Abstract
The apiculate yeasts are the species predominating the first stage of grape must alcoholic fermentation and are important for the production of desired volatile compounds. The aim of the present investigation was to establish a protocol for the enological selection of non-Saccharomyces strains directly isolated from a natural must fermentation during the tumultuous phase. At this scope, fifty Hanseniaspora uvarum isolates were characterized at strain level by employing a new combined PCR-based approach. One isolate representative of each identified strain was used in fermentation assays to assess strain-specific enological properties. The chemical analysis indicated that all the analyzed strains were low producers of acetic acid and hydrogen sulphide, whereas they showed fructophilic character and high glycerol production. Analysis of volatile compounds indicated that one strain could positively affect, during the alcoholic fermentation process, the taste and flavour of alcoholic beverages. The statistical evaluation of obtained results indicated that the selected autochthonous H. uvarum strain possessed physiological and technological properties which satisfy the criteria indicated for non-Saccharomyces wine yeasts selection. Our data suggest that the described protocol could be advantageously applied for the selection of non-Saccharomyces strains suitable for the formulation of mixed or sequential starters together with Saccharomyces cerevisiae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wine is the product derived from a complex of biological and biochemical interactions between grapes (grape must) and diverse micro organisms, such as fungi, yeasts and bacteria (Fleet 2003). Among the micro organisms involved, yeasts play the most important role, because they not only perform the alcoholic fermentation (AF), i.e. the conversion of grape sugar to ethanol and CO2 but they also influence wine flavour and quality by the production and excretion of metabolites during their growth and subsequent autolysis (Fleet 1993, 2003; Lambrechts and Pretorius 2000; Romano et al. 2003; Jolly et al. 2006). In its early stages, the AF is promoted by the action of large number of yeasts belonging to non-Saccharomyces species characterized by a low fermentative power (Heard and Fleet 1985), whereas the final stage is dominated by alcohol-tolerant Saccharomyces cerevisiae strains (Fleet and Heard 1993). The role of non-Saccharomyces species during the AF is very important, since they strongly influence the chemical composition of the final product, suggesting that these autochthonous yeasts give distinct regional and other attractive characteristics to wines (Fleet 1990; Heard 1999; Romano et al. 2003).
In recent years, winemakers and microbiologists have realized that non-Saccharomyces yeasts contribute to the analytical composition and the sensorial characteristics of wine, thus increasing interest in the industrial application of the above yeast species (Charoenchai et al. 1997; Fernandez et al. 1999; Fleet 2003; Ciani et al. 2006). In fact, the addition of non-Saccharomyces yeast species as part of mixed starter formulations, together with S. cerevisiae, has been recently indicated as a way of taking advantage of spontaneous fermentations to improve wine quality (Rojas et al. 2001; Romano et al. 2003; Ciani et al. 2006). Bearing in mind that the current tendency in the wine industry is to develop original and peculiar products, mixed industrial starters may be a good method of conferring a particular aroma and characteristics to wines (Zironi et al. 1993; Mingorance-Cazorla et al. 2003).
The apiculate yeasts, with low fermentative power, Hanseniaspora uvarum and its anamorph Kloeckera apiculata, are the yeast species most present on the external surface of grape berries and they predominate in the first stage of spontaneous AF (Fleet and Heard 1993). These yeasts are important in the production of volatile compounds in wine, and the chemical composition of wines made by Kloeckera spp./S. cerevisiae combinations differs from reference wines (Zironi et al. 1993; Gil et al. 1996; Erten 2002; Ciani et al. 2006). Previous reports indicated that several physiological properties of H. uvarum are a strain-dependent feature, such as the levels of produced ethanol (Caridi and Ramondino 1999), the low volatile acidity (Romano et al. 1992; Ciani and Maccarelli 1998) and, most of all, the production of other primary metabolites, i.e. glycerol, acetaldehyde or secondary such as ethyl acetate and hydrogen sulphide (Romano et al. 1997).
The aim of the present report was to develop and validate a procedure for the enological selection of non-Saccharomyces strains dominating the tumultuous fermentation phase, with the final goal of identifying strains with biotechnological interest and suitable for the formulation of fermentation mixed starter cultures together with Saccharomyces cerevisiae.
Materials and methods
Yeast isolates
A total of 50 Hanseniaspora uvarum isolates were isolated from a spontaneous must fermentation of “Negroamaro” grape must and previously identified at the species level as described (Bleve et al. 2006). Isolates were preserved on YEPD plates (yeast extract 1%, peptone 2%, dextrose 2%, agar 2%) at 4°C and sub cultured every 6 months or kept at −80°C in liquid YEPD medium with glycerol (50% final concentration) as a cryoprotectant. In addition, the type strain of Hanseniaspora uvarum (CBS 314, Centraalbureau voor Schimmelcultures) and a commercial Saccharomyces cerevisiae starter culture (CM) were also used. The identified strains were deposited in the ISPA Culture Collection (http://www.ispa.cnr.it/Collection) (Table 2).
