Abstract
Genome shuffling is a recent development in microbiology. The advantage of this technique is that genetic changes can be made in a microorganism without knowing its genetic background. Genome shuffling was applied to the marine derived bacterium Nocardia sp. ALAA 2000 to achieve rapid improvement of ayamycin production. The initial mutant population was generated by treatment with ethyl methane sulfonate (EMS) combined with UV irradiation of the spores, resulting in an improved population (AL/11, AL/136, AL/213 and AL/277) producing tenfold (150 μg/ml) more ayamycin than the original strain. These mutants were used as the starting strains for three rounds of genome shuffling and after each round improved strains were screened and selected based on their ayamycin productivity. The population after three rounds of genome shuffling exhibited an improved ayamycin yield. Strain F3/22 yielded 285 μg/ml of ayamycin, which was 19-fold higher than that of the initial strain and 1.9-fold higher than the mutants used as the starting point for genome shuffling. We evaluated the genetic effect of UV + EMS-mutagenesis and three rounds of genome shuffling on the nucleotide sequence by random amplified polymorphic DNA (RAPD) analysis. Many differences were noticed in mutant and recombinant strains compared to the wild type strain. These differences in RAPD profiles confirmed the presence of genetic variations in the Nocardia genome after mutagenesis and genome shuffling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past 30–40 years, marine organisms have been a focus of worldwide efforts targeting the discovery of novel natural products (Anada et al. 2006). Marine microorganisms (Actinobacteria) are sources of novel compounds with often unique structures and various potential therapeutic applications. The Actinomycetes are widely distributed in natural and manmade environments and are well known as a rich source of antibiotics and bioactive molecules and these have been of considerable importance in chemotherapy (Mirabelli et al. 1985; Kanzaki et al. 2000). Nocardia species, which are known antibiotic producers, are one of the most promising sources of bioactive compounds. Of the sixteen Actinomycete strains that were tested for their potential to produce bioactive compounds that inhibit the growth of the pathogen Vibrio damsela, Nocardia brasiliensis showed the largest inhibition zone and highest activity (El-Sersy and Abou-Elela 2006). In our previous work, the marine derived bacterium Nocardia sp. ALAA 2000 was identified as a producer of a novel antimicrobial agent ayamycin; 1,1-dichloro-4-ethyl-5-(4-nitro-phenyl)-hexan-2-one, which is unique in containing both chlorination and a rarely observed nitro group (El-Gendy et al. 2008). The structure of ayamycin (Fig. 1) was elucidated by single crystal X-ray diffraction studies. This compound displayed potent antimicrobial activity against both Gram-positive and Gram-negative bacteria as well as fungi with MIC ranging from 0.1 to 10 mg/ml (El-Gendy et al. 2008).
X-ray crystal structure (a) and chemical structure (b) of ayamycin (El-Gendy et al. 2008)
Classical strain improvement methods have succeeded in obtaining many industrial strains, but are time-consuming and laborious due to the need for many repeated rounds of random mutation and selection. Recently, an efficient technology named genome shuffling has advanced the construction of mutants with significantly improved phenotypes (Zhang et al. 2002). Genome shuffling involves the generation of mutant strains that have an improved phenotype, followed by multiple rounds of mating among the mutants to allow recombination between genomes; this is followed by selection to combine the useful alleles of many parental strains into single cells showing the desired phenotype (Zhang et al. 2002; Patnaik et al. 2002; Hida et al. 2007).
Recent studies have successfully demonstrated increased production of the polyketide antibiotic tylosin in Streptomyces fradiae (Zhang et al. 2002) and improved acid tolerance in Lactobacillus (Patnaik et al. 2002) using genome shuffling. This approach has also been used to improve the degradation of pentachlorophenol in Sphingobium chlorophenolicum (MingHua and Shelley 2004) and the production of hydroxycitric acid in Streptomyces (Hida et al. 2007). Moreover, Wang et al. (2007) and Lei et al. (2008) applied genome shuffling to improve the acid tolerance and volumetric productivity in Lactobacillus rhamnosus. A library of shuffled bacteria with genetic changes is repeatedly used in the above process. Where limited knowledge about genome sequences impairs the rational application of recombinant DNA techniques when manipulating strains, genome shuffling has the advantage of recombination between genomes in uncharacterized organisms.
