Abstract
HIV associated tuberculosis (TB) morbidity and mortality is a major concern in sub-Saharan Africa. Understanding the level of HIV infection among TB patients is vital for adequate response. We conducted a systematic review and meta-analysis to estimate the prevalence of HIV in TB patients in sub-Saharan Africa. We searched PubMed, EMBASE, Web of Science and CINAHL databases. A meta-analysis with a random-effects model was performed. Potential sources of heterogeneity in the prevalence estimates were explored using meta-regression analysis. We identified 68 studies that collectively included 62,969 TB patients between 1990 and 2017. The overall estimate of HIV prevalence in TB patients was 31.8% (95% CI 27.8–36.1). There was substantial heterogeneity in the prevalence estimates in Southern, Central, Eastern, and Western sub-Saharan Africa regions (43.7, 41.3, 31.1 and 25.5%, respectively). We noted an apparent reduction in the estimate from 33.7% (95% CI 27.6–40.4) in the period before 2000 to 25.7% (95% CI 17.6–336.6) in the period after 2010. The Eastern and Southern sub-Saharan Africa region had higher prevalence [34.4% (95% CI 29.3–34.4)] than the Western and Central region [27.3% (95% CI 21.6–33.8)]. The prevalence of HIV in TB patients has declined over time in sub-Saharan Africa. We argue that this is due to strengthened HIV prevention and control response and enhanced TB/HIV collaborative activities. Countries and regions with high burdens of HIV and TB should strengthen and sustain efforts in order to achieve the goal of ending both HIV and TB epidemics in line with the Sustainable Development Goals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, it is estimated that around 10.4 million tuberculosis (TB) cases occurred in 2016; out of which 1.3 million people died among HIV-negative people with an additional 374,000 deaths among HIV-positive people [1]. Overall, it is also estimated that 1.7 billion people are infected with M. tuberculosis. However, only 5–15% will develop TB disease during their lifetime. The risk of developing active TB differs markedly according to the presence of HIV infection and other risk factors. People living with HIV are 26–31 times more likely to develop active TB than people without HIV [1]. The burden of TB is greater in areas with higher HIV prevalence [2, 3]. The HIV epidemic poses a paramount challenge to TB prevention and control in sub-Saharan Africa. According to the World Health Organization (WHO), there were an estimated 1.2 million new cases of TB amongst people living with HIV in 2016, 71% of whom were living in Africa [1]. The WHO has recommended a package of collaborative TB/HIV activities that will reduce the burden of HIV infection in presumptive and diagnosed TB patients by providing: HIV testing and counselling, co-trimoxazole preventive therapy, antiretroviral therapy for HIV-positive TB patients, and other HIV prevention interventions, treatment and care [4].
In many high HIV prevalence settings, concerted measures have been taken to prevent and control HIV/AIDS since 2000. These have reduced the burden of HIV-associated TB morbidity and mortality [5]. In 2016, TB incidence and mortality rates have decreased by an average of 2% and 3% per year, respectively, since 2000. The pace of decline has varied among different WHO regions. In sub-Saharan Africa countries including Ethiopia, Kenya, Lesotho, Namibia, the United Republic of Tanzania, Zambia and Zimbabwe, there has been a decline in TB incidence, TB mortality rate and HIV-associated TB between 1990 and 2017 [6, 7]. Nevertheless, HIV is still challenging TB control efforts and an accelerated response is needed to produce a 4–5% annual decline in order to achieve the “End TB” milestone for the region in 2020 [1].
The accelerated response towards “End TB” targets require consolidated evidence on the burden of HIV in TB patients. Two systematic reviews [8, 9] and one narrative review [10] have been published on the burden of HIV in TB and/or TB in HIV coinfection. However, they do not include all studies done in sub-Saharan African and do not attempt to show HIV prevalence in TB patients distinctly. According to a systematic review and meta-analysis of the global prevalence of HIV/TB and/or TB/HIV co-infection in countries (excluding China) in 2013, the prevalence of co-infection [31.2% (95% CI 19.3–43.2)] in African countries was higher than for the rest of the world [8]. Therefore, it is important to exclusively report HIV prevalence and its trend over time in TB patients in the sub-Saharan Africa where about half of the high TB burden countries are located.
In this study we aimed to systematically review the existing literature and quantify the prevalence of HIV in TB patients over three different distinct time periods across regions in sub-Saharan Africa.
Methods
Search Methods for Identifying Studies
We searched PubMed, EMBASE, Web of Science and CINAHL electronic databases using a predefined search strategy (Supplementary material I). Studies reporting HIV infection in TB patients which were published up to September 14, 2017 were included. In addition to the articles we retrieved from our literature search, we hand-searched the references of all the relevant articles to ensure that we did not exclude eligible studies. Searches were limited to human studies (all age groups) conducted in sub-Saharan Africa countries and published in English language.
Eligibility Criteria
Studies were eligible for inclusion if the prevalence of HIV in TB patients was presented or could be calculated from available data. All study designs including prevalence surveys, and published reports of programmatic activities were included.
Studies were excluded if the language of publication was not English, if they only reported the prevalence (not reported people screened for HIV test and HIV tested positive TB patients), were reviews were conducted in a special population (e.g. miners), or if they only reported prevalence of TB or HIV.