PCR-based analysis
Total genomic DNA from the strains was prepared according to the method used by Cappello et al. (2004). The oligonucleotides used were purchased from PRIMM srl (Milan, Italy) and are shown in Table 1. Amplification reactions were performed in a final volume of 50 μl containing 100 ng of genomic DNA, 10 mM Tris HCl, 50 mM KCl, 1.5 mM MgCl2, 0.001% gelatine, 2 mM of each dNTP, 0.8 μM of primer and 1 U of Taq DNA polymerase (Euroclone, Italy). The thermal cycler was programmed for 40 cycles of 1 min at 94°C, 1 min at the primer-specific annealing temperature (Table 1), 2 min at 72°C with a final extension cycle of 10 min at 72°C. To avoid ambiguous results, the amplification reactions of all 50 strains were processed simultaneously from one stock solution of premixed reagents in a single PCR assay. The amplified DNA products were visualized by agarose gel electrophoresis, as previously described (Bleve et al. 2006). The amplicon profiles obtained in the above described PCR reactions were analyzed with the Gel Compar 3.1 software (Applied Math, Kortrijk, Belgium). Similarities between combined fingerprints were calculated using the DICE coefficient (Nei and Li 1979). Cluster analysis of the pair wise values was generated with the UPGMA algorithm, by the NTSYS software (Applied Biostatistic, USA).
Micro-fermentations
To evaluate strain-specific fermentation performances, the identified strains were tested by micro-fermentations in Negroamaro grape must (sugars 243 g/l, 23.6° Brix, pH 3.4, assimilable nitrogen concentration 137.38 g/l), clarified by centrifugation (10 min at 8000 g) and then sterilized by membrane filtration through Millipore system (0.45 mm membrane). One hundred milliliters of treated must were placed in sterile Erlenmeyer 150 ml flasks and then inoculated with 106/ml CFU of H. uvarum pre-cultured in the same must, for 48 h at 25°C. The kinetics of the fermentations were monitored daily by gravimetric determinations, evaluating the loss of weight due to the production of CO2. The samples were incubated at 25°C, and weighted daily to follow the weight loss caused by CO2 production. When the CO2 evolution stopped (i.e. at constant weight), the samples were directly analyzed or stored at −20°C, until required for further analysis. Each fermentation experiment was carried out by performing three simultaneous independent repetitions.
Analytical determinations
Hydrogen sulfide production was evaluated by inserting a lead acetate strip between the plug and inner wall of the Erlenmeyer, above the level of the liquid. The qualitative hydrogen sulfide production was detected by the blackening of the PbAcO paper (Martínez-Rodríguez et al. 2001) and the isolates were classified as high (+++), medium (++), low (+) and no (−) sulphide producers. Fermentation vigour (FV), fermentation rate (FR) and fermentation purity (FP) were calculated as described by Ciani and Maccarelli (1998). Alcohol yield coefficient (AYC) was calculated as the amount of ethanol formed in relationship to sugar consumed. High performance liquid chromatography (HPLC) was employed to assay contents of sugars, ethanol, glycerol and organic acids. Samples were centrifuged (5 min at 5000 g), 1:10 diluted with double distilled water and filtered through a 0.45 μm membrane. The samples were isocratically separated at 0.6 ml/min, using a Bio-Rad Aminex HPX 87H (Bio-Rad Laboratories, USA) at 50°C, with 5 mM sulphuric acid as mobile phase. All samples were analyzed in triplicate by injecting a sample volume of 20 μl. The eluting compounds were monitored by a fixed wavelength ultraviolet (UV–VIS) detector at 210 nm, which was connected in series with a refractive index (RI) detector. Glucose, fructose, glycerol and ethanol were detected by RI. Organic acids were detected by UV–VIS. The components were identified by comparison of their retention times with those of the standard compounds (Sigma, USA) and the produced peaks were quantified using external standard calibration. The major volatile constituents [acetaldehyde, ethyl acetate, 2-methyl-1-propanol, higher alcohols (3-methyl- and 2-methyl-1-butanol), acetoin] were determined by gas-chromatography according to Mallouchos et al. (2003). Samples were analyzed in triplicate by directly injecting 1 μl of filtered fermented must into a Zebron Wax Plus capillary column (30 M × 0.32 mm × 0.50 μm; Phenomenex, USA) and analyzed by the CP-3800 VARIAN using the FID detector. The temperature program was 40°C (5 min), followed by an increase of 5°C/min to 120°C and a final treatment at 25°C/min to 230°C (5 min). Injector and detector temperature were 200 and 240°C, respectively and the carrier gas was helium with a flow rate of 2.05 ml/min. The internal standard solution used was 4-methyl-1-pentanol. Stock solutions of the standards were prepared in an alcoholic solution containing 12% (v/v) ethanol and 4 g/l of tartaric acid (pH 3.2).
