Abstract
Members of the P. boydii species complex (Microascaceae) are frequently involved in human opportunistic disease. Studies indicate that the prevalent habitat of P. boydii sensu lato is in agriculturally exploited or otherwise human-impacted soils. Quantitative analysis of fungal indicators in the environment can be exploited for monitoring of general environmental changes, as well as for understanding local population changes and its epidemiological consequences. In this study we present the development and testing of a semi-selective isolation procedure for P. boydii and related species. Three general media, DG18, rose bengal agar and five variations of modified Leonian’s agar with and without benomyl were tested. Germination percentages of P. boydii, S. prolificans, Petriella spp. and Aspergillus fumigatus (control) were evaluated. Tests were carried out on the success of P. boydii isolation from inoculum mixed with A. fumigatus. Subsequently the procedure was applied to water, sediment and soil samples. On the newly introduced semi-selective medium (SceSel+), the germination of P. boydii was superior or similar to that seen on the other media tested. P. boydii was isolated from mixed cultures only on SceSel+ but not on SceSel without benomyl. Isolation from environmental sources with SceSel+ was successful, and human impacted soil was confirmed as the predominant habitat of P. boydii.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Members of the Pseudallescheria boydii complex (P. boydii (Shear) McGinnis et al. and S. apiospermum) and S. prolificans are members of the Microascaceae, which are frequently involved in human opportunistic disease. Infections occur in immunocompetent patients and can be subcutaneous or deep, such as in the case of mycetoma and arthritis. Also subclinical colonization of the respiratory tract including lungs of patients with cystic fibrosis is common (Cimon et al. 2000). In the immunocompromised patient, systemic infections occur, which often involve the central nervous system (Gueho and de Hoog 1999). A unique clinical entity is brain infection after aspiration of contaminated water after accidents. In one such case, isolation of P. boydii was possible at the site of an accident (Buzina et al. 2006).
Studies so far, seem to indicate that agriculturally heavily exploited or otherwise human impacted environments like cities and industrial areas are main components of the ecological niche of P. boydii (Guarro et al. 2006; Kaltseis and Rainer 2006). Pseudallescheria boydii, is known to be fit for growth in such environments by virtue of its ability to degrade alkanes (April et al. 1998) and to grow in microaerophilic environments (de Hoog et al. 1994). Regardless of the ecological tendencies mentioned above, the entire niche and the in situ biomass levels of P. boydii are not known accurately enough to allow infection risk assessment (Guarro et al. 2006). In addition, we lack understanding of the role of P. boydii as a decomposer, and we are not fully exploiting its potential to serve as indicator organism of environmental changes. Selective isolation may comprise a useful tool to generate data for clinical and ecological purposes. Applications may be designed for diagnosis of mycoses, tracing of routes of infection, detection and monitoring of high-risk environments, and, in the laboratory, determination of the minimal load of fungal propagules needed to cause infection in model organisms.
Quantitative analysis is crucial in the ecological investigation of fungal populations. Changes in microbial communities, such as the loss or gain of certain species, may reflect general environmental changes or changing local influences. In addition, shifts in the population structure may help to monitor the impact of anthropogenic activities on the environment. Studies on the diversity and abundance of soilborne fungi are generally based on culture techniques. Use of such methods has disadvantages. Culture media and growth conditions strongly influence which organisms will germinate and manifest sufficiently vigorous growth to prevail against competitors in the artificial situation. Culture techniques alone therefore do not allow accurate determination of fungal biodiversity in environmental samples. It remains difficult to obtain a realistic picture of the ecological role of particular fungi just from recording “colony forming units” (CFU) in various situations. Another limitation intrinsic to culture methods is the possible over-representation of fast growing strains on the complex isolation media that are mostly used. Selective media have therefore been designed to overcome this problem. One of the advantages of selective isolation is that it allows a reasonable approximation of target organism quantities.
In this study we present the development of a semi-selective isolation procedure for the medically relevant fungi of the genera Pseudallescheria and Scedosporium.
