Abstract
Genetic diversity among 27 isolates (23 from chickpea and 4 from other host crops) of Rhizoctonia bataticola representing 11 different states of India was determined by random amplified polymorphic DNA (RAPD), internal transcribed spacer restriction fragment length polymorphism (ITS-RFLP) and ITS sequencing. The isolates showed variability in virulence test. Unweighted paired group method with arithmetic average cluster analysis was used to group the isolates into distinct clusters. The clusters generated by RAPD grouped all the isolates into six categories at 40% genetic similarity. High level of diversity was observed among the isolates of different as well as same state. Some of the RAPD (OPN 4, OPN 12, and OPN 20) markers clearly distinguished majority of the isolates into the area specific groups. The ITS I, 5.8rDNA and ITS II regions of 11 isolates representing different RAPD groups were amplified with primers ITS 1 and ITS 4 and digested with seven restriction enzymes. The restriction enzymes DraI, MboI, RsaI, and AluI were found to be suitable for differentiating the isolates into five categories by showing isolate specific ITS-RFLP patterns. The isolates were variable in their nucleotide sequences of the ITS regions. This is the first study on genetic diversity among chickpea isolates of R. bataticola.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chickpea (Cicer arietinum L.) is one of the major grain legumes widely grown in India as well as other parts of the world. Among various factors attributing to low productivity of chickpea, susceptibility to diseases is very important. It is estimated that yield loss due to insects and diseases ranges from 5 to 10% in temperate and 50 to 100% in tropical regions (Van Emden et al. 1988). Chickpea is prone to many diseases (Nene et al. 1984) and among them dry root rot caused by Rhizoctonia bataticola (Taub) Butler [synonym-Macrophomina phaseolina (Tassi) Goid] is one of the major constraints in chickpea production and causing 10–20% annual loss (Vishwadhar and Chaudhary 2001). R. bataticola is primarly seed and soil-borne fungal pathogen. In chickpea, infected seeds and sclerotia surviving in the soils are the major source of primary inoculum. The pathogen also has wide host range. Since 75% cultivation of chickpea in India is under rain fed, the crop faces severe moisture stress, which predisposes the crop to dry root rot development.
Prolonged saprophytic survival ability of the pathogen in soil makes chemical control and crop rotation ineffective. Cultivation of resistant varieties is an economical approach for the management of dry root rot of chickpea, but only a few sources with low level of genetic resistance are available. However, commercial cultivars of chickpea with desired level of genetic resistance to dry root rot have not yet been developed (Pande et al. 2004). The information regarding diversity among the populations of the pathogen should enhance the development and release of disease resistant chickpea cultivars. Different molecular methods such as amplified fragment length polymorphism (Vandemark et al. 2000; Mayek-Perez et al. 2001), random amplified polymorphic DNA (Almeida et al. 2003; Jana et al. 2003; Rajkumar and Kuruvinashetti 2007) and restriction fragment length polymorphism (Almeida et al. 2003) have been used to reveal genetic polymorphism within populations of M. phaseolina isolated from other than chickpea host crops. However, polymorphisms at the DNA level among chickpea isolates of R. bataticola have not been described yet.
The objective of this study was to determine the genetic diversity of R. bataticola isolates obtained from different chickpea growing states of India using RAPD, ITS-RFLP markers and ITS sequencing.
Materials and methods
Fungal cultures
Chickpea plants showing characteristic dry root rot symptoms were collected from different chickpea growing states of India (Table 1). The associated fungus was isolated from the roots of diseased plants. Four isolates of Macrophomina phaseolina (synonym- Rhizoctonia bataticola) isolated from the other host crops were also obtained from Indian Type Culture Collection (ITCC), IARI, New Delhi, India for comparison. The cultures were purified by single sclerotium and hyphal tip. On the basis of morphological characters (mycelium and sclerotia) the isolates were identified as R. bataticola (synonym-M. phaseolina) as described earlier (Briton-Jones 1925; Ashby 1927; Holliday 1980). The isolates which produce pycnidia in culture were named as M. phaseolina. The cultures were maintained on potato dextrose agar slants at 4°C for future study.