Enzymatic activities
Biogenic amines formation was determined, as previously described (Nikolaou et al. 2006). Yeast strains were spotted on YEPD agar plates, supplemented with 0.006% bromocresol purple and 1% of chosen amino acid. The amino acids histidine, tyrosine, phenylalanine, tryptophan, lysine, leucine and arginine were used. The plates were incubated at 25°C for 7 days and in the case of amino acid decarboxylation a purple halo appeared around the yeast colony. Screening for detecting β-glucosidase activity was carried out on agar plates to which was added arbutin as a selective substrate (Rosi et al. 1994). Extracellular protease production was determined by replica plating yeast colonies onto YPD plates containing 2% casein (BDH laboratories). The plates were incubated for 5 days at 25°C and a clear zone around the colony identified protease activity (Strauss et al. 2001). Yeasts were screened for polygalacturonase production and pectinase by a plate test where enzymatic activity was associated to a purple halo around the yeast colony after staining the plates with 0.1% Ruthenium Red (Strauss et al. 2001). Glucanase activity was determined by replica plating the yeast onto YPD plates containing either 0.2% pachyman (Sigma, USA) or 0.2% lichenan (Sigma, USA). Glucanase activity was found to be associated to a clear halo around the yeast colony after staining the plates with 0.03% Congo Red. Yeasts were screened for xylanase activity according to Strauss et al. (2001) by replica plating onto SC plates (YNB 6.7 g/l, glucose 20 g/l, agar 20 g/l) containing 0.2% Remazol Brilliant Blue Xylan (RBB-Xylan, Sigma). The plates were incubated for 5 days at 30°C. Colonies showing activity were identified by a clear zone around the colony. All the described plate assays were carried out at pH 3.5.
Statistical treatment of data
Significant differences among selected strains were determined for each chemical (Table 3) and volatile (Table 4) compound by analysis of variance (Turkey, α = 0.05). The contribute of strains was estimated by Principal Component Analysis (PCA). Statistical data processing was performed using the XLSTAT 6.0 statistical pocket.
Results
Molecular characterization
The analyzed population was composed of fifty H. uvarum isolates isolated during the first 24 h of the spontaneous fermentation of Negroamaro grape must and previously identified at species level (Grieco F., unpublished data).The molecular identification of yeast isolates was considered as the first stage of the procedure for selecting strains with desired enological properties, As second step, the characterization of the analyzed population of Hanseniaspora uvarum isolates at strain level was achieved. The discriminating power of four PCR methods, i.e. Random Amplification of Polymorphic DNA (RAPD), intron splice site polymorphism, minisatellites and microsatellites analysis, was compared to assess a method for H. uvarum intraspecies identification. For this purpose, a total number of the nine different primers were tested on the analyzed population (Table 1). The results indicated that the primers OPA1 and OPA13 (RAPD-PCR), (ATG)5 and (GTG)5 (microsatellite) and RM13 (minisatellite) generated a similar profile for almost all the strains (data not shown).
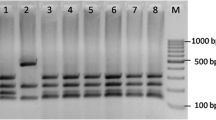
The molecular characterization at strain level was performed by means of a combination of the following PCR-based assays: (i) RAPD-PCR using the primers OPA9 and P80; (ii) minisatellite sequences evaluation, using the primer M13; (iii) analysis of the regions complementary to the intron splice sites, using the primer EI1. In our study, strain-specific patterns were generated by the combined analysis of the molecular markers produced by the OPA9, P80, M13 and EI1 primers, as indicated in Fig. 1. The dendrogram analysis of the data set was based on the presence or absence of major bands produced by the above four primers. The obtained cluster indicated that the analyzed population was composed of nine different strains, thus showing a biodiversity value equal to 18%, within the population itself (Fig. 2). The nine strains identified clustered in two main groups, branching at a similarity value of 60% (Fig. 2). The former biotype grouped four strains, whereas the latter clustered the five remaining strains. One isolate representing each of the nine identified strains was deposited in the International ISPA Collection and from here onwards they will be denoted with their accession number (Table 2).