Material and methods
Media preparation
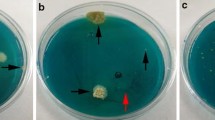
Medium variants tested (Table 1) included several media widely used in soil isolations. Some of these, such as Martin’s Rose Bengal agar or dichloran glycerol agar (DG18), have selective properties or restrict colony diameters of fast-growing fungi (Samson et al. 2004). A benomyl-containing medium was also designed based on the addition of this selective compound to a medium promoting growth and sporulation of a wide range of fungi (modified Leonian’s agar, MLA) (Summerbell 1993). The MLA-benomyl medium supplemented with antibiotics (ciprofloxacin, chloramphenicol, streptomycin, and dichloran) was denoted SceSel+ (Table 1). Before any environmental testing was performed, ten media and variants were examined by comparing the germination percentage of ascospores and conidia achieved on each of them at 37°C. Target organisms, including strains of P. boydii, Pseudallescheria angusta, P. ellipsoidea, and S. apiospermum (referred to as P. boydii complex in Fig. 1) two S. prolificans, and one each of Petriella guttulata and P. setifera, as well as two Aspergillus fumigatus strains representing contaminant organisms (Table 2).
Germination percentage in pure cultures
A sterile cotton swab was used to harvest conidia and, where formed, ascospores from cultures of the isolates listed in Table 2. These propagules were suspended in a Tween 80 0.01%/NaCl 0.85% solution in de-ionized water (w/v). Inoculum counts were determined microscopically with a Thoma chamber (0.1 mm × 0.0025 mm2). The suspensions were serially diluted in a series ranging from 106 to 101 conidia or spores per ml. The dilutions containing 101, 102, and 103 propagules/ml were inoculated on selective and non-selective solid media (Table 2) in triplicate for each dilution. Colonies were counted after incubation at 37°C for 5–7 days on plates where reliably countable numbers of colonies (i.e., 5–50) developed. The mean germination percentage was calculated based on data from the three parallel trials per isolate (Table 3).
Germination in mixed cultures
Two dilutions of an A. fumigatus conidial suspension, 3 × 103 and 3 × 102 conidia/ml, were plated in combination with two suspensions of P. boydii (1.6 × 106 and 1.6 × 104 conidia/ml) on SceSel+ and SceSel−. Each plate was inoculated with 0.2 ml A. fumigatus and 0.2 ml P. boydii suspension resulting in 600 and 60 conidia/plate together with 3.2 × 105 and 3.2 × 103 conidia per plate. Colonies were counted after incubation at 36°C for 4–5 days. The temperature was changed from 37°C to 36°C for technical reasons during this experiment.
Isolation of P. boydii from soil and sediment samples
To concretize the niche of P. boydii and to test the potential performance of SceSel+ medium in a larger ecological study, we assayed the abundance of P. boydii in water, sediment, and soil samples from a single locality. The values (Fig. 2) are illustrated as boxplots calculated from ten replicate plates. The samples were taken from the outflow of a pond frequented by waterfowl in a public park in Innsbruck, Austria. About 5–15 g sediment or soil was collected in sterile 50 ml plastic tubes. Materials were diluted 3.3 × 10−1 (w/v) in Tween 80 0.01%/NaCl 0.85%. Fungal propagules were extracted by shaking the suspension (end-over-end) for 1 h. A total of 250 μl of the suspension were inoculated on SceSel+ on ten replicate plates. After 5–7 days incubation at 37°C, colony counts were obtained; outgrowing fungi were identified based on their colonial and microscopic features (de Hoog et al. 2000).
Isolation of P. boydii from polluted water samples
Water samples were plated directly on ten replicate plates of SceSel+, at 0.5 ml per plate. After 1 week of incubation at 37°C, colonies were counted and identified as described above. The median, minimum, maximum and quartiles of CFU value/ml water were calculated.
Results
Germination percentage in pure culture
The average germination percentage of Pseudallescheria and S. apiospermum propagules on SceSel+ was 44.4% and ranged from 22.4% (CBS 108.54) to 83.9% (CBS 418.73). Average germination was somewhat lower on other media based on MLA and lower on rose bengal agar (28.4% MLA + Bsusp., 32.9% MLBCS; 30.1% rose Bengal, Table 3). On non-selective media, the mean germination levels of P. boydii and S. apiospermum strains lay between 9.5% and 70.4%. Only one strain of P. boydii (CBS 101.22) was found to germinate on DG18 (15.8%).
The mean germination of Scedosporium prolificans conidia ranged from 15.3% on MLA + Bsusp. to 59.5% on DG18. On SceSel+, germination levels of the two tested isolates were 49.0% and 30.3%, values that fall within the range seen for the other benomyl-containing media, except for MLA + Bsusp., for which a lower mean level was calculated. On the non-selective media PDA, SGA, and MEA germination levels were found to fall between 36.0% (SGA) and 50.3% (MEA).