Virulence test
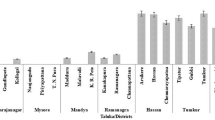
The virulence of 27 isolates of the pathogen was determined by modified sick soil method on susceptible chickpea cultivar Pusa 212 (Nene et al. 1981). Surface sterilized (0.1% formalin) plastic pots (15 cm) were filled (2 kg pot−1) with autoclaved soil (three consecutive sterilization for 3 days at 104 kPa for 1 h) and inoculated 5 days prior to sowing with 15-day old inoculum (20 g kg−1) multiplied on sorghum (Sorghum vulgare L.) grains. Sorghum grains were soaked in tap water for 12 h, strained and filled into 500 ml conical flasks (250 g flask−1). The flasks containing sorghum grains were autoclaved for two subsequent days at 104 kPa for 30 min and inoculated with 3-day old culture of R. bataticola. Inoculated flasks were incubated at 25 ± 1°C for 15 days. Ten seeds of chickpea variety Pusa 212 were sown in each pot in two replications and observed for symptoms development. Re-isolations were made from the roots of diseased plants on PDA medium, and isolated fungus was identified to confirm its identity. Dry root rot incidence was recorded at 20 days interval up to maturity of the crop on the basis of the number of diseased plants out of the total germinated seeds. The data were analysed statistically in completely randomized design (Gomez and Gomez 1984).
DNA extraction
The isolates were grown in potato dextrose broth medium at 25 ± 1°C for 10 days. Mycelia were harvested by filtering through Whatman filter Paper No. 1, washed repeatedly with distilled sterilized water to remove excess of salts adhering to it, blot dried and stored in Al-foils at −80°C for genomic DNA extraction. DNA extraction was based on the cetrimide tetradecyl trimethyl ammonium bromide (CTAB) method (Murray and Thompson 1980). Mycelium (1 g) was crushed in liquid nitrogen and transferred into 7.5 ml pre-warmed (65°C) DNA extraction buffer [1 M Tris–HCl (pH 8.0), 5 M NaCl, 0.5 M EDTA (pH 8.0) and 2% CTAB], mixed well and incubated in a water bath at 65°C with gentle shaking for 1 h. Equal volume of chloroform: isoamyl alcohol (24:1 v/v) was added and mixed gently to denature proteins and centrifuged at 12,857g for 10 min. DNA was precipitated with 0.6 volume of chilled isopropanol and 0.1 volume of 3 M sodium acetate (pH 5.2) and centrifuged at 18,514g for 15 min. The pellets were washed twice with chilled 70% ethanol, dried at room temperature, re-suspended in 100 μl sterile TE (10 mM Tris–HCl buffer and 1 mM EDTA—pH 8) and stored at −20°C. Quantification of DNA was done by spectrophotometer method. The sample (5 μl) was mixed thoroughly with 95 μl distilled water and placed in spectrophotometer. Readings were taken at two wave lengths (260 and 280 nm). Quantity of DNA was determined using the formula as amount of DNA μg/μl = (OD) 260 × 50 × dilution factor/1,000. Based on concentrations of DNA, working solutions having 25 ng/μl were prepared for optimization of PCR conditions.
Random amplified polymorphic DNA marker analyses
Different PCR reactions were tested for obtaining the best amplification (Cobb and Clarkson 1994). Three concentrations for each, template DNA (25, 50, and 75 ng), MgCl2 (1.5, 2.5, and 3.5 mM), dNTPs (0.2, 0.4, and 0.6 mM) and primers (6, 9, and 13 pmol) were tested with 1U Taq polymerase and 1× reaction buffer (Bangalore Genei, India). DNA from 27 R. bataticola isolates was amplified by the RAPD method (Williams et al. 1990) using 46 random oligonucleotide (10-mer) primers. Forty-two primers were from Operon Technologies, USA and only four primers (R2, P1, P3, and Primer 3) were synthesized from Clonitec Inc., USA (Table 2). Reactions were carried out in a final volume of 25 μl containing 1× PCR buffer, 1U Taq polymerase (Bangalore Genei, India), 2.5 mM MgCl2, 0.6 mM dNTP, 9 pmol primer and 75 ng DNA. The PCR was performed in a Mastercycler® ep gradient thermalcycler (Eppendorf AG, Germany). Initial denaturation of 94°C for 5 min was followed by 40 cycles consisting of denaturation at 94°C for 1 min, annealing at 35°C for 1 min and extension at 72°C for 2 min. A final extension of 72°C for 5 min was incorporated into the program followed by cooling at 4°C until recovery of the samples. The annealing temperature (35°C) was found to be the best for most of the primers included in the present study (Dubey and Singh 2008). Amplified products (25 μl) were mixed with 2 μl of 6× loading dye (Bangalore Genei, India) and analyzed by electrophoresis on 1% agarose gel containing 1 μg ml−1 of ethidium bromide in 1× TAE buffer (pH 8) along with a 1 kb DNA ladder (Bangalore Genei, India) as standard molecular weight marker and visualized under ultraviolet light (Bio Rad gel doc system).