PCR amplification profiles of genomic DNA of the identified nine Hanseniaspora uvarum strains primed with P80 (a), RM13 (b), OPA9 (c) and EI1 (d) primers. Lane 1, strain 8795; lane 2, strain 8797; lane 3, strain 8799; lane 4, strain 8801; lane 5, strain 8802; lane 6, strain 8807; lane 7, strain 8810; lane 8, strain 8811; lane 9, strain 8813; lane 10, CBS314. M, molecular weight marker Quick-Load 2-log DNA Ladder (New England Biolabs, USA)
UPGMA cluster analysis of the 50 Hanseniaspora uvarum isolates showing the nine different strains identified, based on combined RAPD-PCR, minisatellite and intron splice site fingerprints. Number of isolates with an identical profile is indicated in brackets on the side of the ISPA accession number of the strain
Fermentation performance
As third step of the proposed selection protocol, the deposited isolate representing each of the nine identified strains was used in microfermentation assays in order to evaluate strain-specific technological and enological properties (Table 2). The fermentation kinetics were similar among all the strains of H. uvarum with the exception of strain 8795, which had a rapid start of fermentation and maintained this pattern throughout the fermentation (Table 2). In fact, this strain showed a high fermentation vigour (4.8%), a parameter that takes into account the maximum amount of ethanol produced from the sugars present in the must and a good fermentation rate. The qualitative analysis of the must fermentations showed that all strains were no or low H2S-producer and moreover, with the exception of strains 8801, 8802, 8810 and 8813, they produced a fermented must significantly clear and with hardly any foam (Table 2). The 8795 strain had an interesting value of fermentation purity and showed to produce a low amount of volatile acidity (Table 2).
The results of the quantitative analysis of the main chemical compounds found in fermented musts, adopted as fourth point of the selection procedure, are shown in Table 3. As expected, all H. uvarum strains showed a fructophilic character, having a preferential consumption of fructose respect to glucose. The production of ethanol was directly proportional to the consumption of sugars, higher for the strains 8795, 8799, 8801 and 8807 (respectively 48.10, 47.32, 46.29, 43.16 g/l), while the lowest production of ethanol (30.11 g/l) was shown by strain 8813. In all fermentations, a fair amount of glycerol was also detected. This is a major secondary compound that contributes to the viscosity and “softness” of wine with a positive effect on taste, with a maximum production by the strain 8795 with 7.25 g/l. All the strains produced concentrations of acetic acid, ranging from 0.95 (strain 8811) to 0.62 g/l (8795). The quantitative analysis of other organic acids revealed that malic acid (ranging from 3.20 to 3.80 g/l) and tartaric acid (with a range of values between 3.62 and 3.87 g/l) were the most abundant, while no significant differences were observed in the amounts of lactic and citric acids (Table 3).
The strain-specific attitude to produce a number of volatile compounds involved in the wine flavour formation (acetaldehyde, ethyl acetate, 2-methyl-1-propanol, higher alcohols and acetoin) was also evaluated and the results obtained are shown in Table 4. The acetaldehyde is one of the most important carbonyl compounds produced during fermentation. All strains of H. uvarum produced a quantity of this compound, within the range between 9.1 mg/l (strain 8795) and 13.6 mg/l (strain 8801). Only free acetaldehyde has a role in flavor formation; at low levels it contributes to fruity flavors, while high concentrations (>200 mg/l) confer ‘flatness’ in wines. The ethyl acetate was detected in quantities ranging from 52.8 (strain 8797) to 151.7 mg/l (strain 8801). Ethyl acetate may contribute with pleasant, fruity fragrance to the general wine aroma at low concentrations, whereas it contributes significantly to defect aroma at a content of 150–200 mg/l (Lambrechts and Pretorius 2000). As regard isoamylic alcohols produced, the amounts detected ranged from 38.2 (strain 8813) to 73.7 mg/l (strain 8795). Since these values, for most of the wines, were below 400 mg/l, it can be considered that these compounds have positive contribution to the global wine aroma. All the H. uvarum strains under study produced a quantity of acetoin (3-hydroxy-2-butanone) within the range of 28.5 (strain 8810) to 54.1 mg/l (strain 8802). Nevertheless, as its odor threshold is quite high (150 mg/l) its sensory meaning for the global aroma is practically insignificant.
Hydrolytic enzyme production
The fifth step of the suggested procedure has assessed the ability of the analyzed strains to produce extracellular enzymes such as β-glucosidase, protease, pectinase, β-glucanase, xylanase, as well as enzymes involved in decarboxylation of amino acids. Appropriate dilutions of yeast cultures were plated on solid media containing the different substrates for the detection of the above enzymatic activities. Enzyme production assays (Table 5) revealed that none of the selected strains showed xylanase and protease activities nor the ability to decarboxylate the tested amino acids. All assayed strains showed they possessed glucanase activity, since they were able to degrade the 1,3:1,4-β-d-glucan (lichenan) but not the 1,3-β-d-glucan (pachyman). It is interesting to note that, the nine H. uvarum isolates revealed the potential to produce extracellular β-glucosidase and pectinase, with the exception of 8801 and 8810 strains, respectively (Table 5).