Data Extraction
All searched studies were imported into EndNote X8 database and duplicate records were removed. YG examined studies using the title and abstract to remove irrelevant studies. The full-text of eligible articles was independently retrieved by two authors (YG and YA) for final inclusion using inclusion/exclusion criteria with a predesigned assessment form.
YG and YA extracted information from selected studies using a data extraction form and inconsistencies were resolved by discussion. For each study, information on geographical location, year of publication, study periods, sample size and sampling strategies, study type, clinical form of TB, age group of the study population, and the diagnostic algorithms of TB and HIV were extracted.
Definitions
For the purpose of this analysis, we categorised HIV prevalence by three research periods and two WHO sub-Saharan Africa regions. We further divided the two regions into four. The research period was defined by the duration of the study: before 2000, 2000 to 2010 and after 2010. These periods were set based on key milestones in the HIV/AIDS and TB/HIV control programs. In the early 2000s, ART scale-up commenced in some health facilities in sub-Saharan Africa countries [11]; between 2000 and 2010, TB/HIV collaborative activities were launched, and HIV/AIDS care and treatment services strengthened in sub-Saharan Africa countries [12]. Since 2010 WHO has been updating its HIV/AIDS treatment guidelines to initiate ART with 350 or less than 350 cell counts. Currently people living with HIV have treatment initiated irrespective of CD4-cells count [13].
Geographic location of studies was categorised as Eastern, Southern, Western and Central regions. These regions were further classified into Central, Eastern, Southern and Western sub-Saharan Africa regions.
Quality Assessment
We used the Newcastle–Ottawa Scale adapted for cross-sectional studies and used by Modesti et al. [14] and Nliwasa et al. [15] to assess the methodological quality of each study. In the scale score of exposure (tuberculosis) and outcome (HIV prevalence in TB patients) measurement of each study was assessed based on the national standard tuberculosis and HIV diagnosis and test procedures of the included countries. Self-reported exposure and outcomes were excluded because TB and HIV disease results are not reported unless confirmed by a health professional. Two stars are given to the studies that assess the outcome (HIV prevalence in active TB patients) with the antibody test and one star is given for medical records linkage to HIV testing (Supplementary Table I).
For each study, the maximum overall score was seven. The overall quality of the studies was categorised into two based on the total scores given using the domain; high quality (studies with an average score or above) and low quality (studies with a score of below average) (Supplementary Table II).
Statistical Analyses
Meta-analysis was undertaken to calculate the pooled HIV prevalence and its 95% CI using a random-effects model (to account for heterogeneity of HIV-prevalence) using the metafor package which carries out meta-analysis for proportions in R (version 3.3.3, the R foundation for Statistical Computing, Vienna, Australia).
A meta-analysis for a proportion includes studies that do not use control groups unlike other types of meta-analysis. We also need to transform data to improve the statistical properties [16]. The logit transformation method was used and back-transformed to calculate the final pooled estimate and 95% CI. The Shapiro–Wilk normality test (W = 0.9811, p value = 0.3905) showed that the transformed data had a normal distribution. Restricted maximum-likelihood estimation (REML) is used to estimate model parameters.
Between-study heterogeneity was assessed with the I2 statistic, which describes the percentage of variation between studies, compared to the overall variation [17]. An influential study diagnostic test, i.e., Cook’s distance (the influence function and baujat plot in R) was used to identify studies which introduced additional residual heterogeneity [18]. Sensitivity analysis was assessed by removing outliers (higher and lower prevalence) and low quality studies. We checked this using the ‘leave one out’ approach (leaveout function of R) [19].
Subgroup analysis was conducted by assuming a common between-study variance component across sub-groups and meta-regression were used to explore heterogeneity further [20, 21]. Subgroup analysis compared Central (reference category) with Eastern, Western, and Southern sub-Saharan African and research periods (before 2000 compared with between 2000 and 2010 inclusive and after 2010) and type of TB (all forms of TB versus Pulmonary TB).
Potential publication bias was investigated using funnel plots. This was further examined using Begg’s test which examines the rank correlation between the log odds ratio and the meta-analysis weight [22]. Trim and fill plot analysis was also used for assessing publication bias.
Data on TB and HIV infections per 100,000 population for each country were obtained from UNAIDS and UN Millennium Development Goals databases [23, 24] and used to extract national estimates for the year of completion of the study period. We included significant variables (p < 0.05) into a meta-regression model using the metareg function of R [25].
Results
Characteristics of Included Studies
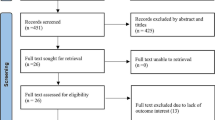
A total of 1, 490 articles were initially identified in our search. After the exclusion of duplicate references (143) and non-relevant studies (1157), a full text review was conducted for 190 articles. Finally, 68 articles were included in the meta-analysis (Fig. 1).
Among the 68 eligible studies published from 1990 to 2017, 26 were conducted before 2000 [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51] were from 2000 to 2010 [52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81], and 12 were after 2010 [82,83,84,85,86,87,88,89,90,91,92,93]. With respect to age group, 36 studies included adults, eight studies included children and 24 studies were unclassified. One-third (22 studies) were conducted on pulmonary tuberculosis (PTB) and 46 studies were not classified by type of TB. Two studies estimated prevalence in special populations: pregnant women [63] and prisoners [88]. The sample size of the studies ranged from 74 to 10, 612, with a combined total of 62,969 TB patients included to estimate HIV prevalence in TB patients. HIV prevalence was 32.5% (95% CI 24.8–41.2) among PTB patients and 31.5% (95% CI 27–34.4%) among uncategorised TB patients.