The primers that produced reproducible and scorable amplifications were used in the analysis. The experiments were repeated twice to confirm the repeatability of the results and only one repeatable result was scored for each primer. DNA bands that could be scored unequivocally for their presence or absence were included in the analysis. Binary matrices were analyzed by NTSYS—PC (Version 2.02; Exeter Biological Software, Setauket, NY). Jaccard’s coefficients were clustered to generate a dendrogram-using SHAN clustering programme selecting unweighted paired group method with arithmetic averages (UPGMA; Rohlf 2005).
Restriction fragment length polymorphisms
The ITS regions and the 5.8S rDNA of 11 isolates (Rb1, Rb3, Rb5, Rb7, Rb11, Rb12, Rb16, Rb17, Rb21, Rb25, and Rb26) of the pathogen representing various RAPD groups were amplified with a set of primers ITS1 (5′ TCCGTAGGTGAACCTGCGG 3′) and ITS4 (5′ TCCTCCGCTTA TTGATATGC 3′) as described by White et al. (1990). The PCR-amplification reaction was optimized with various concentrations of MgCl2, dNTPs, primer and template DNA. The amplification was carried out in a Mastercycler® ep gradient thermalcycler (Eppendorf AG, Germany) in a 50 μl PCR reaction consisting of 1× PCR buffer, 1U Taq polymerase (Bangalore Genei, India), 2.5 mM MgCl2, 0.6 mM dNTPs, 6 pmol of each primer and 50 ng of genomic DNA. Initial denaturation of 94°C for 5 min was followed by 35 cycles of 94°C for 1 min, 55°C for 1 min (primer annealing), 72°C for 2 min (primer extension). A final extension of 72°C for 5 min was incorporated into the program, followed by cooling to 4°C until recovery of the samples. PCR products were visualized using UV light and separated on a 1.4% agarose gel pre-stained with ethidium bromide (1 μg ml−1). 100 kb ladder (Bangalore Genei, India) was used as a marker.
The amplified products were digested with seven restriction enzymes (AluI, PvuII, RsaI, DraI, EcoRV, MboI, and SmaI) according to the manufacturers’ recommendations. After restriction digestion, the DNA products were separated on a 2% agarose gel in TAE buffer and detected as described earlier. The experiment was repeated twice for consistent results.
ITS sequencing
The ITS amplified bands (~500–650 bp) of 6 isolates (Rb1, Rb5, Rb16, Rb17, Rb21, and Rb26) representing different ITS-RFLP groups were eluted and purified using QIAGEN gel extraction and purification kits (Promega, USA) as per manufacturers’ instructions. The cloning of the fragment was performed with pGEM-T Easy vector system (Promega, USA) following standard procedures (Sambrook et al. 2001). The competent cells were prepared by calcium chloride method as described by Mendal and Higa (1970). The transformed colonies were selected and cultured in Luria Agar (LA) plate containing ampicillin, X-gal (5-bromo-4 chloro-3 indolyl-ß-D galactopyranoside) and IPTG (Isopropyl-ß-D thiogalactopyranoside) (100 μl of 1% ampicillin, 200 μl of 2% X-gal and 20 μl of 0.1 M IPTG for 100 ml LA). The plates were incubated overnight at 37°C. The white colonies were selected and subsequently plated on IXA (IPTG, X-gal and ampicillin) plates and incubated at 37°C for overnight. Recombinant plasmid DNA was isolated by modified alkaline lysis method (Brinboim and Dolly 1979). Presence of insert was confirmed by restricting the recombinant DNA with EcoR1. Colony PCR was also used as a second conformation. Cloned ITS inserts were sequenced. For each isolate two clones were sequenced to minimize errors due to amplification. The nucleotide sequences were subjected to BLAST analysis on World Wide Web (http://www.ncbi.nih.gov/index.html) and submitted to GenBank at National Center for Biotechnology Information (NCBI) and obtained accession numbers. The multiple sequence alignment and pair wise alignment were made using BioEdit version 5.09 (Hall 1999). Phylogeny tree was constructed on the based maximum likelihood of nucleotide sequences by using CLUSTALW 1.6 sequence alignment selecting UPGMA by MEGA 4.1 programme.