Data analysis
As sixth and last step of the proposed procedure, a Principal Component Analysis (PCA) was applied on the data set of concentrations of eight compounds (glucose, fructose, acetoin, acetic acid, acetaldehyde, glycerol, ethanol, isoamyl alcohols) for the nine identified strains with the aim of interpreting the different fermentative performances among the above strains (Fig. 3). Data concerning the hydrogen sulphide production (Table 2) were not included in the PCA, since they were not continuous. Along the second component, the strains were clearly grouped in 3 clusters: the first only included the 8795 strain, the higher ethanol-, glycerol- and isoamyl alcohols-producing strain; the second cluster grouped all the other eight strains, which were not distinguishable according the selected variables; the last group consisted of the H. uvarum CBS314 type strain, that was the strain characterized by the worse fermentative performances. The PCA scores plot indicated that the distribution of selected strains on the plane was clearly dependant by their fermentation behaviour, thus indicating that this statistical assay allowed the identification of the best performing strain(s).
Discussion
In the present study, we have proposed and validated a selection procedure aimed to the identification of Hanseniaspora uvarum strains dominating the tumultuous fermentation phase, which could be suitable for fermentation starter cultures preparation. The protocol consisted of the following steps: (i) yeast selection and identification at species level; (ii) yeast typing at strain level; (iii) strain evaluation by fermentation test; (iv) chemical analysis of fermented must; (v) evaluation of strain-specific enzymatic properties; (vi) statistical analysis of obtained data.
The first stage proposed enological selection protocol is required to determine the number of different isolates belonging to the yeast species selected as target of the selection procedure. The taxonomical typing of the considered isolates is performed by application of cheap molecular methods, which have already been assessed for industrial applications (Schuller et al. 2004). Numerous molecular methods have been suggested for the characterization at strain level of yeast isolates: restriction analysis of mitochondrial DNA (Comi et al. 2000), comparative analysis of the karyotype (Cardinali et al. 1995), random amplified polymorphic DNA (RAPD-PCR), analysis of microsatellite sequences (Bujdosó et al. 2001). The first two methods are extremely laborious and have not demonstrated the specificity and sensitivity owned by identification approaches based on gene amplification (Cadez et al. 2002). For the first time in our knowledge, the combined application of RAPD-PCR, microsatellite and intron splice sites (de Barros Lopes et al. 1996) analyses were used for the intraspecific analysis of wine yeasts and it allowed the identification of the different strains composing the population of H. uvarum under investigation.
The statistical analysis of the results showed a biological diversity of the population examined, i.e. 18%, since nine different strains were found out of the 50 analyzed isolates, which is lower than that shown in previously published investigations (Bujdosó et al. 2001; Cadez et al. 2002; Capece et al. 2005). However, this evidence can be easily explained by the fact that these last studies took in consideration H. uvarum strains isolated from different sources, whereas the strains considered in the present paper were all isolated from the same substrate. Indeed, the polymorphism observed among strains of H. uvarum from different geographical origins is not comparable with that observed among strains isolated from the same grape must and therefore it does not reflect the presence of several distinct populations, but simply indicates the genetic variability that can occur within a population derived from different sources.
As expected, all isolates showed levels of fermentative vigour lower than that classically described for S. cerevisiae, thus confirming similar evidence produced in other previous studies (Ciani and Maccarelli 1998; Ciani et al. 2006). However, the strain 8795 showed an interesting fermentation kinetics, revealing a good fermentative vigour significantly higher than that indicated as typical of the H. uvarum species (Ciani and Maccarelli 1998).
The production of hydrogen sulphide, highly undesired wine off-flavour, by Saccharomyces and non-Saccharomyces yeast strains was considered as an important negative trait in the selection procedure (Mendes-Ferreira et al. 2002). We evaluated strain-specific H2S production during natural grape must fermentation and the results obtained indicated that all the tested strains showed low or no production of the above unwelcome off-flavour.
The chemical analysis of the major secondary products of fermentation provided information on several parameters of technological importance in enology. Acetic acid contribution to wine bouquet becomes negative in concentrations close to its flavour threshold (0.7–1.1 g/l), whereas values between 0.2 and 0.7 g/l are likely to be considered the optimal ones (Lambrechts and Pretorius 2000). With the exception of 8807, 8810, 8811 and 8813 strains, the amount of acetic acid produced by the other H. uvarum strains was very close to the 0.7 g/l limit, indicating that they can be suitable for a possible use in mixed starter production. These data are in agreement with previous reports (Romano et al. 1992; Ciani and Maccarelli 1998), that identified low acetic acid-producing H. uvarum strains in natural grape juice. Moreover, the strain 8795 showed the highest value of alcohol yield coefficient and at the same time the lowest amount of acetic acid produced (0.62 g/l), thus indicating that acetic acid production was not correlated with the ethanol formation during fermentation (Ciani and Maccarelli 1998). Apiculate yeasts are also known to be high producers of acetic acid, making them undesirable for wine production (Ciani and Picciotti 1995). However, it has been reported that large strain variability exists and that not all strains of Kloeckera spp. form high levels of acetic acid (Romano et al. 1992). The data here reported confirmed these evidences, since most of the selected strains and, in particular, the 8795 strain produces less than 1 g/l of acetic acid.
