Abstract
We aimed to characterize successful cognitive aging (SCA) among older HIV-infected (HIV+) and HIV-uninfected (HIV−) adults, and to determine associations with positive psychological factors and health-related quality of life (HRQoL). Ninety-nine HIV+ and 46 HIV− older adults (≥ 50 years) completed measures of neurocognition, positive psychological factors, and HRQoL. Using study-defined SCA criteria (i.e., no cognitive or everyday impairment or major depressive disorder), we compared positive psychological factors and HRQoL across four groups: HIV+/SCA+, HIV+/SCA−, HIV−/SCA+, HIV−/SCA−. SCA was identified in 29% of the HIV+ sample compared to 61% of the HIV− sample (p < 0.01). HIV+/SCA+ participants had higher scores on 8 of 10 measures of positive psychological factors as well as better HRQoL (ps < 0.05) as compared to the HIV+/SCA− group. Furthermore, the HIV+/SCA+ participants had comparable scores on these factors as HIV− adults. Fewer HIV+ than HIV− participants met SCA criteria; however, the level of positive psychological factors among the HIV+/SCA+ group was comparable to the HIV− sample. Our findings present opportunities for interventions to optimize positive psychological factors and potentially improve SCA among older HIV+ adults.
Resumen
Nuestro objetivo fue caracterizar el envejecimiento cognitivo exitoso (ECE) entre personas mayores VIH+ y VIH−, y determinar asociaciones con factores psicológicos positivos y con la calidad de vida relacionada a la salud (CVrS). Noventa y nueve personas mayores (de 50 años o más) VIH+ y 46 VIH− completaron indicadores de neurocognición, de factores psicológicos positivos y de CVrS. Mediante la utilización de criterios de ECE definidos por el presente estudio (p. ej. la ausencia de deterioro cognitivo, impedimentos en el funcionamiento cotidiano, o trastorno depresivo mayor) comparamos los factores psicológicos positivos y la CVrS entre cuatro grupos: VIH+/ECE+, VIH+/ECE−, VIH−/ECE+, VIH−/ECE−. El ECE fue identificado en 29% de la muestra de VIH+ comparado con 61% de la muestra de VIH− (p < 0,01). Los participantes VIH+/ECE+ obtuvieron puntuaciones más altas en 8 de los 10 indicadores de factores psicológicos positivos, así como mejor CVrS (ps < 0,05), comparado con el grupo VIH+/ECE−. Además, los participantes VIH+/ECE+ obtuvieron valores comparables a los de los adultos VIH− en estos factores. Una proporción menor de participantes VIH+ que VIH− cumplieron criterios de ECE; sin embargo, el nivel de los factores psicológicos positivos en el grupo VIH+/ECE+ fue comparable a la muestra de la población VIH−. Nuestros resultados presentan oportunidades de intervención para optimizar los factores psicológicos positivos y potencialmente mejorar el ECE entre los adultos mayores con VIH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A large proportion of HIV-infected (HIV+) adults in the United States are 50 years of age or older [1], and it is predicted that by 2030 that 73% of HIV-infected individuals will be over the age of 50 [2]. Aging with HIV involves complex interactions among a variety of person-level factors, including neurocognitive functioning, functional status, mental health, health behaviors, and psychosocial issues [3]. HIV-associated neurocognitive disorders (HAND) are observed in just over half of HIV+ adults [4], and older adults with HIV are at a three-fold risk of HAND compared to their younger counterparts [5]. Older HIV+ adults also face psychosocial challenges, including more social isolation than their HIV-uninfected (HIV−) counterparts [6]. Together, these risk factors translate into a disproportionate risk for disruptions in the ability to remain independent on many instrumental activities of daily living (IADL) [7,8,9,10,11]. Functional disability expenditures have made HIV among the most expensive chronic illnesses and may interfere with health-related quality of life (HRQoL) [12, 13], emotional well-being [14, 15], and successful cognitive aging (SCA) at the individual level.
A great deal of research has focused on the less than optimal outcomes of some older persons living with HIV, however, limited research has examined the risk and protective factors associated with SCA in this growing population. Among HIV− adults, positive psychological factors (e.g., resilience), mental HRQoL, and social support have been shown to facilitate successful aging [16,17,18,19]. Such factors may inform intervention strategies to promote successful aging in older adults living with HIV.