Results
Virulence test
The chickpea as well as other host crops isolates of the pathogen proved pathogenic on chickpea cultivar Pusa 212 (Table 1). The effect of isolates on dry root rot incidence was significant (P < 0.05). Out of 27 isolates, 15 isolates causing more than 75% dry root rot incidence were highly virulent. Among these, four isolates, namely, Rb1 (Karnataka), Rb2 (Haryana), Rb6 (Uttar Pradesh) and Rb19 (Jharkhand) caused cent per cent dry root rot incidence. Eight isolates causing 50–75% dry root rot incidence were considered as virulent whereas, only four isolates causing <50% incidence were considered as less virulent. New Delhi (Rb3) isolate caused minimum disease incidence (31.7%) followed by Rajasthan isolate (Rb15). The dry root rot incidences recorded in these two isolates were statistically similar.
RAPD analysis
Out of 46 RAPD primers screened for amplification of DNA of 27 isolates of R. bataticola (Table 1), 26 primers produced reproducible and scorable bands (Table 2). Number of bands obtained were specific to each primer and ranged from 4 to 12. Out of 226 total bands, 224 bands were polymorphic. Except OPX series, all evaluated primers were found to be suitable for amplification of the isolates of R. bataticola. Out of 11 primers of OPA series, eight primers produced scorable and reproducible bands with the highest polymorphism in OPA4. Out of seven primers of OPM series, four primers produced polymorphic bands with the highest polymorphism in OPM6. Out of seven primers of OPN series, six primers produced polymorphic bands for all the isolates. Primer OPN9 showed the highest polymorphism followed by OPN4. Banding patterns obtained with 26 primers for 27 isolates of R. bataticola were analyzed. The Jaccard’s similarity coefficients were varied from 11.4 to 84.8%. Similarity coefficient was maximum (84.8%) in between Rb26 (Madhya Pradesh) and Rb27 (Chhattisgarh) followed by Rb16 and Rb19; Rb18 and Rb13 isolates from Jharkhand. Majority of the isolates showed <50% similarity coefficient. More than 50% similarity coefficients were observed in between Rb20 and Rb21, both from Haryana; Rb6 (Uttar Pradesh) and Rb21 (Haryana); Rb23 (New Delhi) and Rb27 (Chhattisgarh); Rb1 (Karnataka) and Rb2 (Haryana); Rb9 and Rb10, both from Uttar Pradesh and Rb4 and Rb5, both from Punjab.
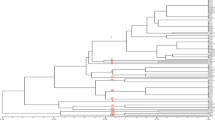
The UPGMA dendrogram demonstrated that the isolates were grouped into two distinct clusters at 0.30 similarity coefficient. First cluster consisted of 10 isolates while the rest 17 isolates were grouped into second cluster. At 0.40 genetic distances, the isolates were grouped into six clusters (Fig. 1) by using unweighted paired group method with arithmetic average. First cluster consisted of six isolates from five states, namely, Karnataka (Rb1), Haryana (Rb2), Delhi (Rb3), Punjab (Rb4 and Rb5) and Assam (Rb22). The second cluster contained 4 isolates one each from Haryana (Rb14) and Uttar Pradesh (Rb8) and two from Rajasthan (Rb15 and Rb17). Two isolates from Haryana (Rb20 and Rb21) and four isolates from Uttar Pradesh (Rb6, Rb7, Rb9, and Rb10) were grouped into third cluster. Fourth cluster consisted of four isolates from Jharkhand (Rb13, Rb16, Rb18, and Rb19) and one isolate from Maharashtra (Rb11). Fifth cluster contained five isolates from Madhya Pradesh (Rb26), Chhattisgarh (Rb27), Delhi (Rb23 and Rb25) and Maharashtra (Rb24). Only one isolate from Uttar Pradesh (Rb12) was grouped separately into sixth cluster.