All isolates showed a significant production of glycerol, in particular the strains 8795 (7.25 g/l) and 8797 (6.84 g/l), above the average that makes it possible to taste the “softness” in the wine (5.2 g/l; Noble and Bursick 1984). The gas chromatographic quantification of main volatile compounds in fermented must was then performed as further point in the selection procedure to determine the possible influence of the H. uvarum strains on the formation of the wine flavour (Suomalainen 1971).
Ethyl acetate, produced by the characterized H. uvarum strains, was found in amounts ranging between 52.8 and 151.7 mg/l, range within which this compound contributes positively to the aroma of the wine (Rapp and Mandery 1986). Different non-Saccharomyces yeasts yielded different levels of higher alcohols, that at low concentrations are desired for their addition to wine complexity (Romano et al. 1992). The here described H. uvarum strains produced amounts of higher alcohols ranging from 38.2 to 73.7 mg/l further indicating that they can potentially contribute to the quality of wine (Lambrechts and Pretorius 2000). Non-Saccharomyces yeasts substantially contribute to the formation of the flavouring in wines and a parameter that makes it possible to evaluate this contribution is the ratio between the ethyl acetate and higher alcohols concentrations (E/A; Mateo et al. 1991). The values of E/A obtained are greater than 1 for all selected strains, confirming that they can potentially make a significant role in the formation of wine aroma. The acetoin is another compound involved in the formation of wine bouquet, which is produced by apiculate yeasts with a threshold taste value around 50 mg/l (Romano et al. 1993; Romano and Suzzi 1996). It is interesting to note that the acetoin levels produced by the strains under study are lower than those described by other studies on H. uvarum (Ciani and Maccarelli 1998; Mingorance-Cazorla et al. 2003).
As fifth step in the selection procedures, the potential enzymatic power of the H. uvarum strains was evaluated by performing different physiological tests, which have been shown to enable identification of non-Saccharomyces yeasts able to produce useful extracellular enzymes during wine fermentation (Charoenchai et al. 1997; Strauss et al. 2001). All the analyzed strains did not show the capacity to decarboxylate several amino acids, thus indicating that the above isolates do not produce biogenic amines. The characterization of yeast strains for the production of biogenic amines has strong applicative importance for the selection of starter cultures, which can contribute to the protection of consumer health (Caruso et al. 2002). This study has also revealed the ability of our nine H. uvarum strains to produce extracellular enzymes with enological significance, in particular, β-glucosidase, pectinase and lichenase. The data obtained confirmed the occurrence of β-glucosidase activity in enological strains of non-Saccharomyces (Charoenchai et al. 1997), thus indicating that these yeasts may potentially have a greater role in flavour development. The strains examined were able to produce the enzyme specifically degrading pectin, which may cause problems in the wine industry by giving rise to turbidity and viscosity during vinification process (Whitaker 1984). Finally, the lichenase activity detected has high technological relevance, because it can favour the wine filterability since the greater part of the grape glucans contain 1,3–1,4 linkages (Strauss et al. 2001). The optimization of a procedure for the enological selection of non-Saccharomyces fermentation starter cultures is of great importance, since their role in enhancing the aromatic quality of wines (Zironi et al. 1993; Ciani and Ferraro 1998; Erten 2002; Toro and Vazquez 2002). Indeed, the formulation of a fermentation starter added with a selected H. uvarum strain could play an important role during grape must fermentation, so that the desired enzymes can be directly produced by the non-Saccharomyces yeast during the fermentation process rather than having to be added to the must (Van Rensburg and Pretorius 2000). In conclusion for the first time, a general and flexible procedure for the enological selection of non-Saccharomyces yeast strains has been here described. However, further investigations performed by small-scale fermentations test are now underway in order to fully validate the selected H. uvarum 8795 strain as industrial starter for wine mixed fermentations.