In one of the first studies to systematically examine factors associated with successful aging among older HIV+ adults, it was found that despite HIV+ adults having a lower mean self-rated successful aging (SRSA) score than their HIV− counterparts, nonetheless two-thirds of the HIV+ adults reported scores in the top half (≥ 5) of a 10-point scale of SRSA [20]. SRSA was related to higher positive psychological factors, better emotional functioning, and better HRQoL. SRSA was unrelated to HIV disease characteristics, including duration of HIV disease, current and nadir CD4 counts, plasma HIV RNA, and AIDS status. The limitation of this previous study, however, was that that successful aging was only defined using a likert-type SRSA scale from 1 to 10. Among studies using more detailed successful cognitive aging criteria (defined as the absence of objective neurocognitive deficits and subjective cognitive symptoms) in those with HIV, a stair-step decline in successful cognitive aging in accordance with increasing age and HIV serostatus has been observed, ranging from approximately 50% of younger HIV− adults meeting criteria for successful cognitive aging to only approximately 20–30% of older HIV+ adults meeting this criteria [17, 18, 21]. These previous studies show that successful aging, specifically successful cognitive aging, is observed among those living with HIV and provide insights to some potential risk and protective factors. The present expands upon these previous studies and uses similar, yet broader, multi-domain, study-defined, SCA criteria.
Although much of the broad successful aging literature has focused on freedom from disease and high physical functioning as criteria for successful aging, such criteria may be overly restrictive and would prevent classification of successful aging among those living with HIV. For the purposes of the present study, we defined a SCA phenotype that includes the absence of the following disease-related morbidities that are common in HIV: cognitive impairment, IADL dependence, and major depressive disorder (MDD). This conceptualization of SCA is not entirely novel in that it partially and inversely overlaps with HAND diagnostic criteria, however, the proposed SCA definition allows us to explore how levels of various positive psychological factors and HRQoL are associated with our study-defined phenotype of SCA.
The purpose of this study was to (1) estimate the proportion of older HIV+ and HIV− adults who meet this study-defined phenotype of SCA (see Methods), and (2) to compare subjective self-rated successful aging (SRSA), positive psychological factors, and HRQoL across these objective study-defined SCA groups.
Methods
Participants and Procedure
The present study examined 99 community-dwelling, older (i.e., aged 50 years and above), HIV+ and 46 HIV− adults. Participants were recruited from Southern California (mostly San Diego County) via flyers and presentations at various community-based meetings. The current study, Successfully Aging Seniors with HIV (SASH), was approved by the UC San Diego Institutional Review Board. Informed consent was obtained from all individual participants included in the study. Exclusion criteria were generally minimal with the exception of acute drug or alcohol intoxication (e.g., positive urine toxicology screen), significant neurologic/neurodegenerative disorders conditions (e.g., Parkinson’s Disease), and serious psychotic disorders (e.g., schizophrenia). Our goal was to enroll a representative cohort of both HIV+ and HIV− subjects, rather than exclude participants with certain conditions prior to enrollment. All participants were reviewed for potential confounding comorbid psychiatric, medical, and neurological conditions known to adversely impact neurocognitive functioning (e.g., stroke, brain injury with loss of consciousness, history of substance use disorders) using established methods in the HIV literature [4, 22], which yielded the following four confound statuses: no confounds, minimal, moderate, and severe. Severe confound status was determined by two independent raters blinded to neuropsychological status (Master’s- and Doctoral-level psychology trainees) and confirmed by a clinical neuropsychologist (DJM). Severely confounded comorbidities in this sample included myocardial infraction, significant head injury, and other factors known to be associated with declines in neuropsychological functioning. Given the relatively small subset of participants classified as severely confounded [n = 16 (16%) of HIV+ sample and n = 7 (15%) of HIV− sample] as well as the high prevalence of neurological comorbidities in the general HIV population, we chose to include all participants in the present study to enhance the ecological validity and generalizability of our findings to the broader HIV population.
