Abstract
The continuum of care for successful HIV treatment includes HIV testing, linkage, engagement in care, and retention on antiretroviral therapy (ART). Loss to follow-up (LTFU) is a significant disruption to this pathway and a common outcome in sub-Saharan Africa. This review of literature identified interventions that have reduced LTFU in the HIV care continuum. A search was conducted utilizing terms that combined the disease state, stages of the HIV care continuum, interventions, and LTFU in sub-Saharan Africa and articles published between January 2010 and July 2015. Thirteen articles were included in the final review. Use of point of care CD4 testing and community-supported programs improved linkage, engagement, and retention in care. There are few interventions directed at LTFU and none that span across the entire continuum of HIV care. Further research could focus on devising programs that include a series of interventions that will be effective through the entire continuum.
Resumen
La continuidad de la atención para el éxito del tratamiento del VIH incluye la prueba del VIH, la vinculación y el compromiso en el cuidado y mantenimiento de la terapia antirretroviral (TAR). Las pérdidas durante el seguimiento (LTFU) es una alteración significativa de esta vía y un resultado común en el África subsahariana. Esta revisión de la literatura identificó intervenciones que han reducido LTFU en el continuo de la atención del VIH. Se realizó una búsqueda utilizando términos que combinaban el estado de la enfermedad, las etapas del continuo de la atención del VIH, las intervenciones y LTFU en el África subsahariana y los artículos publicados entre enero de 2010 y julio de 2015. Trece artículos fueron incluidos en la revisión final. El uso del punto de atención pruebas de CD4 y los programas apoyados por la comunidad mejorar la articulación, compromiso, y la retención de los pacientes. Hay pocas intervenciones dirigidas a LTFU y ninguno que se extienden a lo largo de todo el continuo de la atención del VIH. La investigación adicional podría centrarse en la elaboración de programas que incluyen una serie de intervenciones que serán efectivas a través de toda la cadena.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The continuum of care for successful HIV treatment includes HIV testing, linkage, engagement in care, and retention on antiretroviral therapy (ART) [1]. Over the last decade, millions of individuals in sub-Saharan Africa have started ART. However, low HIV testing rates and losses between the point of testing and the initiation of ART have mitigated this success, and the majority of people in need of treatment are not receiving it [2]. By linking and engaging a patient in care, patient outcomes are improved and the system costs for delivering healthcare are reduced [3, 4]. The importance of engagement and retention in care can be quantified; patients that only received care in one quarter of their first year after diagnosis had twice the risk of death as patients who made visits to their healthcare provider in every quarter of the first year [5, 6].
Engagement in care is a global problem. In 2014, the Centers for Disease Control and Prevention reported that while 86 % of individuals in the United States living with HIV had been diagnosed and 80 % were linked to care within 3 months of their diagnosis, only 40 % were engaged in care [7]. Similar results were reported in a 2015 analysis of a screening program in Bunyala, Kenya. Of the segment that discovered their HIV-positive status during the screening program, only 15 % sought treatment for HIV over 3 years [8]. These statistics also highlight the gap that exists between testing and engagement in care.
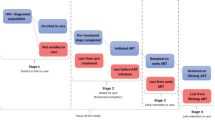
While the continuum of care seems like it should be a linear pathway, it often is less simple than it appears. There are many opportunities for a patient to be “loss to follow-up” (LTFU) in the care cascade. Once an individual tests positive for HIV, they need their CD4 count tested. Failure to determine CD4 counts at the time of testing provides one of the earliest opportunities where a patient can fail to be engaged and retained [9]. Individuals who receive their CD4 counts will either begin ART or will not yet be eligible depending on treatment protocols. Those who do not begin therapy must remain engaged until they begin ART or this is another stage where LTFU can occur [2]. The third stage where losses can occur is after ART begins, because an individual will need lifelong treatment. Challenges with initial ART initiation and retention in care include: fear of stigma, transportation costs, not feeling sick, or opting for traditional medicine [10]. The pathway is dynamic in that a patient that drops out of care can be re-engaged at the same or later point in the continuum, so LTFU or disengagement from care is not necessarily a permanent endpoint [11].
Constructs of the Social Cognitive Theory (SCT) can be used to identify many of the reasons that a person becomes LTFU, which may reside within the person (i.e. fear), within the structural environment (i.e. lack of transportation), or the social environment (i.e. stigma about HIV-positive people) [12]. Financial problems and poor health are also common reasons that patients are LFTU, along with the inability to find HIV patients for follow-up that stems from incorrect or missing telephone numbers and addresses [13].