References
Bleve G, Grieco F, Cozzi G, Logrieco A, Visconti A (2006) Isolation of epiphytic yeasts with potential for biocontrol of Aspergillus carbonarius and A. niger on grape. Int J Food Microbiol 108:204–209
Bujdosó G, Egli CM, Henick-Kling T (2001) Inter- and intra-specific differentiation of natural wine strains of Hanseniaspora (Kloeckera) by physiological and molecular methods. Food Technol Biotechnol 39:19–28
Cadez N, Raspor P, de Cock AWAM, Boekhout T, Smith MT (2002) Molecular identification and genetic diversity within species of the genera Hanseniaspora and Kloeckera. FEMS Yeast Res 1:279–289
Capece A, Fiore C, Maraz A, Romano P (2005) Molecular and technological approaches to evaluate strain biodiversity in Hanseniaspora uvarum of wine origin. J Appl Microbiol 98:136–144
Cappello MS, Bleve G, Grieco F, Dellaglio F, Zacheo G (2004) Characterization of Saccharomyces cerevisiae strains isolated from must of grape grown in experimental vineyard. J Appl Microbiol 97:1274–1280
Cardinali G, Pellegrini L, Martini A (1995) Improvement of chromosomal DNA extraction from different yeast species by analysis of single preparation steps. Yeast 11:1027–1029
Caridi A, Ramondino D (1999) Biodiversità fenotipica in ceppi di Hanseniaspora di origine enologica. Enotecnico 45:71–74
Caruso M, Fiore C, Contursi M, Salzano G, Paparella A, Romano P (2002) Formation of biogenic amines as criteria for the selection of wine yeasts. World J Microbiol Biotechnol 18:159–163
Charoenchai C, Fleet GH, Henschke PA, Tood BEN (1997) Screening of non-Saccharomyces wine yeasts for the presence of extra cellular hydrolytic enzymes. Aust J Grape Wine Res 3:2–8
Ciani M, Ferraro L (1998) Combined use of immobilized Candida stellata cells and Saccharomyces cerevisiae to improve the quality of wines. J Appl Microbiol 85:247–254
Ciani M, Maccarelli F (1998) Enological properties of non-Saccharomyces yeasts associated with wine-making. World J Microbiol Biotechnol 14:199–203
Ciani M, Picciotti G (1995) The growth kinetics and fermentation behaviour of some non-Saccharomyces yeasts associated with wine-making. Biotechnol Lett 17:1247–1250
Ciani M, Beco L, Comitini F (2006) Fermentation behaviour and metabolic interactions of multistarter wine yeast fermentations. Int J Food Microbiol 108:239–245
Comi G, Maifreni M, Manzano M, Lagazio C, Cocolin L (2000) Mitochondrial DNA restriction enzyme analysis and evaluation of the enological characteristics of Saccharomyces cerevisiae strains isolated from grapes of the wine-producing area of Collio (Italy). Int J Food Microbiol 58:117–121
de Barros Lopes M, Soden A, Henschke PA, Langridge P (1996) PCR differentiation of commercial yeast strains using intron splice site primers. Appl Environ Microbiol 62:4514–4520
Erten H (2002) Relations between elevated temperatures and fermentation behaviour of Kloeckera apiculata and Saccharomyces cerevisiae associated with winemaking in mixed cultures. World J Microbiol Biotechnol 18:373–378
Fernandez MT, Ubeda JF, Briones AI (1999) Comparative study of non-Saccharomyces microflora of musts in fermentation, by physiological and molecular methods. FEMS Microbiol Lett 173:223–229
Fleet GH (1990) Growth of yeasts during wine fermentations. J Wine Res 1:211–223
Fleet GH (1993) The microorganisms of winemaking—isolation, enumeration and identification. In: Fleet GH (ed) Wine microbiology and biotechnology. Harwood Academic Publishers, Switzerland, pp 1–25
Fleet GH (2003) Yeast interactions and wine flavour. Int J Food Microbiol 86:11–22
Fleet GH, Heard GM (1993) Yeasts: growth during fermentation. In: Fleet GH (ed) Wine microbiology and biotechnology. Harwood Academic Publishers, Chur., Switzerland, pp 27–54
Gil JV, Mateo JJ, Jiménez M, Pastor A, Huerta T (1996) Aroma compounds in wine ad influenced by apiculate yeasts. J Food Sci 61:1247–1266
Heard GM (1999) Novel yeast in winemaking—looking to the future. Food Aust 51:347–352
Heard GM, Fleet GH (1985) Growth of natural yeast flora during the fermentation of inoculated wines. Appl Environ Microbiol 50:727–728
Jolly NP, Augustyn OPH, Pretorius IS (2006) The role and use of non-Saccharomyces yeasts in wine production. S Afr J Enol Vitic 27:15–39
Lambrechts MG, Pretorius IS (2000) Yeast and its importance to wine aroma. S Afr J Enol Vitic 21:97–129