Measures
Successful Cognitive Aging
Study-defined SCA was operationalized as the absence of: neurocognitive impairment, current MDD diagnosis, and dependence in instrumental activities of daily living (IADL). Neurocognitive impairment was determined via a comprehensive, seven-domain, neurocognitive battery. Raw test scores were converted to demographically-corrected T-scores using the most appropriate normative corrections [4]. Neurocognitive impairment was defined using the published clinical ratings approach, such that those with a global clinical rating of 5 or greater (which is indicative of at least two cognitive domains in the impaired range) were classified as impaired [23]. Current MDD was diagnosed with the Composite International Diagnostic Interview (CIDI) [24, 25], which is based upon DSM-IV diagnostic criteria. IADL dependence was determined based on decline and need for assistance in ≥ 2 IADL domains (e.g., finances, managing medications) on the Lawton and Brody ADL questionnaire; irrespective of whether subjects attributed these declines to cognitive or physical factors [26,27,28]. Any participant who had at least one of these three criteria was deemed SCA−, whereas the absence of all of these three criteria was given the assignment of SCA+ . This yielded four primary groups: HIV+/SCA+, HIV+/SCA−, HIV−/SCA+, HIV−/SCA (Table 1).
Correlates of SCA
In order to examine correlates of SCA, we used questionnaires measuring positive psychological factors, SRSA, and HRQoL. The ten positive psychological factors are listed in Table 2 along with detailed information on the constructs assessed and range of scores. SRSA was measured via a single question asking subjects to rate how successfully they felt they were aging using their own definition on a 10-point Likert scale, where 1 is the worst and 10 is the best [29]. Finally, HRQoL was assessed with the Mental and Physical Health Composite Scores of the Medical Outcomes Study SF-36 [30]. Other medical covariates (e.g., diabetes, hyperlipidemia, etc.) were identified via clinical interviews with our medical staff (e.g., nurse). The lay-administered CIDI was also used to diagnose DSM-IV substance abuse or dependence. Current and lifetime substance use disorder was assigned as present if abuse or dependence criteria were met for any the following substances: cocaine, methamphetamine, heroin, hallucinogen, opioid, PCP, or sedative.
Statistical Analyses
Chi square was used to examine the differences in SCA prevalence between the HIV+ and HIV− samples, including prevalence of each of the individual components (i.e., neurocognitive impairment, current MDD, and IADL dependence). Analysis of variance (ANOVA), and Chi square when appropriate, was used to compare differences between the four SCA/HIV groups on demographic and health variables. Subsequently, we conducted an analysis of covariance (ANCOVA) to determine whether the difference in SCA between HIV+ and HIV− subjects remained after adjustment for potential covariates (i.e., variables associated with either HIV status or SCA status). We then compared the overall HIV+ and HIV− groups on each of the positive psychological factors and SRSA, and similarly used ANOVA with Student’s t pairwise tests to compare differences between the four groups on each of the positive psychological factors, SRSA, as well as HRQoL. Follow-up confirmatory analyses were conducted on SRSA, positive psychological factors, and HRQoL by employing a 2 × 2 ANCOVA (including relevant covariates), examining main effects of HIV, SCA, and the HIV × SCA interaction.
Results
The overall sample was primarily white (76%) males (79%), ranging from 50 to 79 years of age (M (SD) = 58.70 (6.81)) with between 6 and 20 years of education (M (SD) = 14.28 (2.64)). All HIV+ participants except two were currently taking antiretroviral therapy. Descriptive information on the sample divided by HIV and SCA status is provided in Table 1.