More than half of included studies were from the Eastern region; eighteen were in Ethiopia [26, 44, 48, 51, 53, 59, 63, 67, 72, 75, 77, 78, 80, 81, 83, 84, 88, 90], seven in Tanzania [32, 37, 38, 47, 60, 71, 79], five in Kenya [31, 62, 65, 73, 74], one in Eretria [61] and one in Uganda [28]. Twelve articles were included from the Southern region; four in South Africa, four in Zambia [30, 34, 64, 91], two in Zimbabwe [29, 33], one in Angola [76] and one in Malawi [46].
Nearly one-third of included studies were from the Western Sub-Saharan Africa region; fourteen in Nigeria [43, 49, 50, 52, 54, 55, 57, 58, 68, 69, 86, 87, 89, 92], three in Cote d’Ivoire [27, 35, 36], two in Ghana [56, 93], one in Burkina Faso [39] and one in Togo [70]. Only three studies were included in the Central sub-Saharan Africa regions; two in Cameroon [66, 82] and one in Republic of Congo [85] (Table 1).
Prevalence of HIV in Tuberculosis Patients
The overall estimates of HIV prevalence ranged from 6% in Ethiopia [88] to 72% in Zambia [64] study. Most estimates were between 20 and 30% of the population. Low estimates (less than 20%) occurred in 16 studies [26, 28, 35, 43, 49, 50, 52,53,54, 58, 61, 63, 75, 87, 88, 93]. Forty-four studies presented age-specific HIV prevalence. The prevalence among children and adults were 31.4% (95% CI 17.1–50.2) and 31.1% (95% CI 26.5–36.0), respectively.
The overall estimates of HIV prevalence in TB patients was 31.8% (95% CI 27.8–36.1) using a random-effects model. The I2 statistic (98%, P < 0.01) indicated substantial heterogeneity among the studies.
The highest HIV prevalence in TB patients was estimated to be in the Southern region, 43.7% (95% CI 35.05–52.70; I2 = 99%) with a range of 23% [91] to 72% [64] in the Zambia followed by the Central region (41.3%, 95% CI 30.4–51.2; I2 = 98%) ranging from 31% in Republic of Congo [85] to 51% in Cameroon [66]; Eastern region 31.1% (95% CI 25.4–37.5; I2 = 98%) ranging from 6% in Ethiopia to 60% [88] in Kenya [65] and Western region 25.5% (95% CI 19.7–32.3; I2 = 98%) ranging from 12% [43] to 72% in the Nigeria [86] (Fig. 2).
There were an apparent reduction in prevalence of HIV in TB from before 2000, [33.7% (95% CI 27.6–40.4); I2 = 98%, p < 0.01) to after 2010, 25.7% (95% CI 17.6–36.0); I2 = 98%, p < 0.01)] (Fig. 3).
HIV prevalence in TB patients was 34.41% (95% CI 29.27–39.94%) in Eastern and Southern regions and 27.30% (95% CI 21.63–33.82%) in Western and Central regions of sub-Saharan Africa. A significant decline was observed in Southern and Eastern Africa region, where HIV prevalence is highest, from before 2000, 38.15% (95% CI 31.25–45.55, I2 = 97%), to after 2010, 18.87%, 95% CI 11.01–30.42, I2 = 95%) (Fig. 4).
In the Western and Central region, where HIV prevalence is relatively low, an increase was observed from before 2000, 23.00% (95% CI 15.07–33.43; I2 = 99%) to after 2010, 31.5% (95% CI 19.41–46.76; I2 = 99%) (Fig. 5).
Sub-group Analysis and Meta-regression
Sub-group analysis showed that research period (p = 0.34), population category (p = 0.89), type of TB (p = 0.81), sample size (p = 0.28) and study type (p = 0.76) were not significantly associated with HIV prevalence. Regions within sub-Saharan Africa (p = 0.02, R2 = 9.05%) and study quality (p = 0.042, R2 = 5.01%) were significantly associated with HIV prevalence; together, they explained 12.04% of heterogeneity.
In univariate meta-regression analyses, type of TB (PTB or uncategorised TB), population category (adults compared with children and all age groups), sample size (≤ 500 or > 500) and TB prevalence per 100,000 population were not significantly associated with HIV prevalence in TB (Table 2). However, significant variation was found for geographical region (Central or other regions) and HIV prevalence per 100,000 population. These variables accounted for variation, measured by R2 as follows: \(R_{\text{region}}^{2} = 9.05\%\), Pregion = 0.021; \(R_{\text{HIV}}^{2} = 7.87\%\), PHIV = 0.011. A multivariate mixed-effects meta-regression model was fitted using geographical region and HIV prevalence per 100,000 population as covariates. These two variables accounted for 13.3% of the heterogeneity in the HIV prevalence in TB patients estimates (\(R_{{{\text{region}} + {\text{HIV}}}}^{2} = 13.3\%\), Pregion + HIV = 0.0070).