The random amplified polymorphic DNA (RAPD) technique is based on the polymerase chain reaction (PCR) and has been a commonly used molecular technique for the development of DNA markers. Random amplified polymorphic DNA markers are amplification products of DNA sequences using short and arbitrary oligonucleotide primers and do not require prior knowledge of a DNA sequence. Unlike other techniques, which are labour-intensive when they are used for identification of organisms in multiple microbiological samples, the RAPD technique is fast and reliable. Low cost, efficiency in developing a large number of DNA markers in a short time and a requirement for less sophisticated equipment has made the RAPD technique very valuable (Cocconcelli et al. 1995; Williams et al. 1990).
In the present study, we applied genome shuffling to the marine derived bacterium Nocardia sp. ALAA 2000 to achieve rapid improvement of ayamycin production. Moreover, molecular analysis of the genetic effect of UV + EMS-mutagenesis and genome shuffling on nucleotide sequence by RAPD analysis was also carried out.
Materials and methods
Microorganisms
The marine bacterium Nocardia sp. ALAA 2000 was derived from the marine red alga Laurencia spectabilis collected off the Ras-Gharib coast of the Red Sea, Egypt and is the producer of of ayamycin. This species was used as the initial strain in subsequent genome shuffling (El-Gendy et al. 2008). Escherichia coli JM 109 carrying plasmid (PCBH1-hph-2.2) which contains an ampicillin resistance gene (EL-Bondkly 2002), E. coli NRRL B-766, Micrococcus luteus NRRL 287 and Bacillus subtilis NRRL B-543 were used as indicator strains.
Preparation of the starting mutants for genome shuffling
Nocardia sp. ALAA 2000 was used as the initial strain and mutagenized with UV irradiation and ethyl methane sulfonate (EMS) to obtain an initial mutant library as follows: 10 ml sample of spore suspension (slants 8 days old) in sterilized 0.2 M potassium phosphate buffer, pH 7.0, was transferred to an aseptic plate. The plate, with the cover removed, was exposed to UV irradiation for 3 min at a distance of 25 cm from a UV lamp (Philips T–UV–30 W lamp type number 57413). Then 50 μl/ml EMS (Sigma Co.) was added into the suspension and the sample shaken for 30 min. at 28°C in shaker water bath. The mutagenized suspension was diluted and spread onto agar culture medium and cultured at 35°C for 8 days. The agar medium contained (per liter of 100% Sea water) 10 g starch, 2 g peptone, 4 g yeast extract and 20 g agar and the pH was adjusted to 7.0–7.5.
Single colonies that grew on the plates were transferred into a 250 ml Erlenmeyer flask with 50 ml fermentation medium. The medium contained starch 1.0%, glucose 1.0%, glycerol 1.0%, corn steep powder 0.25%, peptone 0.5%, yeast extract 0.2%, NaNO3 0.1% and CaCO3 0.3% in 100% sea water, and the pH was adjusted to 7.2 before autoclaving. The culture was incubated at 35°C for 7 days on a rotary shaker (180 rpm) (El-Gendy et al. 2008). After fermentation, the ayamycin yield of each colony was analyzed by a diffusion bioassay test using various Gram-positive and Gram-negative target strains. The mutants with high productivity were preserved and selected as the starting strains for the genome shuffling.
Antibiotic bioassay
The indicator strains were sensitive to ayamycin and completely resistant to the other bioactive secondary metabolites (chrysophanol 8-methyl ether, asphodelin; 4,7′-bichrysophanol, justicidin B) which are produced by Nocardia sp. ALAA 2000 (El-Gendy et al. 2008). Consequently, the ayamycin antibiotic bioassay was carried out according to the paper-disc diffusion method using the fermentation broth of mutant or genome shuffled isolates. Indicator bacteria were grown in Luria Broth (LB) medium contains (g/l) tryptone, 10; yeast extract, 5; NaCl, 10; Agar, 15 with the pH adjusted to pH 7.0. Antibiotic assay discs (Whatman product No. 2017, six mm diameter) were saturated with 25 μl of each isolate supernatant and placed on the surface of indicator test plates then incubated overnight at 37°C. The inhibition zone diameter was measured in mm and the antibiotic concentration (μg/ml) was determined using a standard calibration curve based on the purified antibiotic (ayamycin). Ayamycin production in mutant or fusant isolates was compared to the original strain.