The primers OPN4, OPN12, and OPN20 (Figs. 2, 3 for both repetitions) were found to be suitable for area specific grouping of the majority of the isolates of the pathogen. The dendrogram obtained from the analysis of banding pattern of OPN4 primer indicated that two isolates from Punjab (Rb4 and Rb5), four isolates from Jharkhand (Rb13, Rb16, Rb18 and Rb19), three isolates one each from Madhya Pradesh (Rb26), Chhattisgarh (Rb27), and Delhi (Rb23) and two isolates, one each from Haryana (Rb21) and Uttar Pradesh (Rb6) showed 100% similarity coefficient. At 0.35 genetic similarity the isolates were grouped into three clusters. The first cluster contained 12 isolates from Karnataka, Punjab, Haryana and Uttar Pradesh, and second cluster consisted of 13 isolates from Rajasthan, Maharashtra, Jharkhand, Delhi, Madhya Pradesh and Chhattisgarh whereas, third cluster had only two isolates one each from Haryana and Assam (Fig. 4). The dendrogram obtained with OPN12 grouped all the isolates into two clusters at 0.25 genetic similarity. Thirteen isolates were grouped in first cluster while 14 isolates were placed in second cluster (Fig. 5). In first cluster, two isolates from Punjab (Rb4 and Rb5) and in second cluster, two isolates from Jharkhand (Rb16 and Rb18) and two isolates one each from Uttar Pradesh (Rb12) and Jharkhand (Rb18) showed 100% similarity coefficient.
The dendrogram derived from the analysis of banding pattern obtained with primer OPN20 (Fig. 6) divided the isolates of the pathogen into two clusters. First cluster contained 10 isolates. Two sub-clusters consisting of two isolates one each from Karnataka (Rb1) and Haryana (Rb2) and 2 bean isolates from Assam (Rb22) and Maharashtra (Rb24) showed identical banding patterns were placed in this cluster. Second cluster consisted of 17 isolates. There were two sub-clusters of 12 (Rb20 and Rb21 from Haryana, Rb6, Rb7, Rb9, Rb10, and Rb12 from Uttar Pradesh, Rb11 from Maharashtra and Rb13, Rb16, Rb18, and Rb19 from Jharkhand) and three (Rb4 from Punjab, Rb26 from Madhya Pradesh and Rb27 from Chhattisgarh) isolates with identical banding patterns showing cent per cent similarity coefficient. Urdbean and groundnut isolates of the pathogen from Delhi (Rb23 and Rb25) were also included in second cluster with 80% similarity coefficient.
ITS-RFLP analysis
PCR amplification with primer set ITS1 and ITS4 yielded single DNA fragment, present in all isolates with ~500–650 bp in size. The fragment pattern generated from seven restriction digestion of ITS regions demonstrated high levels of variability in the isolates of the R. bataticola. ITS-RFLP patterns (1–3 visible bands) clearly differentiated the isolates into five groups. Karnataka isolate (Rb1) was placed separately in first group. The second group consisted of three isolates one each from Punjab (Rb4), Uttar Pradesh (Rb7) and Haryana (Rb21). Group third and fourth contained one isolate in each from Punjab (Rb5) and Rajasthan (Rb17), respectively, whereas the rest of the five isolates from Madhya Pradesh (Rb26), Delhi (Rb3 and Rb25), Jharkhand (Rb16) and Maharashtra (Rb11) were included in fifth group. Except Rb25 (groundnut isolate), all isolates were isolated from chickpea. The DraI differentiated ITS-RFLP pattern of Rb1 (Karnataka) from Rb5 (Punjab) by producing two fragments in latter (~200 and 500 bp) isolate whereas, in former (Rb1) it was only one (~500 bp). Endonuclease MboI differentiated Rb17 (Rajasthan) from rest of the isolates. Endonuclease RsaI differentiated Rb21 (Haryana), Rb7 (Uttar Pradesh) and Rb4 (Punjab) from other isolates whereas, rest of the isolates namely Rb3 and Rb25 (Delhi), Rb11 (Maharashtra), Rb16 (Jharkhand) and Rb26 (Madhya Pradesh) grouped separately by endonuclease AluI. Therefore, out of seven restriction enzymes evaluated, only four namely DraI, MboI, RsaI, and AluI were found to be suitable for distinguishable ITS-RFLP patterns (Fig. 7).