Influence of Study Quality on HIV Prevalence in TB Patients
Sensitivity analysis was assessed by systematically removing outliers (high and lower prevalence) and low-quality studies. There was no material difference in the estimate of HIV prevalence [31.9% (95% CI 28.6–35.4) and 33.63% (95% CI 29.3–38.2)] when removing outliers and low-quality studies, respectively (Supplementary Table III).
Publication bias was assessed using a funnel plot (Fig. 6). Each point represents an individual study. The points are distributed asymmetrically, indicating the existence of publication bias. However, Begg’s test demonstrated non-significant publication bias (p = 0.2518).
Discussion
The overall pooled prevalence of HIV infection in TB patients in sub-Saharan Africa was 31.8% (95% CI 27.8–36.1) with an apparent reduction from 33.7% (95% CI 27.5–40.4) before 2000 to 25.7% (95% CI 17.6–36.0) after 2010. The Eastern and Southern sub-Saharan Africa region had a higher HIV prevalence [34.4% (95% CI 29.3–34.4)] than the Western and Central sub-Saharan Africa region [27.3% (95% CI 21.6–33.8)]. The prevalence of HIV dropped significantly in the Eastern and Southern sub-Saharan African region, from 38.15% (95% CI 31.25–45.55) before 2000 to 18.87% (95% CI 11.01–30.42) after 2010, while it increased in Western and Central sub-Saharan African region over time, from 23.00% (95% CI 15.07–33.43) before 2000 to 31.5% (95% CI 19.41–46.76) after 2010.
A previous meta-analysis of HIV/TB co-infection prevalence in countries excluding China [9] reported a lower prevalence (25%) than the present review. This is because our analysis included only studies conducted in the sub-Saharan region where HIV prevalence is higher compared with regions included in the previous studies. However, the WHO series of global TB reports showed that the incidence of HIV infection in Africa region has gradually decreased from 130 in 2000 to 75 per 100,000 population in 2016 [1].
Globally, HIV/AIDS and TB/HIV control measures have been strengthened and services scaled up since 2000 [94]. The prevalence of HIV in TB declined progressively in South-East regions, while it remains high in West-Central region from before 2000 to the after 2010 [6]. We argue that the discrepancy in the implementation of these activities explains our findings that the trend of HIV prevalence among TB patients varies across regions in sub-Saharan Africa.
The 2018 UNAIDS report indicated that in Eastern and Southern sub-Saharan Africa regions antiretroviral therapy (ART) coverage has increased from about 26% in 2010 to about 66% in 2017 [6], whereas in Western and Central region ART coverage has increased from about 14% in 2010 to 40% in 2017, which is lagging behind the rest of sub-Saharan Africa [23]. Moreover, the high number of people who do not know their HIV status is a key barrier [95]. Despite these gaps, huge decisive steps have been made towards meeting the 90–90–90 targets. In 2016, in the Eastern and Southern sub-Saharan Africa, almost all people living with HIV in the region, who were aware of their status were on treatment [95]. Therefore, the substantial reduction in HIV prevalence among TB patients in the Eastern and Southern region is likely to be due to effective scaling up of HIV prevention and ART programmes among the general population in addition to other similar activities, including TB/HIV collaborative activities.
Our meta-regression analysis showed that geographical region and population prevalence of HIV are sources of heterogeneity. Western sub-Saharan Africa region had a significantly lower prevalence of HIV in TB patients than Central, Eastern or Southern regions. Prevalence of HIV among TB patients is positively associated with HIV prevalence in the general population in countries of origin of the studies, which is consistent with a previous systematic review and successive WHO reports [1, 8, 96]. However, these two variables explained only 13.3% of the heterogeneity. Other characteristics that were not reported in the original articles such as types of diagnostic tools for TB and HIV, types of HIV, types of TB, data collection methods, or by study methods could cause this heterogeneity.
The findings of this study have important practical relevance and implications toward achieving the goal of ending TB in line with the WHO’s ‘‘End TB’’ Strategy [97]. This is especially so for countries with high TB and HIV burden. The evidence that HIV prevalence among TB patients has fallen from the level before 2000 to that after 2010 in regions with high HIV prevalence and better HIV and TB/HIV response implies that the response has been effective. Countries and regions with high burdens of TB and HIV, which are lagging in response, need to strengthen their HIV and TB/HIV response packages. This is important to reduce the burden of HIV among TB patients, and vice versa [4].
The fast-tracking of the HIV response has been a focus of HIV high-burden countries mainly in South and East sub-Saharan Africa, while most countries in the Western and Central Africa region have neglected to provide adequate response [98, 99]. We argue, based on the results of this review, that fast-tracking the HIV response including TB/HIV collaborative activities in these sub-Saharan Africa regions would reduce the overwhelming double burden of these infections on the health care system [1]. This review calls for strengthened HIV prevention, routine HIV testing, treatment, and care for TB patients in Western and Central regions in sub-Saharan Africa [4, 97, 100].