Application of the genome shuffling technique
Genome shuffling was carried out using modifications of the described methods (Zhang et al. 2002; Patnaik et al. 2002; MingHua and Shelley 2004). The starting mutants for genome shuffling were grown in 25 ml of culture medium containing 1.0% glycine in a 250 ml Erlenmeyer flask at 35°C for 36 h. Cells were harvested by centrifugation at 5000 rpm for 10 min at 4°C, washed twice with 10 ml of P buffer (Hopwood et al. 1985; EL-Bondkly and El-Gendy 2010) and treated with lysozyme (5 mg/ml in P buffer) at 30°C for 1.5 h. After protoplast formation had been observed by a phase-contrast microscopy, the lysozyme was removed by washing twice with 10 ml of P buffer and equal ratio from each highest ayamycin isolate protoplasts pooled all of them together as well as protoplasts fused for 5 min in 5 ml of 40% PEG 4000. Then the suspension was diluted fivefold with P buffer, the protoplasts were harvested by centrifugation at 3000 rpm for 5 min at 20°C and resuspended in 10 ml of P buffer. Next the suspension was diluted and spread onto regeneration medium and cultured at 35°C for 3–8 days. The regeneration medium contained (per liter) 100 g sucrose, 10 g glucose, 5 g yeast extract, 0.1 g peptone, 10 g MgCl2·6H2O, 0.25 g KH2PO4, 3 g CaCl2·2H2O, 20 ml N-Tris (hydroxymethyl) methyl-2-aminoethanesulfonic acid (TES) buffer (5.73%, adjusted to pH 7.2) (Hopwood et al. 1985) and 20 g agar. The pH value was adjusted to 6.5 before autoclaving. Colonies that grew on the regeneration medium were selected for further fermentation tests and the recombinants with the highest production were used as the starter strains for the second and third rounds of genome shuffling by the same method.
Genomic DNA preparation
Genomic DNA was extracted and purified using the QIAGEN DNeasy Tissue Kit following the manufacturer’s protocol for Gram-positive bacteria.
Random primers and polymerase chain reaction (PCR) amplification conditions
Polymerase chain reaction was performed using puReTaqTM Ready-To-GoTM PCR Beads (Amersham Biosciences). Each bead contains all of the necessary reagents, except primer and DNA template, for performing a 25 μl PCR amplification reactions. Table 1 presents the five different arbitrary primers (10-mers) that were used in the present study and supplied by Operon Technologies Company, Netherlands. To each Ready-To-Go PCR bead, 15 ng of the used random primer and 50 ng of the purified DNA sample were added. The total volume of the amplification reaction was made up to 25 μl using sterile distilled water. The conditions for this PCR were: initial denaturation (5 min at 94°C) followed by 45 cycles of primer annealing (40 s at 50°C), primer extension (90 s at 72°C) and denaturation (40 s at 94°C), a final primer annealing (1 min at 42°C) and a final extension phase (5 min at 72°C). The amplified DNA products from the RAPD amplification were separated by electrophoresis on a 1% Tris–borate–EDTA (TBE) agarose gel (1% agarose, 8.9 mM Tris, 8.9 mM borate, 0.2 mM EDTA), stained with ethidium bromide and visualized under UV illumination. The different band sizes were determined against a 100 bp ladder and photographed using a Polaroid Instant Camera with UV Transilluminator.
Results and discussion
Induction of genetic variations and selection of starting mutants for genome shuffling
Genome shuffling accelerates directed evolution by facilitating recombination within a diverse mutant population. Utilization of this method thus requires, as a starting point, a diverse population of bacterial mutants that already show some improvement in the trait of interest compared with the same trait in the wild type strain (Hida et al. 2007; Xu et al. 2009). To fulfill this need, genetic variability was introduced using ultraviolet irradiation (UV) and EMS mutagenesis. In spite of the fact that induction of mutations is occasionally associated with appearance of morphological differences and nutritional requirements compared with the parental strain, a multistep selection procedure was adopted. This method is favored not only for the selection of high productivity strains, but also has some practical advantages such as stability, fast growth, and good sporulation that are needed throughout the breeding programme. Therefore either large initial population diversity or original strains with superior performance need to be used to start genome shuffling.