Twenty-nine percent (29/99) of the HIV+ sample met study-defined SCA criteria as compared to 61% (28/46) in the HIV− sample (p < 0.01). Table 3 shows the prevalence of each of the three individual risk factors by HIV status. Each of the three risk factors was significantly more prevalent (ps < 0.05) in the HIV+ compared to the HIV− group. Table 1 shows sample demographics by the four HIV/SCA groups, as well as HIV characteristics between the HIV+/SCA+ and HIV+/SCA− groups. An omnibus one-way ANOVA of the four HIV/SCA groups showed that the only significant (p < 0.05) demographic difference between HIV/SCA groups was sex, with the HIV+ group as a whole having more men. There were trends for race and age, such that the HIV+ group had overall more white subjects, while for age, the HIV+/SCA+ was younger than the HIV−/SCA+ group. Omnibus examination of lifetime MDD showed that rates differed between the groups (p < 0.01); however, this factor was not included in subsequent models since current MDD was one of our defining criteria for the successful cognitive aging groups (Lifetime MDD rates; HIV+/SCA+ = 45%, HIV+/SCA− = 66%, HIV−/SCA+ = 18%, and HIV−/SCA− = 44%). There were no significant differences on HIV disease characteristics between the HIV+/SCA+ and HIV+/SCA− groups. Confound rating was significantly different between the four groups (χ2 (9, N = 148) = 41.9, p < 0.01). Most of the confound ratings in the HIV+ group were “minimal” or “moderate” (80%), whereas the majority of confound ratings in the HIV− group were rated as “none” or “minimal” (73%) (Table 1). Finally, hyperlipidemia was significant different between the four groups, with the HIV+ group overall having a higher prevalence of this condition. We also examined whether any Table 1 variables were associated at p < 0.10 with HIV status (minus HIV variables) and SCA status independently to determine whether any potential covariates might affect the association of HIV status with SCA. We found that hyperlipidemia, gender, race, lifetime substance diagnoses, and confound rating were associated with HIV status, and gender was associated with SCA status. We entered these covariates along with HIV status into an ANCOVA, and found that the main effect of HIV status on SCA remained.
For the SRSA variable, there was a significant difference between the overall HIV+ and HIV− samples (p = 0.03, HIV+ M (SD) = 7.05 (2.29); HIV− M (SD) = 7.87 (1.82)), with the HIV+ sample having a lower (worse) SRSA. Using a cut-point based on the median SRSA in a previous paper (i.e., ≥ 7 on the 10 point SRSA scale was coded as successful aging; [17, 18]), 67% percent of the HIV+ group and 82% of the HIV− group rated themselves as successfully aging. As compared to HIV− participants, HIV+ persons had significantly worse scores on eight of 10 of the positive psychological factors (all ps < 0.05) except for posttraumatic growth (p = 0.19) and religiosity, (p = 0.08). The HIV+ group also had lower scores on both mental and physical HRQoL (ps < 0.05) as compared to the HIV− group.
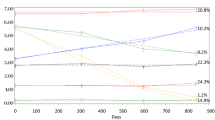
We used omnibus one-way ANOVA to compare the four HIV/SCA groups on the SRSA variable, psychological factor questionnaires (Table 4), and HRQoL (Fig. 1). SRSA was lowest among the HIV+/SCA− group; however, we found no significant difference between the four SCA/HIV groups (HIV+/SCA+ = 7.4, HIV+/SCA− = 6.9, HIV−/SCA+ = 7.7, HIV−/SCA− = 8.2; p = 0.10). For the positive psychological factors we found a significant difference between the HIV/SCA groups on seven of the ten psychological factor questionnaires (i.e., emotional support, hardiness, optimism, personal mastery, attitude towards aging, resilience, and life satisfaction), with HIV+/SCA− participants reporting the lowest scores (ps < 0.05) (Table 4). Religiosity (p = 0.09), social support (p = 0.06), and posttraumatic growth (p = 0.32) were not significantly associated with study-defined SCA groups at the omnibus level. Although SCA was not associated with HIV disease characteristics, we investigated whether any of the positive psychological factors were associated with the HIV characteristics. Hardiness was positively associated with current CD4 count (r = 0.22 p = 0.04), satisfaction with life and resilience were negatively associated with nadir CD4 count (r = − 0.31, p < 0.01; r = − 0.25, p = 0.01, respectively). Lastly, HIV+/SCA− participants had significantly (ps < 0.01) lower HRQoL scores than HIV+/SCA+, HIV−/SCA+, and HIV−/SCA− groups (who did not differ from one another, all p-values > 0.05) on both the physical and mental health composite scores (Fig. 1).