Petriella species either did not grow at 37°C or were found to form much smaller colonies than P. boydii or S. prolificans within 5–7 days at 37°C on all tested media. Aspergillus fumigatus did not germinate on any of the benomyl-containing media, although a germination level of 91.4–100% was observed on non-selective media.
Germination in mixed cultures: comparison of SceSel with and without benomyl
Inoculating A. fumigatus and P. boydii together on SceSel− in concentrations of 3 × 102 to 1.6 × 106 resulted in a large number of small P. boydii colonies observable (1–2 mm diameter) between large, sporulating A. fumigatus colonies. On the SceSel+ plates only P. boydii was detectable (no growth of A. fumigatus) (Table 4). The detection limit of P. boydii in the presence of A. fumigatus on SceSel+ was found to lie below 1.6 × 103 conidia/ml. When A. fumigatus was not suppressed by benomyl, P. boydii was either not detectable or was too extensively overgrown to be enumerated. The germination percentage of P. boydii ascospores/conidia in the presence of A. fumigatus on SceSel+ was lower than in pure culture (Tables 3, 4).
Application of SceSel+ for the detection of P. boydii in soil, sediment, and water
The median value of CFU counts of P. boydii were 975.6 CFU/g dry weight (d.w.) in soil and 242 CFU/g d.w. in sediment. The fungus was not detected in water on this occasion (Fig. 2). In earlier experimental samplings (data not shown) the water of this pond was found to contain low CFU numbers of P. boydii.
Discussion
In comparisons of various general and selective media, it was shown that benomyl-amended MLA media do not exert negative effects on germination levels of Pseudallescheria and Scedosporium strains (Table 3, Fig. 1). Similarly, antibacterial and antimycotic agents had no adverse effect on the germination of P. boydii and S. prolificans at 37°C. Diluting benomyl in methanol instead of suspending it directly in the 55°C agar was introduced successfully and provides improved dispersal of this component. Other Microascaceae that might be isolated from soil or wood, e.g., Petriella spp., did not grow on SceSel+, or grew much slower at 37°C than at room temperature. Whereas a high proportion of A. fumigatus conidia germinated on media without benomyl, only a small proportion of P. boydii ascospores and conidia did so. Likewise, in mixed cultures of P. boydii and A. fumigatus, a more than 50-fold higher inoculum level of P. boydii led to CFU counts differing from A. fumigatus by a factor of only 2.3.
No growth of P. boydii was found on DG18; we therefore tested dichloran separately on MLBCS + D. In this test, germination levels were similar to those observed on other MLA-based media. The factor causing the inhibition of P. boydii on DG18 obviously is the very low water activity of this medium and not the presence of dichloran. To reduce the chance that SceSel+ would be overgrown by benomyl-tolerant Zygomycetes, dichloran was added to the medium. Because S. prolificans differed from P. boydii by growing on DG18, this medium can be used supplementary to microscopy for rapid non-molecular species distinction.
In addition to the problem of overgrowth by fast growing fungi, fungal soil isolation studies are complicated by problems posed by soil bacteria, especially Pseudomonas species. In the present study, it was shown that various antibiotic mixtures did not affect the germination of P. boydii ascospores/conidia but decreased bacterial growth in cultures of all sample types.
In experiments with artificially mixed inocula, it was demonstrated that the selective effect of SceSel+ is high, and that other thermotolerant fungi like A. fumigatus did not overgrow P. boydii on this medium. Therefore, underestimation of P. boydii in environmental samples is minimized when SceSel+ is used for isolation.
During field sampling, strains of P. boydii, some yeasts, and highly resistant bacteria were isolated. These isolates were not identified. Summerbell et al. (1993) stated, that several genera of fungi are resistant to benomyl, among them common ascomycetous and basidiomycetous yeasts (Candida, Cryptococcus, Hansenula, Kloeckera, Malassezia, Pichia, Rhodotorula, Saccharomyces, Trichosporon), members of the Pleosporaceae as well as Geotrichum candidum and Microascaceae. In our experience, these fungi do not represent a practical problem during isolation of P. boydii, because it grows in conditions where most competing organisms are inhibited by antibiotics or temperature. A minor problem was met with certain Zygomycetes which were resistant to the added antimycotics. As their growth was inhibited to a large extent, enumeration of P. boydii was always possible. Such overgrowth makes subculturing and microscopic investigation difficult, and therefore complicates species identification of members of the Pseudallescheria/Scedosporium clade.