ITS sequencing
Six isolates of R. bataticola representing 5 different ITS-RFLP groups were variable (499–670 bp) in respect of their nucleotide sequences of ITSI, 5.8S and ITSII regions. The ITS of Karnataka isolate (Rb1) was largest (670 bp), whereas it was smallest (499 bp) in Haryana (Rb21) isolate. The sizes of ITS regions of Rb17 (Rajasthan), Rb26 (Madhya Pradesh), Rb16 (Jharkhand) and Rb5 (Punjab) isolates were 537, 545, 557, and 652 bp, respectively. The size of two fragments obtained in the isolate Rb5 (Punjab) by restriction with DraI was about 700 bp when compared with 100 bp marker (Fig. 7), but after sequencing its actual size was 652 bp. The sequences of the isolates were deposited to GenBank nucleotide database with accession numbers EU375545, EU375546, EU375547, EU375548, EU375549, and EU375550, for isolates Rb5, Rb1, Rb16, Rb17, Rb21, and Rb26, respectively. The phylogeny tree constructed from the nucleotide sequence similarity of these six isolates along with 27 other ITS sequences of fungi which showed 96–100% similarity during blast analysis clustered them into three major groups (Fig. 8). The isolates Rb16 (Jharkhand), Rb21 (Haryana) and Rb26 (Madhya Pradesh) clustered in one group and showed 96-100% ITS nucleotide sequences similarity with the nucleotide sequences of M. phaseolina (R. bataticola) available in the GenBank. The isolate Rb17 (Rajasthan) clustered separately along with the isolates of Diaporthe and Phomopsis species and linked with the group containing M. phaseolina isolates. The nucleotide sequences of ITS regions of Rb1 (Karnataka) and Rb5 (Punjab) also clustered separately with 100% similarity to the sequences of R. solani available in GenBank and this group was linked with other earlier mentioned clusters.
Discussion
The aim of the present study was to assess the genetic diversity among 27 isolates of R. bataticola obtained from 11 major chickpea growing states of India by using PCR based markers. This is the first study on genetic diversity among chickpea isolates of R. bataticola. All the isolates proved pathogenic on chickpea cultivar Pusa 212. The results revealed that while there was significant variation in the virulence of the pathogen, there was no clear pattern of distribution linked to the virulence of the isolates. In contrast to the present results, Beas-Fernandiz et al. (2006) observed that pathogenic specialization of M. phaseolina was related to the geographic origin of the isolates. Above 20% root rot incidence is consider as susceptible reaction therefore, all the isolates tested in the present study caused susceptible reaction on cultivar Pusa 212, but they differed in degree of virulence. The virulent and less virulent isolates were not concentrated in any one particular state/region. Interestingly, all other host crops isolates (Rb22 to Rb25) were placed into virulent group with 50–75% disease incidence.
Random amplified polymorphic DNA and ITS-RFLP analyses performed on genomic DNA of 27 isolates of R. bataticola (M. phaseolina) revealed the presence of high level of genetic diversity. RAPD analysis potentially provides information across an entire genome, while ITS-RFLP analysis can reveal variation only within small region. ITS-RFLP banding patterns are very suitable and consistant. In contrast RAPD banding patterns are greatly influenced by components and conditions of PCR (Arabi and Jawhar 2007). In the present study both analyses revealed similar relationship among R. bataticola isolates. The isolates shared varied level of genetic similarity within a range of 11.4–89.9% with maximum in between isolates from Madhya Pradesh (Rb26) and Chhattisgarh (Rb27). It was followed by four isolates from Jharkhand (Rb16 and Rb19; Rb13 and Rb18). Four isolates from Haryana were placed into three different clusters indicated high level of variability among themselves. First RAPD cluster contained six isolates from five different states whereas, cluster six contained only one isolate from Uttar Pradesh also indicated high level of variability among the isolates from different states as well as within a state. Out of six isolates from Uttar Pradesh, four were placed in third cluster along with one Haryana isolate. South Indian isolate from Karnataka (Rb1) was grouped with north Indian isolates. The existence of genetic diversity among the isolates from the same as well as different states might be due to movement of R. bataticola through infected seeds and soil with planting materials by the means of vegetative stage and sclerotia.
No relationship was found between RAPD groups and the geographical origin of the isolates. Earlier workers also tried to establish relationship in between isolates of M. phaseolina obtained from other host crops and their hosts as well as geographical origin. In spite of high level of genetic heterogeneity, in some cases isolates could be grouped according to geographical locations by AFLP (Mayek-Perez et al. 2001) and SSR (Jana et al. 2005) markers, however in others they could not clearly be grouped according to their geographical locations (Purkayastha et al. 2006; Rajkumar and Kuruvinashetti 2007; Vandemark et al. 2000; Pecina-Quintero et al. 2002). The present findings are partially in accordance with the earlier results that the M. phaseolina isolates of same host were genetically similar and differed distinctly from the isolates of other hosts (Su et al. 2001; Purkayastha et al. 2006). In the present study, out of four non-chickpea host isolates of the pathogen, three were grouped together into one cluster along with two chickpea isolates from Chhattisgarh and Madhya Pradesh (cluster 5). They were also in same virulence group. Vandemark et al. (2000) and Mayek-Perez et al. (2001) used AFLP to determine variability and grouped the isolates, but the results obtained from both the groups differed. Thus, the differences in the findings may be due to the genetic variations in the populations of M. phaseolina obtained from different agroclimatic areas and host crops. The presence of high level variations among the isolates of R. bataticola in India may be attributed to variations in the climatic conditions and cropping patterns in different parts of the country, besides movement of the pathogen. Some of the isolates within a state showed similarity suggesting that these isolates did not evolve independently from each other and therefore, they may be considered as a part of the same ancestral population (Almeida et al. 2003). The primers like OPN 4, OPN 12, and OPN 20 showed area specific grouping of the isolates would be further utilized in development of molecular markers for detection of area specific isolates.