Finally, to confirm the previous analyses, we conducted 2-way ANCOVAs on SRSA, positive psychological factors, and HRQoL, including HIV, SCA, HIV × SCA interaction, and the aforementioned covariates. For SRSA, we found no main effect for HIV status or SCA status. Moreover, there was no HIV × SCA interaction. For the positive psychological factors, we found: (1) SCA main effect only at p < 0.05: hardiness; (2) HIV main effect only at p < 0.05: life satisfaction, personal mastery, attitude towards aging, religiosity, optimism; (3) no HIV or SCA main effects or interaction: emotional support, social support, posttraumatic growth; (4) For resilience, there were trends toward HIV and SCA main effects (ps < 0.10). For physical HRQoL, there was an HIV main effect trend (p < 0.10) and a HIV × SCA interaction trend (p < 0.10). For mental HRQoL there were HIV and SCA main effects and a significant HIV × SCA interaction (ps < 0.05).
Discussion
As individuals live longer with HIV, there is an increasing need to understand how persons age successfully with HIV. In this study, we found that 29% of older HIV+ persons met study-defined criteria for SCA. This proportion was lower than that observed among a demographically-comparable cohort of HIV− persons (61%). Although fewer HIV+ persons met SCA criteria than HIV− persons there is nonetheless a sizeable portion of the HIV+ cohort that were considered to be aging successfully. Among the HIV+ persons who met criteria for SCA, levels of various positive psychological factors, and mental and physical HRQoL, were comparable to the HIV− sample.
We recognize that there is currently no gold standard definition of SCA. With that said, neurocognitive impairment, IADL dependence, and depressed mood are common negative outcomes associated with aging with HIV, with a higher prevalence of each of these found in older adults with HIV compared to HIV− counterparts. Specifically, HAND estimates range from 30 to 50% of adults with HIV [4] and IADL impairment estimates are similar [41]. Elevated depressed mood has been reported as 30–46% among older (60 years or older) HIV-infected persons [42]. Consistent with previous findings of elevated prevalence of each of these components individually, the present study found similar elevations of each of these factors in our HIV+ cohort. Given the association between these factors, HIV-infection, and aging, using this operationalization of SCA allowed us to examine their combined association with self-rated successful cognitive aging, positive psychological factors and HRQoL.
Our finding that self-rated successful aging was higher than study-defined SCA for both HIV+ and HIV− older adults is consistent with the findings among HIV− older adults [43]. Although not statistically significant, the self-rated successful aging score was lowest in the HIV+/SCA− group. However, even among the HIV+/SCA− group, the mean self-rated successful aging was 6.9 out of 10, indicating that, on average, participants felt they were experiencing successful aging despite not meeting the study-defined criteria for SCA. These findings highlight the complex interdependencies among objective and subjective experiences of SCA. From a clinical, public health, and epidemiological standpoint, subjective assessment of self-reported successful aging may be less important than objective measurements anchored to real-world outcomes. From the patient’s perspective; however, it may be beneficial and motivate positive health behaviors to perceive his or her aging as successful [19, 43, 44].
The majority of the positive psychological factors examined in this study were associated with study-defined SCA, including: emotional support, hardiness, optimism, personal mastery, attitudes toward aging, resilience, and life satisfaction. The general pattern of results was such that the HIV+/SCA− group had the lowest scores on these factors compared to the other groups whose scores were similar to each other. These findings suggest that positive psychological factors may be particularly protective for HIV+ older adults, as compared to HIV− older adults. Further analysis controlling for important variables that might affect SCA (e.g., history of substance use disorders), yielded mostly HIV main effects, suggesting the differences across the HIV/SCA groups may have been primarily a function of HIV. Moreover, some of these positive psychological factors (e.g., hardiness, satisfaction with life, resilience) were associated with better HIV disease outcomes. This same pattern of results was found with mental and physical HRQoL, with the HIV+/SCA− group having the poorest self-reported HRQoL as compared to the other groups. When controlling for confounders, we found HIV and SCA main effects as well as an HIV × SCA interaction with mental HRQoL. This latter synergistic effect of HIV and SCA shows specificity with mental HRQoL, and is consistent with previous reports in HIV and aging [17, 18]. Altogether these findings highlight that those HIV+ older adults classified as SCA had similar levels of positive psychological factors and HRQoL as HIV− persons.