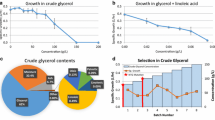
In this work, the marine Nocardia sp. ALAA 2000 was used as the initial strain and its production of ayamycin in a shaking flask culture was only 15 μg/ml. Consequently, we generated an initial library of marine Nocardia sp. ALAA 2000 mutants and selected mutants that showed a higher yield of ayamycin than the wild type strain. UV irradiation and EMS were used to generate populations of mutants of Nocardia sp. ALAA 2000. After UV irradiation and EMS mutation, 338 colonies were randomly selected and examined for ayamycin production by shake-flask fermentation test and antibiotic assay. Results are presented in Fig. 2, and after application of UV irradiation and EMS to generate population of mutants, the mutants formed four ranges. A total of 110 mutants (32.5%) had lost completely their ability to produce ayamycin (class A). On the other hand, 135 mutants (39.9%) produced as much or more ayamycin than original strain (ranging from 10 to 50 μg/ml, class B) and 78 mutants (23.1%) produced ayamycin in titres ranging from 51 to 100 μg/ml (class C). Furthermore, 15 mutants (4.4%, class D) produced a much higher yield of ayamycin (101–150 μg/ml) than the original strain. Amongst these were seven mutants with the highest ayamycin production (AL/11, AL/28, AL/136, AL/178, AL/213, AL/277and AL/332) which were selected, and their ayamycin yields were quantified in triplicate to be 150, 125, 150, 130, 150, 150 and 145 μg/ml, Fig. 3. Four mutants (AL/11, AL/136, AL/213 and AL/277) produced tenfold more ayamycin than the original strain. Consequently, these were selected as the starting population for genome shuffling.
Improvement of ayamycin production by genome shuffling
Four mutants (AL/11, AL/136, AL/213 and AL/277) were subjected to three rounds of protoplast fusion. Genome shuffling amplifies genetic diversity by homologous recombination using protoplast fusion within the selected mutant population. The selected four mutants of our starting population showed different growth rates and morphological characters; therefore we prepared individual protoplasts, counted their numbers with a compound light microscope, and pooled all of them together in 1:1 ratio.
All of the single colonies that appeared on the regeneration medium after genome shuffling were selected for fermentation analysis. In the first round of genome shuffling, 135 colonies tested for ayamycin production and all produced more ayamycin than the original strain. The values ranged from 51 to 200 μg/ml ayamycin (classes C, D and E, Fig. 2). Five recombinants (F1/27, F1/53, F1/87, F1/95, and F1/121) exhibited a further improvement in the yield of ayamycin (195, 180, 180, 190, and 195 μg/ml, respectively, Fig. 3). These recombinants were then used as the starter strains for the second round of genome shuffling.
The second round of genome shuffling produced 115 colonies that were assayed for ayamycin production. As shown in Fig. 2 the results indicated that, 7 isolates (6.1%), 27 isolates (23.5%), 44 isolates (38.3%) and 37 isolates (32.2%) of the tested isolates produced ayamycin within the ranges of 51–100, 101–150, 151–200 and 201–250 μg/ml, respectively (class C, D, E and F, respectively, Fig. 2). In total, five of these recombinants (F2/13, F2/33, F2/47, F2/72 and F2/101) showed a further improvement in the yield of ayamycin (235, 245, 250, 245 and 250 μg/ml, respectively, Fig. 3). These were subjected to a third round of genome shuffling producing 85 colonies that were assayed. The obtained results showed that, 3 (3.5%), 21 (24.7%), 28 (32.9%) and 33 isolates (38.8%) exhibited ayamycin productivity within the ranges of 101–150, 151–200, 201–250 and 251–300 μg/ml (class D, E, F and G, respectively, Fig. 2). Three recombinants (F3/22, F3/42, and F3/65) had a further improvement in yield of ayamycin (285, 275, and 275 μg/ml, respectively, Fig. 3) over the second round. Strain F3/22 yielded 285 μg/ml of ayamycin, which is 19-fold higher than that of the initial strain and 1.9-fold higher than that of initial starting parent mutants AL/11, AL/136, AL/213 and AL/277 with a yield of 150 μg/ml.
