid Surface B 67:59–66
Kendall M (1938) A new measure of rank correlation. Biometrica 30(1–2):81–89
Lai MC, Chen SC, Shu CM, Chiou MS, Wang CC, Chuang MJ, Hong TY, Liu CC, Lai LJ, Hua JJ (2002) Methanocalculus taiwanensis sp. nov., isolated from an estuarine environment. Int J Syst Evol Microbiol 52:1799–1806
Lai MC, Lin CC, Yu PH, Huang YF, Chen SC (2004) Methanocalculus chunghsingensis sp. nov., isolated from an estuary and a marine fishpond in Taiwan. Int J Syst Evol Microbiol 54:183–189
Lai Q, Yuan J, Wu C, Shao Z (2009) Oceanibaculum indicum gen. nov., sp. nov., isolated from deep seawater of the Indian Ocean. Int J Syst Evol Microbiol 59:1733–1737
Li Q, Kang C, Wang H, Liu C, Zhang C (2002) Application of microbial enhanced oil recovery technique to Daqing oil field. Biochem Eng J 11:197–199
Liang Q, Lloyd-Jones G (2010) Sphingobium scionense sp. nov., an aromatic hydrocarbon-degrading bacterium isolated from contaminated sawmill soil. Int J Syst Evol Micr 60:413–416
Lomans BP, Maas R, Luderer R, Op den Camp HJM, Pol A, van der Drift C, Vogels GD (1999) Isolation and characterization of Methanomethylovorans hollandica gen nov, sp nov, isolated from freshwater sediment, a methylotrophic methanogen able to grow on dimethyl sulfide and methanethiol. Appl Environ Microbiol 65(8):3641–3650
Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235
Marmur J (1961) A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol 3:208–218
McInerney MJ, Nagle DP, Knapp RM (2005) Microbially enhanced oil recovery: past, present, and future. In: Ollivier B, Magot M (eds) Petroleum microbiology. ASM Press, Washington, pp 215–237
Mikucki JA, Liu Y, Delwiche M, Colwell FS, Boone DR (2003) Isolation of a methanogen from deep marine sediments that contain methane hydrates and description of Methanoculleus submarinus sp nov. Appl Environ Microbiol 69(6):3311–3316
Planckaert M (2005) Oil reservoirs and oil production. In: Ollivier B, Magot M (eds) Petroleum microbiology. ASM Press, Washington, pp 3–19
Pruesse E, Quast C, Knittel K, Fuchs B, Ludwig W, Peplies J, Glöckner FO (2007) SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35:7188–7196
Reinhold-Hurek B, Hurek T (2006) The Genera Azoarcus, Azovibrio, Azospira and Azonexus. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The Prokaryotes, vol 5. Springer, New York, pp 873–891
Rosenberg M (2006) Microbial adhesion to hydrocarbons: twenty-five years of doing MATH. FEMS Microbiol Lett 262:129–134
Rosenberg M, Gutnick D, Rosenberg E (1980) Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett 9:29–33
Schloss PD, Westcott SL (2011) Assessing and improving methods used in operational taxonomic unit-based approaches for 16S rRNA gene sequence analysis. Appl Environ Microb 77(10):3219–3226
Schloss PD, Westcott SL, Ryabin T et al (2009) Introducing Mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microb 75:7537–7541
Tan T, Wang B, Shao Z (2009) Donghicola xiamenensis sp. nov., a marine bacterium isolated from seawater of the Taiwan Strait in China. Int J Syst Evol Micr 59:1143–1147
Teramoto M, Suzuki M, Hatmanti A, Harayama S (2010) The potential of Cycloclasticus and Altererythrobacter strains for use in bioremediation of petroleum-aromatic-contaminated tropical marine environments. J Biosci Bioeng 110(1):48–52
Van der Mei HC, Van de Belt-Gritter B, Busscher HJ (1995) Implications of microbial adhesion to hydrocarbons for evaluating cell surface hydrophobicity. Colloids Surf B 5:117–126
Voordouw G, Grigoryan AA, Lambo A et al (2009) Sulfide remediation by pulsed injection of nitrate into a low temperature Canadian heavy oil reservoir. Environ Sci Technol 43(24):9512–9518
Yakimov MM, Genovese M, Denaro R (2010) Thalassolituus. In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology. Springer, Berlin, pp 1765–1772
Youssef N, Simpson DR, Duncan KE, McInerney MJ, Folmsbee M, Fincher T, Knapp RM (2007) In situ biosurfactant production by Bacillus strains injected into a limestone petroleum reservoir. Appl Environ Microb 73(4):1239–1247
Yue JC, Clayton MK (2005) A similarity measure based on species proportions. Commun Stat A -Theor 34:2123–2131
Zengler K, Richnow HH, Rosselló-Mora R, Michaelis W, Widdel F (1999) Methane formation from long-chain alkanes by anaerobic microorganisms. Nature 401:266–269
Zhang X, Yue S, Zhong H, Hua W, Chen R, Cao Y, Zhao L (2011) A diverse bacterial community in an anoxic quinoline-degrading bioreactor determined by using pyrosequencing and clone library analysis. Appl Microbiol Biotechnol 91:425–434
Zhao H-P, Wang L, Ren J-R, Li Z, Li M, Gao H-W (2008) Isolation and characterization of phenanthrene-degrading strains Sphingomonas sp. ZP1 and Tistrella sp. ZP5. J Hazard Mater 152:1293–1300
Zoueki CW, Ghoshal S, Tufenkji N (2010) Bacterial adhesion to hydrocarbons: role of asphaltenes and resins. Colloids Surf B 79:219–226
Acknowledgments
This work was supported by funding from Genome Canada, Genome Alberta, the Government of Alberta, Genome BC and a Natural Sciences and Engineering Research Council (NSERC) Industrial Research Chair Award to GV. The latter was also supported by Baker Hughes Canada, Commercial Microbiology Ltd., the Computer Modelling Group Ltd., ConocoPhillips Co., YPF, Saudi Aramco, Shell Canada Ltd., Suncor Energy Developments Inc. and Yara. We thank Ryan Ertmoed from Baker Hughes Canada for providing samples of produced oil–water mixtures, Dr. Sean Caffrey for organizational efforts and recommendations on the applications of statistical methods and Drs. Indranil Chatterjee and Rhonda Clark for their coordinating contributions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kryachko, Y., Dong, X., Sensen, C.W. et al. Compositions of microbial communities associated with oil and water in a mesothermic oil field. Antonie van Leeuwenhoek 101, 493–506 (2012). https://doi.org/10.1007/s10482-011-9658-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-011-9658-y