A patient is considered LTFU when they do not come in for appointments for a period of time without having been transferred to the service of another facility for ART and it is not known if the patient has died or has disengaged from care. Although the period of time varies, a study by Chi and colleague found that a period of 180 days since the last visit resulted in the fewest misclassification of LTFU [14]. One systematic review concluded that 40 % of sub-Saharan patients believed to have been LTFU were actually deceased [13].
Studies report that 41–59 % of patients are retained in pre-ART care [15–19]. An average of 65 % of patients in sub-Saharan Africa who begin ART are retained at 3 years, so linking and engaging a patient to care between testing and ART initiation is crucial in reducing LTFU [20]. One estimate of the proportion of patients LTFU after testing positive for HIV, but before initiation of ART, is 80 % [2]. This estimate included patients at three stages: testing to staging, staging to ART eligibility, and ART eligibility to ART initiation.
Additional studies have shown that a patient’s CD4 count can impact retention in care and LTFU. A study by Wang and colleagues in South Africa found that pregnant women with a CD4 count of less than 200/μl had 6 times the risk of being lost to follow-up, and they hypothesize that this might be due to increased burden of health that pregnant women experience [21]. In the same study, non-pregnant women with a CD4 count of greater than 200/μl were more likely to be LTFU than men, possibly due to stigma associated with HIV for women.
Much research has focused on either interventions to increase HIV testing and initiation of ART or the risk factors for and the rates of LTFU rather than examining interventions to engage and retain individuals [9, 20, 22–33]. While other systematic reviews have identified poor linkage to care after HIV testing or poor retention after initiation of ART, few have focused on interventions to reduce LTFU [2, 34]. The purpose of this systematic review was to identify and analyze interventions that reduced the LTFU of HIV patients in sub-Saharan Africa at each of the stages between testing, linkage and engagement, and retention in ART. Utilizing constructs of the SCT, we identified interventions that reduced barriers within the person or within the person’s structural or social environment.
Methods
Using a study protocol devised with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, a detailed search was conducted to find published interventions to reduce LTFU in the HIV care continuum [35].
Search Strategy
A compound Boolean search was conducted utilizing terms that combined the disease state, stages of the HIV care continuum, interventions, and LTFU (Table 1). Many terms were truncated with the asterisk operator to allow for matching of alternate tenses of a word or plurals. Acronyms such as HIV, AIDS, and LTFU were used as were the full phrases for these terms.
Searches were limited to sub-Saharan Africa and publication dates between 1 January 2010 and 2 July 2015. The searches were conducted in PubMed, MEDLINE, Scopus, Web of Science, and Cochrane Library. Inclusion criteria were peer reviewed publications featuring an intervention to reduce LTFU in any stage of the HIV care continuum that had outcomes data, focused on sub-Saharan Africa and that were published between January 2010 and July 2015 in English. The World Bank listing of sub-Saharan African countries was used as a filter [36]. Studies that did not have interventions, were set in a region other than sub-Saharan Africa, were unrelated to LTFU, or failed to produce an outcome were excluded. Study design was not an exclusion criteria if the study had outcomes data; however, study design was an element of evaluation in the quality control analysis. In an effort to increase generalizability, interventions focused solely on men who have sex with men, intravenous drug users, or sex workers, were excluded.
The search results were exported to RefWorks where duplicate entries were removed. Two authors (JK, JRP) screened the titles and abstracts independently and blinded, to remove articles obviously not related to HIV interventions, LTFU, or sub-Saharan Africa. Studies with relevant titles and abstracts were read in full-text to determine if the inclusion criteria were met. Final selection of studies was also carried out independently and blinded (JK, JRP). Inter-rater agreement was 89 %. When there was a disagreement, a third author (EE) was consulted and the three authors reached an agreement about whether to include the study.
Lastly, a quality control analysis of the included studies was conducted to evaluate reliability, validity, and biases within studies. We utilized the Quality Assessment Tool for Quantitative Studies provided by the Effective Public Health Practice Project (EPHPP) [37].
Data Extraction
From each study, information regarding the study type, location, and design were extracted as were data on the participants, stage of the HIV care continuum in which the study was conducted, intervention, and the outcomes. Each study was rated as strong, moderate or weak based on the EPHPP assessment guidelines.
Results
The search returned 5508 potentially relevant citations. After removing duplicates and utilizing filters, 639 articles remained. Titles and abstracts were read by two reviewers who eliminated 578 articles. The full-text of 61 articles was read. After removing studies that did not meet the inclusion criteria, thirteen articles were retained (Fig. 1). Of the thirteen studies, five investigated the impact of interventions in the testing phase on retention in care, six studied interventions designed to improve retention during ART, and two studies looked at retention in care after testing and diagnosis but before the initiation of ART. Full characteristics are summarized in Table 2.