Several publications (Pinto et al. 2001) have suggested that the preferred habitat of P. boydii is mainly in polluted water. Rainer and de Hoog (2006) postulated that ascomata of Pseudallescheria were adapted to release the ascospores in moist environments. Our application of ScelSel+ allowed assigning the main habitat of P. boydii to soil. But, this does not exclude the dispersal of ascospores by water: high P. boydii counts in soil may lead to an accumulation in the sediment through carriage by water.
One of the main types of events giving rise to Scedosporium infection is a near-drowning incident followed by coma (Guarro et al. 2006). Apparently, a mechanical disturbance of the sediment layers is necessary to suspend infectious particles into the water, which is subsequently aspirated. Probably this circumstantial infection route is the reason why infections with P. boydii are relatively uncommon.
Detection of P. boydii in the environment is relevant because this fungus is able to mineralize hydrocarbons and because of its clinical significance (Guarro et al. 2006). Our data demonstrate that non-selective isolation procedures underestimate the in situ frequency of Pseudallescheria and Scedosporium. Therefore, methods similar to the ones introduced here are crucial to studies on ecological reservoirs and populations of P. boydii. They facilitate risk assessment of the potential P. boydii habitats that are ever more abundant as a result of human activities.
References
April TM, Abbott SP, Foght JM, Currah RS (1998) Degradation of hydrocarbons in crude oil by the ascomycete Pseudallescheria boydii (Microascaceae). Can J Microbiol 44(3):270–278
Buzina W, Feierl G, Haas D et al (2006) Lethal brain abscess due to the fungus Scedosporium apiospermum (teleomorph Pseudallescheria boydii) after a near-drowning incident: case report and review of the literature. Med Mycol 44(5):473–477
Cimon B, Carrère J, Vinatier JF et al (2000) Clinical significance of Scedosporium apiospermum in patients with cystic fibrosis. Eur J Clin Microbiol Infect Dis 19(1):53–56
de Hoog GS, Guarro J, Gené J et al (2000) Atlas of clinical fungi, 2nd edn. Centraalbureau voor Schimmelcultures, Utrecht; Universitat Rovira i Virgili, Reus
de Hoog GS, Marvin-Sikkema FD, Lahpoor GA et al (1994) Ecology and physiology of Pseudallescheria boydii, an emerging opportunistic fungus. Mycoses 37:71–78
Guarro J, Kantarcioglu SA, Horré R et al (2006) Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Med Mycol 44:295–327
Horré R, de Hoog GS (1999) Primary cerebral infections by melanised fungi: a review. Stud Mycol 43:176–193
Kaltseis J, Rainer J (2006) Abundance of Pseudallescheria boydii, an opportunistic pathogen, in urban habitats. Mycoses 49(5):359
Pinto MR, Mulloy B, Haido RM et al (2001) A peptidorhamnomannan from the mycelium of Pseudallescheria boydii is a potential diagnostic antigen of this emerging human pathogen. Microbiology 147(6):1499–1506
Rainer J, de Hoog GS (2006) Molecular taxonomy and ecology of Pseudallescheria, Petriella and Scedosporium prolificans (Microascaceae) containing opportunistic agents on humans. Mycol Res 110:151–160
Samson RA, Hoekstra ES, Frisvad JC (eds) (2004) Introduction to food- and airborne fungi, 7th edn. Centraalbureau voor Schimmelcultures, Utrecht
Summerbell RC (1993) The benomyl test as a fundamental diagnostic method for medical mycology. J Clin Microbiol 31(3):572–577
Acknowledgements
The work was supported by the Austrian Science Fund P16289, the ECMM/ISHAM working group on Pseudallescheria and Scedosporium infections and the Mycology Network Innsbruck. We also acknowledge R. Pöder for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
A communication of the ECMM-ISHAM Working Group on Pseudallescheria/Scedosporium infections.
Rights and permissions
About this article
Cite this article
Rainer, J., Kaltseis, J., de Hoog, S.G. et al. Efficacy of a selective isolation procedure for members of the Pseudallescheria boydii complex. Antonie van Leeuwenhoek 93, 315–322 (2008). https://doi.org/10.1007/s10482-007-9206-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-007-9206-y