Earlier, it was observed that restriction analysis of the ITS regions was not suitable for detecting variability among M. phaseolina isolates (Su et al. 2001; Almeida et al. 2003). But, in the present study, similar to RAPD, ITS-RFLP was also able to detect diversity in the chickpea isolates of R. bataticola. Restriction enzymes DraI, MboI, RsaI, and AluI differentiated the isolates of the pathogen by showing different banding patterns. The Nucleotide sequences of ITS regions of the isolates were also variable. Similar to RAPD group, Karnataka (Rb1) isolate was grouped with Punjab (Rb5) isolate. Jharkhand isolate (Rb16) was clustered separately in RAPD, now grouped with Haryana (Rb21) and Madhya Pradesh (Rb26) isolates. The isolate from Rajasthan (Rb17) was grouped separately similar to RAPD groups. The differences observed in the size of ITS regions of the isolates may be responsible for variations. The RAPD banding patterns of three isolates, Rb1 (Karnataka), Rb5 (Punjab) and Rb17 (Rajasthan) were found to be similar with other isolates of R. bataticola (M. phaseolina) included in the present study, but their nucleotide sequence similarity was not identical with the nucleotide sequences of M. phaseolina available in GenBank. The aligned scores of Rb1 (Karnataka) and Rb5 (Punjab) with other isolates were only up to 32%, similarly aligned score of Rb17 (Rajasthan) with others was up to 70% clearly indicated that they were distinct from others. Both Rb1 and Rb5 clustered together with R. solani isolates whereas; Rb17 clustered separately with Diaporthe and Phomopsis species. The isolates Rb1 and Rb5 were fast growing, largest sclerotia (200–600 μm) producing and highly virulent (74.5–100% dry root rot incidence) on chickpea cultivar Pusa 212. The extensive diversity was observed among the populations of R. bataticola in the present study with molecular markers could also be contributed by mutation in the pathogen in the field, seed and soil-borne nature of the pathogen, parasexuality and wide host range. Cultural practices as well as transport of seeds, soils and planting materials have contributed to the genetic diversity observed within a state.
The results of the present findings clearly demonstrated that chickpea isolates of R. bataticola representing different part of the country were highly variable and RAPD and ITS-RFLP markers are suitable to determine genetic diversity among the populations of R. bataticola. Sequencing of ITS region also showed diversity among the populations of the pathogen. High level of variability in the populations of R. bataticola may be one of the causes of lack of resistance in the present commercial cultivars of chickpea. Thus, the information would also be utilized for the screening of genotypes of chickpea against group/area specific isolate to obtained resistance against variable populations of R. bataticola.