Mallouchos A, Skandamis P, Loukatos P, Komaitis M, Koutinas A, Kanellaki M (2003) Volatile compounds of wines produced by cells immobilized on grape skins. J Agric Food Chem 51:3060–3066
Martínez-Rodríguez A, Carrascosa AV, Barcenilla JM, Pozo-Bayón M, Polo MC (2001) Autolytic capacity and foam analysis as additional criteria for the selection of yeast strains for sparkling wine production. Food Microbiol 18:183–191
Mateo JJ, Jiménez M, Huerta T, Pastor A (1991) Contribution of different yeasts isolated from musts of monastrell grapes to the aroma of wine. Int J Food Microbiol 14:153–160
Mendes-Ferreira A, Mendes-Faia A, Leão C (2002) Survey of hydrogen sulphide production by wine yeasts. J Food Prot 65:1033–1037
Mingorance-Cazorla L, Clemente-Jiménez JM, Martınez-Rodrıguez S, Las Heras-Vazquez FJ, Rodrıguez-Vico F (2003) Contribution of different natural yeasts to the aroma of two alcoholic beverages. World J Microbiol Biotechnol 19:297–304
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76:5269–5273
Nikolaou E, Soufleros EH, Bouloumpasi E, Tzanetakis N (2006) Selection of indigenous Saccharomyces cerevisiae strains according to their enological characteristics and vinification results. Food Microbiol 23:205–211
Noble AC, Bursick GF (1984) The contribution of glycerol to perceived viscosity and sweetness in white wine. Am J Enol Vitic 39:110–112
Rapp A, Mandery H (1986) Wine aroma. Experientia 42:873–884
Rojas V, Gil JV, Pinaga F, Manzanares P (2001) Studies on acetate ester production by non-Saccharomyces wine yeasts. Int J Food Microbiol 70:283–289
Romano P, Suzzi G (1996) Origin and production of acetoin during wine yeast fermentation. Appl Environ Microbiol 62:309–315
Romano P, Suzzi G, Comi G, Zironi R (1992) Higher alcohol and acetic acid production by apiculate wine yeasts. J Appl Bacteriol 73:126–130
Romano P, Suzzi G, Zironi R, Comi G (1993) Biometric study of acetoin production in Hanseniaspora guilliermondii and Kloeckera apiculata. Appl Environ Microbiol 59:1838–1841
Romano P, Suzzi G, Domizio P, Fatichenti F (1997) Secondary products formation as a tool for discriminating non-Saccharomyces wine strains. Strain diversity in non-Saccharomyces wine yeasts. Antonie Van Leeuwenhoek 71:239–242
Romano P, Fiore C, Paraggio M, Caruso M, Capece A (2003) Function of yeast species and strains in wine flavour. Int J Food Microbiol 86:169–180
Rosi I, Vinella M, Domizio P (1994) Characterization of β-glucosidase activity in yeasts of enological origin. J Appl Bacteriol 77:519–527
Schuller D, Valero E, Dequin S, Casal M (2004) Survey of molecular methods for the typing of wine yeast strains. FEMS Microbiol Lett 231:19–26
Strauss MLA, Jolly NP, Lambrechts MG, van Rensburg P (2001) Screening for the production of extracellular hydrolytic enzymes by non-Saccharomyces wine yeasts. J Appl Microbiol 91:182–190
Suomalainen H (1971) Yeasts and its effect on the flavour of alcoholic beverages. J Inst Brew 77:164–177
Toro ME, Vazquez F (2002) Fermentation behaviour of controlled mixed and sequential cultures of Candida cantarellii and Saccharomyces cerevisiae wine yeasts. World J Microbiol Biotechnol 18:347–354
Van Rensburg P, Pretorius IS (2000) Enzymes in winemaking, harnessing natural catalyst for efficient biotransformations—a review. S Afr J Enol Vitic 21:52–73
Whitaker JR (1984) Pectic substances, pectic enzymes and haze formation in fruit juices. Enzyme Microb Technol 6:341–349
Zironi R, Romano P, Suzzi G, Battistuta F, Comi G (1993) Volatile metabolites produced in wine by mixed and sequential cultures of Hanseniaspora guilliermondii or Kloeckera apiculata and Saccharomyces cerevisiae. Biotechnol Lett 15:235–238
Acknowledgements
This research was partially supported by a grant from the Regione Puglia Project PS_008—INNOWINE—“Biotecnologie innovative per il miglioramento della qualità e sicurezza dei vini tipici pugliesi”. The authors wish to thank Mr. Giovanni Colella for his valuable technical assistance. We would also like to thank the native speaker Prof. H. Caffery for proofreading and providing valuable linguistic advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Benedictis, M., Bleve, G., Grieco, F. et al. An optimized procedure for the enological selection of non-Saccharomyces starter cultures. Antonie van Leeuwenhoek 99, 189–200 (2011). https://doi.org/10.1007/s10482-010-9475-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-010-9475-8