More recent models of successful aging include a focus on malleable, self-report factors that can enhance functioning and facilitate successful aging, including positive psychological factors, mental HRQoL, and social support (e.g., [19]), yet these models still tend to be less focused on the importance of cognitive and emotional health for successful aging. Maintaining cognitive and emotional health in the face of disease or physical disability is clearly attainable and the capability of accomplishing this is likely a strong indicator of successful aging [43].
We believe that our findings may serve as a resource for both clinicians providing care for PLWH, as well as PLWH themselves, in terms of possible factors associated with SCA. Working to improve levels of positive psychological factors (e.g., hardiness, resilience, optimism) and HRQoL may, in turn, improve SCA. Several studies, both in the context of HIV as well as among older adults without HIV, have suggested the possible benefits of interventions to improve resilience [45, 46], optimism [47] and other positive psychological constructs [48, 49]. These interventions may be appropriate next steps in efforts to improve daily functioning and quality of life among older persons living with HIV. In the absence of interventions designed to improve these positive psychological factors, it is difficult to know the true clinical meaningfulness of observed differences in the present study.
There are limitations to this study. On average, study participants were relatively well-educated, white males, which certainly limits the generalizability of this study to other subgroups of individuals aging with HIV; however, older white men who have sex with men represent the greatest majority of older HIV+ persons living with HIV in San Diego. Expanding the age range of our study to include younger HIV+ and HIV− adults would have enhanced our ability to examine age-related differences in, and perceptions of, SCA. Moreover, our sample of “older” persons was relatively “young” in that the mean age across the entire sample was in the late 50 s. A greater proportion of individuals who are 60 to 80 years old would be ideal. Additionally, our definition of SCA was circumscribed and is limited in that it focuses in on one aspect of emotional health (i.e., depression) and overlaps with two of the components used in the assessment of HAND. A more comprehensive definition might use a broader definition of emotional health and might focus on the more subjective experiences of the participant. The data gathered in this study were cross-sectional in nature and prevent inferences of causality. With that stated, this study served as preliminary data for an ongoing longitudinal study examining SCA among HIV+ adults. The ongoing study includes a more diverse sample (including a broader age range, range of education, more ethnic/racial minorities, and more women). These longitudinal data will allow us to examine predictors of change in SCA within individuals in future studies. Finally, the number of individuals in some of our SCA/HIV groups is relatively small (i.e., we only have 18 persons who are HIV− and not aging successfully); future studies will endeavor to have a greater sample size in each of these SCA/HIV groups.
Conclusions
This study extends the limited literature on SCA with HIV by using study-defined criteria for SCA that included outcomes-based phenotypes that are both vulnerable in HIV, and are modifiable. Subjective self-rated successful aging and positive psychological factors were associated with our study-defined criteria suggesting that future research is warranted to better understand the course of SCA the longitudinal predictors of SCA, and the interventions best suited to achieve SCA among people living with HIV.
References
United States Senate Special Committee on Aging. Hearing: older Americans: the changing face of HIV/AIDS in America. Washington, DC: United States Senate Special Committee on Aging; 2013.
Smit M, Brinkman K, Geerlings S, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15(7):810–8.
High KP, Brennan-Ing M, Clifford DB, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH office of AIDS research by the HIV and aging working group. J Acquir Immune Defic Syndr. 2012;60:S1–18.
Heaton RK, Clifford DB, Franklin DR Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96.
Valcour V, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii aging with HIV-1 cohort. Neurology. 2004;63:822–7.
Greysen SR, Horwitz LI, Covinsky KE, Gordon K, Ohl ME, Justice AC. Does social isolation predict hospitalization and mortality among HIV+ and uninfected older veterans? J Am Geriatr Soc. 2013;61(9):1456–63.
Foley JM, Gooding AL, Thames AD, et al. Visuospatial and Attentional abilities predict driving simulator performance among older HIV-infected adults. Am J Alzheimer’s Dis Other Demen. 2013;28(2):185–94.
Morgan EE, Iudicello JE, Weber E, et al. Synergistic effects of HIV infection and older age on daily functioning. J Acquir Immune Defic Syndr. 2012;61(3):341–8.