This review has acknowledged the following potential limitations: (1) all published reports and articles might not have been included in our database search, particularly those in country-specific journals; (2) the pooled prevalence of HIV is estimated among TB patients who have access to HIV testing in TB diagnostic and treatment health facilities and likely overestimate the real prevalence; (3) types of TB, types of HIV, mean age and age group were not reported in most articles; (4) TB diagnostic methods and HIV testing methods also varied between studies and over time and by country. Thus, the findings from this review may not be generalizable to countries not included in this review. Nevertheless, the review has strengths: it identified 68 studies published from 1990 to 2017, which allowed us to pool results from 62,969 TB patients tested for HIV. The review provides evidence to enable countries in the sub-Saharan Africa region to evaluate their TB/HIV collaborative activities, particularly routine HIV testing, prevention, treatment, care and support, and to strengthen their efforts to achieve the goal of ending TB by 2035 and HIV by 2030.
Conclusion
Overall, the prevalence of HIV infection among TB patients has steadily declined in sub-Saharan Africa from 1990 to 2017. This reduction is most pronounced in Eastern and Southern sub-Saharan Africa regions, showing the effectiveness of the HIV response and extended TB/HIV collaborative activities in these regions. On the contrary, there was an increase in HIV prevalence in TB patients in Western and Central regions of sub-Saharan Africa, where the response to HIV and TB/HIV has been relatively inadequate. Our systematic review and meta-analysis sheds light on the significance of the HIV and TB/HIV response toward the goal of ending TB and HIV in sub-Saharan Africa and beyond.
References
World Health Organization. Global Tuberculosis Report 2017. WHO; 2017.
Jacobson LM, de Lourdes Garcia-Garcia M, Hernandez-Avila JE, et al. Changes in the geographical distribution of tuberculosis patients in Veracruz, Mexico, after reinforcement of a tuberculosis control programme. Trop Med Int Health. 2005;10(4):305–11.
World Health Organization. Global tuberculosis report 2013. World Health Organization; 2013.
World Health Organization. WHO policy on collaborative TB/HIV activities: guidelines for national programmes and other stakeholders. Geneva: WHO; 2014.
Unaids U. The gap report. Geneva: Switzerland; 2014.
UNAIDS. UNAIDS Data 2018.
World Health Organization. Tuberculosis (TB). Tuberculosis data; 2017; http://www.who.int/tb/data/en/.
Gao J, Zheng P, Fu H. Prevalence of TB/HIV co-infection in countries except China: a systematic review and meta-analysis. PLoS ONE. 2013;8(5):e64915.
Gao L, Zhou F, Li X, Jin Q. HIV/TB co-infection in mainland China: a meta-analysis. PLoS ONE. 2010;5(5):e10736.
Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet. 2006;367:926–37.
United Nations (UN). Millennium Summit (6–8 September 2000); 2000.
World Health Organization. Towards universal access by 2010: how WHO is working with countries to scale-up HIV prevention, treatment, care and support. Geneva: World Health Organization; 2006.
Wilkinson D, Davies GR. The increasing burden of tuberculosis in rural South Africa—impact of the HIV epidemic. S Afr Med J. 1997;87(4):447–50.
Modesti PA, Reboldi G, Cappuccio FP, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS ONE. 2016;11(1):e0147601.
Nliwasa M, MacPherson P, Gupta-Wright A, et al. High HIV and active tuberculosis prevalence and increased mortality risk in adults with symptoms of TB: a systematic review and meta-analyses. J Int AIDS Soc. 2018;21(7):e25162.
Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013:jech-2013-203104.
Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Hampel FR, Ronchetti EM, Rousseeuw PJ, Stahel WA. Robust statistics: the approach based on influence functions, vol. 114. New York: Wiley; 2011.
Sutton AJ, Abrams KR, Jones DR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. Chichester: Wiley; 2000.
Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. Br Med J. 2001;323(7304):101.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
UNAIDS. AIDS data; 2017. http://www.unaids.org/en/resources/aids_data. Accessed 24 Oct 2017.
UN. Millennium Development Goals database; 2017. https://data.worldbank.org/data-catalog/millennium-development-indicators. Accessed 24 Oct 2017.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48.
Hailu K, Debrework Z, Bekure D, et al. The prevalence of HIV-1 antibodies in 106 tuberculosis patients. Ethiop J Health Dev. 1990;4(2):197–200.
De Cock KM, Gnaore E, Adjorlolo G, et al. Risk of tuberculosis in patients with HIV-I and HIV-II infections in Abidjan. Ivory Coast. BMJ. 1991;302(6775):496–9.
Migliori GB, Borghesi A, Adriko C, et al. Tuberculosis and HIV infection association in a rural district of northern Uganda: epidemiological and clinical considerations. Tuber Lung Dis. 1992;73(5):285–90.
Pozniak AL, MacLeod GA, Mahari M, Legg W, Weinberg J. The influence of HIV status on single and multiple drug reactions to antituberculous therapy in Africa. Aids. 1992;6(8):809–14.
Chintu C, Bhat G, Luo C, et al. Seroprevalence of human immunodeficiency virus type 1 infection in Zambian children with tuberculosis. Pediatr Infect Dis J. 1993;12(6):499–504.
Nunn P, Gathua S, Kibuga D, et al. The impact of HIV on resource utilization by patients with tuberculosis in a tertiary referral hospital, Nairobi, Kenya. Tuber Lung Dis. 1993;74(4):273–9.
Van den Broek J, Borgdorff M, Pakker N, et al. HIV-1 infection as a risk factor for the development of tuberculosis: a case–control study in Tanzania. Int J Epidemiol. 1993;22(6):1159–65.