The results illustrates that genome shuffling is an efficient means by which organisms can be obtained with improved traits. Genome shuffling is based on genetic recombination without knowledge of detailed genetic information. The different genes, which may be associated with production, can be recombined over several rounds of genome shuffling and the desirable phenotypes can be selected (Hida et al. 2007; Xu et al. 2009; Gong et al. 2009; Zhanga et al. 2010). The recombinants obtained after genome shuffling show substantial improvement in ayamycin production. Moreover, the genetic stability of the highest ayamycin productivity recombinant F3/22 was evaluated by twenty-five successive subcultivation tests. The yield of ayamycin among the twenty-five generations ranged from 280 to 285 μg/ml which indicate the hereditary characteristics of this high ayamycin-producing recombinant F3/22 strain are stable.
Random amplified polymorphic DNA (RAPD) analysis
Random amplified polymorphic DNA is a PCR-based system that can be used to provide DNA fingerprints to identify differences between strains and species. The process uses single primers of arbitrary sequence, usually ten bases long, and these can be used on any DNA target irrespective of any knowledge of its DNA sequence. This offers a substantial time saving as DNA regions need not be identified, cloned, and sequenced before these use a DNA markers. The genetic effect of the mutagenic treatment and protoplast fusion on the DNA nucleotide sequence of the mutants and protoplast fusants was compared to the original strain Nocardia sp. ALAA 2000 by RAPD analysis. Five primers were used (Table 1) with a GC content 60–70% to amplify genomic DNA fragments with reproducible polymorphisms suitable for strain differentiation in the marine Nocardia sp. isolates. Of the total amplified products, product size ranged from 225 to 2000 bp; with the highest number of bands (85) within the 250–550 bp range, followed by 64 bands within the 700–2000 bp range. Primers OpB-13 and OpI-02 with GC content 70% were found to produce more polymorphic bands than the other primers and was used to obtain RAPD profiles of the selected marine Nocardia sp. isolates (Figs. 2, 3).
Nine amplified bands could be clearly observed when DNA from the original strain was used as a template with primer OpB-13 (Fig. 4a). The band sizes were 2000, 1100, 700, 650, 500, 475, 450, 425 and 225 bp as shown in lane 4. Furthermore, the first band 2000 bp did not appear in mutants AL/11 and AL/213 (lane 5 and 6) and derived strain F1/27 (lane 3) and the second band (1100 bp) was not detected in the mutant AL/11 (lane 5). On the other hand, one mutant (AL/213) and three derived strains (F1/27, F2/47 and F3/22) contained a new band with a size of 1500 bp (lanes 6, 3, 2 and 1) which was not present in the original strain or parental strains. In addition, two new amplified bands of 550 and 325 bp in size were detected with all tested isolates. Using the primer OpI-02 (Fig. 4b), it was clearly noticed that most of the tested mutants and derived strains contained two distinct amplified bands (375 and 325 bp) that were identical to the original strain. Moreover, all mutants and derived strains presented with different banding patterns, when compared with the original strain Nocardia sp. ALAA 2000. The above differences in RAPD profiles confirm that genetic variation had been obtained in the Nocardia sp. ALAA 2000 genome after the UV-mutagensis and three rounds of protoplast fusion. Furthermore, some of these differences, based on the RAPD technique, could be used as genetic markers for an analysis of genetic diversity relative to ayamycin productivity.
Conclusion
The successful application of genome shuffling was demonstrated by showing an improvement in ayamycin production by the marine derived Nocardia sp. ALAA 2000. Isolation of mutants by treatment of EMS combined with UV contributed to an initial improvement in ayamycin production and these high producing mutants were used as a starting point for three rounds of genome shuffling. Genome shuffling was able to achieve a further increase in ayamycin production, with strain F3/22 yielding 285 μg/ml ayamycin (19- and 1.9-fold higher than that of the original strain and the highest starting mutants, respectively). Random amplified polymorphic DNA analysis identified many differences in mutant and recombinant strains compared to the wild type strain. These differences in RAPD profiles confirmed the presence of genetic variations in the Nocardia genome after mutagenesis and genome shuffling. Moreover, these differences may be useful as genetic markers when studying the effect of genetic diversity on ayamycin production.