Thames AD, Kim MS, Becker BW, et al. Medication and finance management among HIV-infected adults: the impact of age and cognition. J Clin Exp Neuropsychol. 2011;33(2):200–9.
Vance DE, Fazeli PL, Gakumo CA. The impact of neuropsychological performance on everyday functioning between older and younger adults with and without HIV. J Assoc Nurses AIDS Care. 2013;24(2):112–25.
Vance DE, Wadley VG, Crowe MG, Raper JL, Ball KK. Cognitive and Everyday Functioning in Older and Younger Adults with and without HIV. Clin Gerontol. 2011;34(5):413–26.
Doyle K, Weber E, Atkinson JH, Grant I, Woods SP, the HIV Neurobehavioral Research Program (HNRP) Group. Aging, prospective memory, and health-related quality of life in HIV infection. AIDS Behav. 2012;16(8):2309–18.
Tozzi V, Balestra P, Galgani S, et al. Neurocognitive performance and quality of life in patients with HIV infection. AIDS Res Hum Retroviruses. 2003;19(8):643–52.
Castellon SA, Hardy DJ, Hinkin CH, et al. Components of depression in HIV-1 infection: their differential relaitonship to neurocognitive performance. J Clin Exp Neuropsychol. 2006;28(3):420–37.
Shimizu SM, Chow DC, Valcour V, et al. The impact of depressive symptoms on neuropsychological performance tests in HIV-infected adults: a study of the Hawaii Aging with HIV cohort. World J AIDS. 2011;1(4):139–45.
Jeste DV, Savla GN, Thompson WK, et al. Association between older age and more successful aging: critical role of resilience and depression. Am J Psychiatry. 2013;170:188–96.
Moore RC, Fazeli PL, Jeste DV, Moore DJ, Grant I, Woods SP, the HNRP Group. Successful cognitive aging and health-related quality of life in younger and older adults infected with HIV. AIDS Behav. 2014;18(6):1186–97.
Moore RC, Eyler LT, Mausbach BT, et al. Complex interplay between health and successful aging: role of perceived stress, resilience, and social support. Am J Geriatr Psychiatry. 2014;23(6):622–32.
Vahia I, Thompson WK, Depp CA, Allison MA, Jeste DV. Developing a dimensional model for successful cognitive and emotional aging. Int Psychogeriatr. 2012;24(4):515–23.
Moore RC, Moore DJ, Thompson WK, Vahia IV, Grant I, Jeste DV. A case-controlled study of successful aging in older adults with HIV. J Clin Psychiatry. 2013;14(5):e417–23.
Malaspina L, Woods SP, Moore DJ, et al. Successful cognitive aging in persons living with HIV infection. J Neurovirol. 2011;17(1):110–9.
Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitinve disorders. Neurology. 2007;69(18):1789–99.
Blackstone K, Moore DJ, Franklin DR, et al. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol. 2012;26(6):894–908.
Robins LN, Wing J, Wittchen HU, et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1998;45(12):1069–77.
Wittchen HU. Reliability and validity studies of the WHO–Composite International Diagnostic Interview (CIDI): a critical review. J Psychiatr Res. 1994;28(1):57–84.
Fazeli PL, Doyle KL, Scott JC, et al. Shallow encoding and forgetting are associated with dependence in instrumental activities of daily living among older adults living with HIV infection. Arch Clin Neuropsychol. 2014;29(3):278–88.
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86.
Obermeit LC, Beltran J, Casaletto KB, Franklin DR, Letendre S, Ellis R, Fennema-Notestine C, Vaida F, Collier AC, Marra CM, Clifford D, Gelman B, Sacktor N, Morgello S, Simpson D, McCutchan JA, Grant I, Heaton RK, CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) Group. Evaluating the accuracy of self-report for the diagnosis of HIV-associated neurocognitive disorder (HAND): defining “symptomatic” versus “asymptomatic” HAND. J Neurovirol. 2017;23(1):67–78.
Montross LP, Depp CA, Daly J, et al. Correlates of self-rated successful aging among community-dwelling older adults. Am J Geriatr Psychiatry. 2006;14:43–51.