Houston S, Ray S, Mahari M, et al. The association of tuberculosis and HIV infection in Harare, Zimbabwe. Tuber Lung Dis. 1994;75(3):220–6.
Luo C, Chintu C, Bhat G, et al. Human immunodeficiency virus type-1 infection in zambian children with tuberculosis: changing seroprevalance and evaluation of a thioacetazone-free regimen. Tuber Lung Dis. 1994;75(2):110–5.
Sassan-Morokro M, De Cock KM, Ackah A, et al. Tuberculosis and HIV infection in children in Abidjan, Cote d’Ivoire. Trans R Soc Trop Med Hyg. 1994;88(2):178–81.
Richards SB, St Louis ME, Nieburg P, et al. Impact of the HIV epidemic on trends in tuberculosis in Abidjan, Cote d’Ivoire. Tuber Lung Dis. 1995;76(1):11–6.
van Cleeff MR, Chum HJ. The proportion of tuberculosis cases in Tanzania attributable to human immunodeficiency virus. Int J Epidemiol. 1995;24(3):637–42.
Chum HJ, O’brien RJ, Chonde TM, Graf P, Rieder HL. An epidemiological study of tuberculosis and HIV infection in Tanzania, 1991–1993. AIDS. 1996;10(3):299–310.
Malkin JE, Prazuck T, Simonnet F, et al. Tuberculosis and human immunodeficiency virus infection in west Burkina Faso: clinical presentation and clinical evolution. Int J Tuberc Lung Dis. 1997;1(1):68–74.
Colvin M, Karim Abdool SS. HIV infection among patients with tuberculosis in KwaZulu/Natal, South Africa. Int J Tuberc Lung Dis. 1998;2(2):172.
Karstaedt AS, Jones N, Khoosal M, Crewe-Brown HH. The bacteriology of pulmonary tuberculosis in a population with high human immunodeficiency virus seroprevalence. Int J Tuberc Lung Dis. 1998;2(4):312–6.
Churchyard GJ, Kleinschmidt I, Corbett EL, Mulder D, De Cock KM. Mycobacterial disease in South African gold miners in the era of HIV infection. Int J Tuberc Lung Dis. 1999;3(9):791–8.
Onipede A, Idigbe O, Ako-Nai A, et al. Sero-prevalence of HIV antibodies in tuberculosis patients in Ile-Ife, Nigeria. East Afr Med J. 1999;76(3):127–32.
Demissie M, Lindtjørn B, Tegbaru B. Human Immunodeficiency virus (HIV) infection in tuberculosis patients in Addis Ababa. Ethiop J Health Dev. 2000;14(3):277–82.
Madhi SA, Huebner RE, Doedens L, Aduc T, Wesley D, Cooper PA. HIV-1 co-infection in children hospitalised with tuberculosis in South Africa. Int J Tuberc Lung Dis. 2000;4(5):448–54.
Kiwanuka J, Graham SM, Coulter JB, et al. Diagnosis of pulmonary tuberculosis in children in an HIV-endemic area, Malawi. Ann Trop Paediatr. 2001;21(1):5–14.
Range N, Ipuge YA, O’Brien RJ, et al. Trend in HIV prevalence among tuberculosis patients in Tanzania, 1991–1998. Int J Tuberc Lung Dis. 2001;5(5):405–12.
Bruchfeld J, Aderaye G, Palme IB, et al. Evaluation of outpatients with suspected pulmonary tuberculosis in a high HIV prevalence setting in Ethiopia: clinical, diagnostic and epidemiological characteristics. Scand J Infect Dis. 2002;34(5):331–7.
Moses A, Adelowo K, Ajayi B. Prevalence of HIV-1 infection among patients with leprosy and pulmonary tuberculosis in a semi-arid region, Nigeria. J R Soc Promot Health. 2003;123(2):117–9.
Odaibo GN, Gboun MF, Ekanem EE, et al. HIV infection among patients with pulmonary tuberculosis in Nigeria. Afr J Med Med Sci. 2006;35(Suppl):93–8.
Gellete A, Kebede D, Berhane Y. Tuberculosis and HIV infection in southern Ethiopia. Ethiop J Health Dev. 2017;11(1):51–9.
Daniel O, Salako A, Oluwole F, Alausa O, Oladapo O. HIV sero-prevalence among newly diagnosed adult pulmonary tuberculosis patients in Sagamu. Niger J Med. 2004;13(4):393.
Yassin MA, Takele L, Gebresenbet S, et al. HIV and tuberculosis coinfection in the southern region of Ethiopia: a prospective epidemiological study. Scand J Infect Dis. 2004;36(9):670–3.
Daniel O, Ogunfowora O, Oladapo O. HIV and tuberculosis co-infection in children: presentation and treatment outcome. Niger J Paediatr. 2005;32(4):83–7.
Ige OM, Sogaolu OM, Ogunlade OA. Pattern of presentation of tuberculosis and the hospital prevalence of tuberculosis and HIVco-infection in University College Hospital, Ibadan: a review of five years (1998–2002). Afr J Med Med Sci. 2005;34(4):329–33.