References
Anada TP, Abdul WB, Yogesh SS, Upal R, Jay S, Siddhartha PS (2006) Antimicrobial activity of marine bacteria from the waters off the coast of South East India. Microbiol Res 161:252–262
Cocconcelli PS, Porro D, Galandini S, Senini L (1995) Development of RAPD protocol for typing of strains of lactic acid bacteria and enterococci. Lett Appl Microbiol 21:376–379
EL-Bondkly AM (2002) Genetic transformation in Trichoderma reesei for the improvement of cellulase production. Tanta University, Egypt
EL-Bondkly AM, El-Gendy MMA (2010) Keratinolytic activity from new recombinant fusant AYA2000, derived from endophytic Micromonospora strain. Can J Microbiol 56:748–760
EL-Gendy MMA, Hawas UW, Jaspars M (2008) Novel bioactive metabolites from a marine derived bacterium Nocardia sp. ALAA 2000. Antibiotics 61:379–386
El-Sersy NA, Abou-Elela MG (2006) Antagonistic effect of marine Nocardia brasiliensis against the fish pathogen Vibrio damsela: application of Plackett-Burman experimental design to evaluate factors affecting the production of the antibacterial agent. Int J Oceans Oceanogr 1:141–150
Gong J, Huijie Z, Zhijun W, Tao C, Xueming Z (2009) Genome shuffling: progress and applications for phenotype improvement. Biotechnol Adv 27:996–1005
Hida H, Yamada T, Yamada Y (2007) Genome shuffling of Streptomyces sp. U121 for improved production of hydroxycitric acid. Appl Microbiol Biotechnol 73:1387–1393
Hopwood DA, Bibb MJ, Chater KF, Kieser T, Bruton CJ, Kieser THM, Lydiate DJ, Smith CP, Ward JM, Schrempf H (1985) Genetic manipulation of Streptomyces: a laboratory manual. The John Innes Foundation, Norwich
Kanzaki H, Yanagisawa S, Nitoda T (2000) Biosynthetic intermediates of the tetradehydro cyclic dipeptide albonoursin produced by Streptomyces albulus KO-23. J Antibiot 53:1257–1264
MingHua D, Shelley DC (2004) Genome Shuffling improves degradation of the anthropogenic pesticide pentachlorophenol by Sphingobium chlorophenolicum ATCC 39723. Appl Environ Microbiol 70:2391–2397
Mirabelli CK, Bartus H, Bartus JL, Johnson R, Mong SM, Sung SP, Crooke ST (1985) Application of tissue culture microtitre test for the detection of cytotoxic agents from natural products. J Antibiot 38:758–766
Patnaik R, Louie S, Gavrilovic V, Perry K, Stemmer WPC, Ryan CM, Cardayr′e S (2002) Genome shuffling of Lactobacillus for improved acid tolerance. Nat Biotechnol 20:707–712
Wang Y, Li Y, Pei X, Yu L, Feng Y (2007) Genome-shuffling improved acid tolerance and L-lactic acid volumetric productivity in Lactobacillus rhamnosus. J Biotech 129:510–515
Williams JGK, Kubelik AR, Livak KJ, rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Xu B, Jin Z, Jin Q, Li N, Cen P (2009) Improvement of pristinamycin production by genome shuffling and medium optimization for Streptomyces Pristinaespiralis. Biotechnol Bioproc Eng 14:175–179
Yu L, Pei X, Lei T, Wang Y, feng Y (2008) Genome shuffling enhanced l-lactic acid production by improving glucose tolerance of Lactobacillus rhamnosus. J Biotechnol 134:154–159
Zhang YX, Perry K, Vinci VA, Powell K, Stemmer WPC, Cardayr′e SB (2002) Genome shuffling leads to rapid phenotypic improvement in bacteria. Nat Lett 415:644–646
Zhanga Y, Jian-Zhong L, Jun-Sheng H, Zong-Wan M (2010) Genome shuffling of Propionibacterium shermanii for improving vitamin B12 production and comparative proteome analysis. J Biotechnol 148:139–143
Acknowledgment
The authors would like to thank Prof. Marcel Jaspars (Marine Biodiscovery Centre, Department of Chemistry, University of Aberdeen, Scotland, UK) for his continuous help and moral support with respect to technical discussion as well as the revision and editing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Gendy, M.M.A., EL-Bondkly, A.M.A. Genome shuffling of marine derived bacterium Nocardia sp. ALAA 2000 for improved ayamycin production. Antonie van Leeuwenhoek 99, 773–780 (2011). https://doi.org/10.1007/s10482-011-9551-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-011-9551-8