Ware JE Jr, Sherbourne CD. The MOS 26-item short-form health survey (SF-36): conceptual framework and item selection. Med Care. 1992;30:473–83.
Duckworth AL, Quinn PD. Development and validation of the short grit scale (Grit-S). J Pers Assess. 2009;91(2):166–74.
Koening HG, Westlund RE, George LK, Hughes DC, Blazer DG, Hybels C. Abbreviating the Duke Socialy Support Index for use in chronically III elderly individuals. Psychosomatics. 1993;34(1):61–9.
Cambell-Sills L, Stein MB. Psychometric analysis and refinement of the Connor-davidson Resilience Scale (CD-RISC): validation of a 10-item measure of resilience. J Trauma Stress. 2007;20(6):1019–28.
Lawton P. The philadelphia geriatric center morale scale: a revision. J Gerontol. 1975;30(1):85–9.
Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Personal Soc Psychol. 1994;67(6):1063–78.
Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess. 1985;49(1):71–5.
Pearlin LI, Lieberman MA, Menaghan EG, Mullan JT. The stress process. J Health Soc Behav. 1981;22(4):337–56.
Seeman TE, Lusignolo TM, Albert M, Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychol. 2001;20(4):243–55.
Tedeschi RG, Calhoun LG. The Posttraumatic Growth Inventory: measuring the positive legacy of trauma. J Trauma Stress. 1996;9(3):455–71.
McNamara P, Durso R, Brown A. Religiosity in patients with Parkinson’s disease. Neuropsychiatr Dis Treat. 2006;2(3):341–8.
Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10(3):317–31.
Milanini B, Castella S, Perkovich B, et al. Psychiatric symptom burden in older people living with HIV with and without cognitive impairment: the UCSF HIV over 60 cohort study. AIDS Care. 2017;. https://doi.org/10.1080/09540121.2017.1281877.
Jeste DV, Depp CA, Vahia IV. Successful cognitive and emotional aging. World Psychiatry. 2010;9(2):78–84.
Pruchno RA, Wilson-Genderson M, Cartwright F. A two-factor model of successful aging. J Gerontol Ser B Psychol Sci Soc Sci. 2010;65(6):671–9.
Fang X, Vincent W, Calabrese SK, Heckman TG, Sikkema KJ, Humphries DL, Hansen NB. Resilience, stress, and life quality in older adults living with HIV/AIDS. Aging Ment Health. 2015;19(11):1015–21.
Vance DE, Burrage J Jr, Couch A, Raper J. Promoting successful aging with HIV through hardiness: implications for nursing practice and research. J Gerontol Nurs. 2008;34(6):22–9.
Sergeant S, Mongrain M. An online optimism intervention reduces depression in pessimistic individuals. J Consult Clin Psychol. 2014;82(2):263–74.
Depp CA, Vahia IV, Jeste DV. Successful aging: focus on cognitive and emotional health. Annu Rev Clin Psychol. 2010;6:527–50.
Carver LF, Buchanan D. Successful aging: considering non-biomedical constructs. Clin Interv Aging. 2016;11:1623–30.
Acknowledgments
The San Diego HIV Neurobehavioral Research Program [HNRP] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Reena Deutsch, Ph.D., Anya Umlauf, M.S. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Funding
This work was primarily supported by ID10-SD-057 from California HIV/AIDS Research Program (CHRP) (D.J. Moore, PI) and the University of California San Diego (UCSD) Stein Institute for Research on Aging Faculty Pilot Research Grant (D.J. Moore, PI). Additional support was provided by the following National Institutes of Health (NIH) Grants: R01MH099987 (D.J. Moore & D.V. Jeste, MPI), P30MH062512 (R.K. Heaton, PI), R00 AG048762 (P.L. Fazeli, PI), K23 MH107260 (R.C. Moore, PI).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Moore, D.J., Fazeli, P.L., Moore, R.C. et al. Positive Psychological Factors are Linked to Successful Cognitive Aging Among Older Persons Living with HIV/AIDS. AIDS Behav 22, 1551–1561 (2018). https://doi.org/10.1007/s10461-017-2001-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-017-2001-5