Adjei AA, Adiku TK, Ayeh-Kumi PF, Hesse IF. Prevalence of human immunodeficiency virus infection among tuberculosis suspect patients in Accra, Ghana. West Afr J Med. 2006;25(1):38–41.
Salami AK, Katibi IA. Human immunodeficiency virus-associated tuberculosis: pattern and trend in the University of Ilorin Teaching Hospital. Afr J Med Med Sci. 2006;35(4):457–60.
Daniel OJ, Ogunfowora OB, Oladapo OT. HIV sero-prevalence among children diagnosed with TB in Nigeria. Trop Doct. 2007;37(4):268–9.
Kassu A, Mengistu G, Ayele B, et al. HIV and intestinal parasites in adult TB patients in a teaching hospital in Northwest Ethiopia. Trop Doct. 2007;37(4):222–4.
Range N, Magnussen P, Mugomela A, et al. HIV and parasitic co-infections in tuberculosis patients: a cross-sectional study in Mwanza, Tanzania. Ann Trop Med Parasitol. 2007;101(4):343–51.
van der Werf MJ, Sebhatu M, Weldegergis T, Tesfazion A, Borgdorff MW. TB-HIV co-infection in Eritrea. Int J Tuberc Lung Dis. 2007;11(7):823–6.
Chakaya J, Uplekar M, Mansoer J, et al. Public-private mix for control of tuberculosis and TB-HIV in Nairobi, Kenya: outcomes, opportunities and obstacles. Int J Tuberc Lung Dis. 2008;12(11):1274–8.
Datiko DG, Yassin MA, Chekol LT, Kabeto LE, Lindtjorn B. The rate of TB-HIV co-infection depends on the prevalence of HIV infection in a community. BMC Public Health. 2008;8:266.
Mwinga A, Mwananyambe N, Kanene C, et al. Provider-initiated HIV testing and counseling of TB patients—Livingstone District, Zambia, September 2004–December 2006. MMWR Morb Mortal Wkly Rep. 2008;57(11):285–9.
Odhiambo J, Kizito W, Njoroge A, et al. Provider-initiated HIV testing and counselling for TB patients and suspects in Nairobi, Kenya. Int J Tuberc Lung Dis. 2008;12(3 Suppl 1):63–8.
Sume GE, Etogo D, Kabore S, Gnigninanjouena O, Epome SS, Metchendje JN. Seroprevalence of human immunodeficiency virus infection among tuberculosis patients in the Nylon district hospital tuberculosis treatment centre. East Afr Med J. 2008;85(11):529–36.
Ayenew A, Leykun A, Colebunders R, Deribew A. Predictors of HIV testing among patients with tuberculosis in North West Ethiopia: a case–control study. PLoS ONE. 2010;5(3):e9702.
Erhabor O, Jeremiah Z, Adias T, Okere C. The prevalence of human immunodeficiency virus infection among TB patients in Port Harcourt Nigeria. HIV/AIDS (Auckland, NZ). 2010;2:1.
Pennap G, Makpa S, Ogbu S. Sero-prevalence of HIV infection among tuberculosis patients in a rural tuberculosis referral clinic in northern nigeria. Pan Afr Med J. 2010;5:22.
Dagnra A, Adjoh K, Tchaptchet HS, et al. Prevalence of HIV-TB co-infection and impact of HIV infection on pulmonary tuberculosis outcome in Togo. Bull Soc Pathol Exot. 2011;104(5):342–6.
Kamenju P, Aboud S. Tuberculosis-HIV co-infection among patients admitted at Muhimbili National hospital in Dar es salaam, Tanzania. J Health Res. 2011;13(1):25–31.
Ligidi T, Gebre-Selassie S, Tsegaye A. The immunological status of newly diagnosed tuberculosis patients co-infected with human immunodeficiency virus-1 in Adama Hospital, Ethiopia. Ethiop Med J. 2011;49(2):75–83.
van’t Hoog AH, Laserson KF, Githui WA, et al. High prevalence of pulmonary tuberculosis and inadequate case finding in rural western Kenya. Am J Respir Crit Care Med. 2011;183(9):1245–53.
Nyamogoba HDN, Mbuthia G, Mining S, et al. HIV co-infection with tuberculous and non-tuberculous mycobacteria in western Kenya: challenges in the diagnosis and management. Afr Health Sci. 2012;12(3):305–11.
Teklu T, Belyhun Y, Tesfaye S, Medhin G. Trends of tuberculosis and HIV infections between 2004 and 2008 in Wolaita Sodo, southern Ethiopia. Ethiop Med J. 2012;50(1):1–11.
Valadas E, Gomes A, Sutre A, et al. Tuberculosis with malaria or HIV co-infection in a large hospital in Luanda, Angola. J Infect Dev Ctries. 2013;7(3):269–72.
Kebede W, Keno F, Ewunetu T, Mamo G. Acceptance of provider initiated HIV testing and counseling among tuberculosis patients in East Wollega administrative zone, Oromia regional state, western Ethiopia. Tuberc Res Treat. 2014. https://doi.org/10.1155/2014/935713.
Keflie TS, Ameni G. Microscopic examination and smear negative pulmonary tuberculosis in Ethiopia. Pan Afr Med J. 2014;19:162.
Kishimba RS, Mghamba J, Mmbaga VM. Trend of HIV infection among pediatric tuberculosis patients in Tanzania, 2006-2010. Int J Infect Dis. 2014;21:125.