References
Almeida AMR, Abdelnoor RV, Arias CAA, Carvalho VP, Jacoud Filho DS, Marin SRR, Benato LC, Pinto MC, Carvalho CGP (2003) Genotypic diversity among Brazilian isolates of Macrophomina phaseolina revealed by RAPD. Fitopatol Bras 28:279–285
Arabi MIE, Jawhar M (2007) Molecular and pathogenic variation identified among isolates of Cochliobolus sativus. Australas Plant Pathol 36:17–21
Ashby SF (1927) Macrophomina phaseoli (Maubl) Com. Nov. the pycnidial stage of Rhizoctonia bataticola (Taub.) Butler. Trans Br Mycol Soc 12:141–142
Beas-Fernandiz R, De Santiago AD, Hernandez-Delgado S, Mayek-Perez N (2006) Characterization of mexican and non-mexican isolates of Macrophomina phaseolina based on morphological characteristics, pathogenicity on bean seeds and endoglucanase genes. J Plant Pathol 88:53–60
Brinboim HC, Dolly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523
Briton-Jones HR (1925) Mycological work in Egypt during the period 1920–1922. Egypt Ministry of Agriculture Technical and Science Bulletin, Cairo 49:129
Cobb BD, Clarkson JM (1994) A simple procedure for optimizing the polymerase chain reaction (PCR) using modified Tuguchi methods. Nucleic Acids Res 22:3801–3805
Dubey SC, Singh SR (2008) Virulence analysis and oligonucleotide fingerprinting to detect diversity among Indian isolates of Fusarium oxysporum f. sp. ciceris causing chickpea wilt. Mycopathologia 165:389–406
Gomez KA, Gomez AA (1984) Statistical procedure for agricultural research. Wiley, Singapore, pp 139–153
Hall TA (1999) BIOEDIT: a user-friendly biological sequences alignment, editor and analysis of program for windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Holliday P (1980) Fungus diseases of tropical crops. Cambridge university press, Cambridge, NY10022, p 607
Jana TK, Sharma TR, Prasad RD, Arora DK (2003) Molecular characterization of Macrophomina phaseolina and Fusarium species by single primer RAPD technique. Microbiol Res 158:249–257
Jana TK, Sharma TR, Singh NK (2005) SSR-based detection of genetic variability in the charcoal root rot pathogen Macrophomina phaseolina. Mycol Res 109:81–86
Mayek-Perez N, Lopez-Castaneda C, Gonzalez-Chavira M, Garcia-Espinoa R, Acosta-Gallegos J, Martinez de la Vega OM, Simpson A (2001) Variability of mexican isolates of macrophomina phaseolina based on pathogenesis and AFLP genotype. Physiol Mol Plant Pathol 59:257–264
Mendal M, Higa A (1970) Calcium dependent bacteriophase DNA isolation. J Mol Biol 53:159–162
Murray MG, Thompson WE (1980) RAPD isolation of high molecular weight DNA. Nucleic Acids Res 8:4321–4325
Nene YL, Hawara MP, Reddy MV (1981) Chickpea disease resistance screening technique. ICRISAT Inf Bull 10:4–5
Nene YL, Shiela VK, Sharma SB (1984) A world list of chickpea (Cicer arietinum) and pigeon pea (Cajanus cajan) pathogen. ICRISAT Pulse Pathol Prog Rep 32:19
Pande S, Kumar KK, Rao JN (2004) Evaluation of chickpea lines for resistance to dry root rot caused by Rhizoctonia bataticola. Int Chickpea Pigeonpea Newsl 11:37–38
Pecina-Quintero V, De la Vega O, Alvarado Balleza M, Vandemark G, Williams-Alanis H (2002) Comparacion de dos Sistemas de Marcadores Moleculares en el Analisis de Lasrelaciones Geneticas de Macrophomina phaseolina. Rev Mex Fitopatol 19:72–80
Purkayastha S, Kaur B, Chaudhury D (2006) Characterization of Macrophomina phaseolina, the charcoal rot pathogen of cluster bean, using conventional techniques and PCR-based molecular markers. Plant Pathol 55:106
Rajkumar BF, Kuruvinashetti MS (2007) Genetic variability of sorghum charcoal rot pathogen (Macrophomina phaseolina) assessed by random DNA markers. Korean Plant Pathol J 23:45–50
Rohlf JF (2005) NTSYS-pc: numerical taxonomy and multivariate analysis system version 2.2. Exter Software, Setauket, NY
Sambrook JE, Fritsch F, Maniatis T (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor
Su G, Suh SO, Schneider RW, Russian JS (2001) Host specialization in the charcoal rot fungus, Macrophomina phaseolina. Phytopathology 91:120–126
Van Emden HF, Ball SL, Rao MR (1988) Pest diseases and weed problems in pea, lentil and faba bean and chickpea. In: Summerfield RJ (ed) World crops: cool season food legumes. Kluwer, Dordrecht, pp 519–534
Vandemark G, Martinez O, Pecina V, de Jesus Alvarado M (2000) Assessment of genetic relationships among isolates of Macrophomina phaseolina using a simplified AFLP technique and two different methods of analysis. Mycologia 92:656–664
Vishwadhar, Chaudhary RG (2001) Disease resistance in pulse crop-current status and future approaches. In: Nagarajan S, Singh DP (eds) The role of resistance in intensive agriculture. Kalyani, New Delhi, pp 144–157
White TJ, Bruns T, Lee S, Talyor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninisky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aghakhani, M., Dubey, S.C. Determination of genetic diversity among Indian isolates of Rhizoctonia bataticola causing dry root rot of chickpea. Antonie van Leeuwenhoek 96, 607–619 (2009). https://doi.org/10.1007/s10482-009-9375-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-009-9375-y