Mihret A, Bekele Y, Aytenew M, et al. Human immunodeficiency virus infection among new smear positive pulmonary tuberculosis patients in Addis Ababa, Ethiopia. Ethiop Med J. 2014;2014(Suppl 1):1–6.
Belay M, Bjune G, Abebe F. Prevalence of tuberculosis, HIV, and TB-HIV co-infection among pulmonary tuberculosis suspects in a predominantly pastoralist area, northeast Ethiopia. Global Health Action. 2015;8:27949.
Namme LH, Marie-Solange D, Bertrand MNH, Elvis T, Achu JH, Christopher K. Extrapulmonary tuberculosis and HIV coinfection in patients treated for tuberculosis at the Douala General Hospital in Cameroon. Ann Trop Med Public Health. 2013;6(1):100.
Yadeta D, Alemseged F, Biadgilign S. Provider-initiated HIV testing and counseling among tuberculosis patients in a hospital in the Oromia region of Ethiopia. J Infect Public Health. 2013;6(3):222–9.
Denegetu AW, Dolamo BL. HIV screening among TB patients and co-trimoxazole preventive therapy for TB/HIV patients in Addis Ababa: facility based descriptive study. PLoS ONE. 2014;9(2):e86614.
Linguissi LS, Mayengue PI, Sidibe A, et al. Prevalence of national treatment algorithm defined smear positive pulmonary tuberculosis in HIV positive patients in Brazzaville, Republic of Congo. BMC Res Notes. 2014;7:578.
Gomerep SS, Eze UA, Chiegboka LO, et al. Sputum smear pattern among patients diagnosed with pulmonary tuberculosis in Makurdi, North Central Nigeria. Niger J Med. 2015;24(3):201–6.
Ojiezeh TI, Ogundipe OO, Adefosoye VA. A retrospective study on incidence of pulmonary tuberculosis and human immunodeficiency virus co-infection among patients attending National Tuberculosis and Leprosy Control Programme, Owo centre. Pan Afr Med J. 2015;20:345.
Gebrecherkos T, Gelaw B, Tessema B. Smear positive pulmonary tuberculosis and HIV co-infection in prison settings of North Gondar Zone, Northwest Ethiopia. BMC Public Health. 2016;16(1):1091.
Ranti KO, Glory AO, Victoria BT, Isaac KO. Prevalence of HIV infection among tuberculosis patients in a teaching hospital in south-west Nigeria: a four-year retrospective study. HIV Rev. 2016;15(4):136–40.
Tarekegne D, Jemal M, Atanaw T, et al. Prevalence of human immunodeficiency virus infection in a cohort of tuberculosis patients at Metema Hospital, Northwest Ethiopia: a 3 years retrospective study. BMC Res Notes. 2016;9(1):192.
Chanda-Kapata P, Kapata N, Klinkenberg E, Grobusch MP, Cobelens F. The prevalence of HIV among adults with pulmonary TB at a population level in Zambia. BMC Infect Dis. 2017;17(1):236.
Chinedu K, Takim AE, Obeagu EI, Chinazor UD, Eloghosa O, Ojong OE. HIV and TB co-infection among patients who used Directly Observed Treatment Short-course centres in Yenagoa, Nigeria. IOSR J Pharm Biol Sci. 2017;12(4):70–5.
Osei E, Der J, Owusu R, Kofie P, Axame WK. The burden of HIV on tuberculosis patients in the Volta region of Ghana from 2012 to 2015: implication for tuberculosis control. BMC Infect Dis. 2017;17(1):504.
World Health Organization. Strategic framework to decrease the burden of TB/HIV. Geneva: World Health Organization; 2002.
MSF. Out of focus: how millions of people in West and Central Africa are being left out of the global HIV response; 2016.
World Health Organization. Global tuberculosis report 2016; 2016.
Uplekar M, Weil D, Lonnroth K, et al. WHO’s new end TB strategy. Lancet. 2015;385(9979):1799–801.
UNAIDS, Fast-track. Ending the AIDS epidemic by 2030. UNAIDS, Geneva. 2014.
Medecins Sans Frontires. How millions of people in west and central Africa are being left out of the global HIV response: MSF; 2016.
Harries AD, Boxshall M, Phiri S, Kwanjana J. Managing HIV and tuberculosis in sub-Saharan Africa. Lancet. 2006;367(9525):1817–8.
Acknowledgements
YG is supported by University of Queensland International Scholarship (UQI). We thank Mr. Scott Macintyre for his professional support during electronic database search.
Author information
Authors and Affiliations
Contributions
YAG, YA, GW and RSM conceived the research question and study design. YAG performed the electronic database search, data abstraction, analysis and wrote the first draft of the article. YAG supervised and reviewed the database search, data analysis and interpretation. GW, CG, RSM and HG supervised the research process and provided comments. All authors reviewed subsequent versions and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gelaw, Y.A., Williams, G., Soares Magalhães, R.J. et al. HIV Prevalence Among Tuberculosis Patients in Sub-Saharan Africa: A Systematic Review and Meta-analysis. AIDS Behav 23, 1561–1575 (2019). https://doi.org/10.1007/s10461-018-02386-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-018-02386-4