Nine countries (Chad, Côte d’Ivoire, the Democratic Republic of the Congo, Kenya, Malawi, Mozambique, Rwanda, South Africa, and Uganda) were represented in the selected studies. South Africa was the most common country with five of the interventions having been conducted there. Both rural and peri-urban environments were present, but urban was the most common with eleven studies having at least one urban setting. Most research was done retrospectively or observationally, but three studies were randomized controlled trials and one study was conducted as a cost-effectiveness survey.
Two articles analyzed how point of care CD4 testing impacts linkage to care, retention in other phases of the continuum of HIV care and reduces LTFU by having eligibility for ART determined quickly [9, 38]. A pair of published interventions analyzed the benefits of provider-initiated and inpatient HIV testing and counseling (HTC), while a third looked at the impact of abbreviated HTC on LTFU [39–41]. Three studies examined community-based interventions and how they compare to clinic-based programs in terms of LTFU and mortality [42–45]. One study looked at the cost-effectiveness of using a patient tracer to find patients LTFU [46]. Two papers examined the reduction in LTFU after free prophylaxis or free and continuous antiretroviral programs were implemented [29, 47]. The other articles studied the effect of an enhanced follow-up program on LTFU between 6 and 18 months after the initiation of ART [48].
Of the thirteen articles reviewed, twelve of the studies had a global quality rating of moderate or strong based on Effective Public Health Practice Project’s Quality Assessment Tool for Quantitative Studies (Table 3). The one study that had a global quality rating of weak was a prospective cohort study and will be identified in the discussion.
Discussion
Most of the studies focused on a single intervention designed to reduce LTFU at a particular stage in the continuum of HIV care. Since effective interventions have been identified at different stages, future research could focus on developing a series of interventions designed to keep someone engaged and retained in care through the entire continuum of HIV care.
Point of care CD4 testing, whether conducted at a clinic or in a mobile HTC setting, decreased the likelihood that a patient would be LTFU [9, 38]. In an observational study, the introduction of point of care CD4 testing reduced the LTFU from 64 to 33 % [9]. On average, point of care CD4 testing reduced the time to stage eligible patients from enrollment to ART initiation by tenfold (from 1 month to 3 days). Patients who received a point of care CD4 count were more likely to complete a referral visit than individuals who did not have a CD4 count at the time of diagnosis [38]. As an intervention for LTFU, point of care CD4 testing is effective in reducing the number of visits required for staging. It is an intervention that occurs in the testing and diagnosis phase, but has impacts that can reduce LTFU in the linkage and engagement to care phases. By decreasing the amount of time elapsed before one is linked to care and potentially initiates ART, there is less chance for pre-treatment LTFU [9, 38]. Since almost two-thirds of individuals that begin ART remain in treatment after 3 years [20], moving eligible individuals through the staging process increases the likelihood that they will be retained over time. Point of care CD4 testing is also more cost effective and decentralizes care since the device can be operated by nurses or other non-physician professionals in rural areas [49].
One study compared linkage to care of provider-initiated HTC against HTC that was initiated at the voluntary request of patients [39]. Both the intervention and the control groups showed poor linkage to care with almost one-third of patients in each group not receiving CD4 testing. Furthermore, two-thirds of the individuals in the provider-initiated HTC and 78.9 % of those requesting voluntary HTC did not receive ART [39]. When initiated on an inpatient basis, individuals receiving provider-initiated HTC were less likely than those receiving voluntary HTC to attend an HIV clinic after discharge (55.8 vs. 73.6 %) [40]. This result may be due to motivation. As one study showed, 84 % of individuals who self-referred for testing were engaged in care after 3 years while just 15 % of individuals who did not voluntarily seek out testing received any HIV care over the next 3 years [8].
An intervention designed to reduce LTFU for patients undergoing ART was the use of a patient tracer [46]. A patient tracer is a social worker/case manager who utilized all available contact information to find patients listed as LTFU. The patient tracer was empowered to offer assistance to patients in an effort to return them to care. Assistance included making appointments for the patient, going with the patient to an appointment or consultation, obtaining any documentation required for the patient’s treatments, referring the patient to other facilities that might be closer or open at more convenient hours, and helping the patient disclose HIV status to others. The patient tracer also had the ability to offer reimbursement for transport to and from the patient’s home and the clinic. In the study, the results of the patient tracer’s work revealed that one fifth of patients believed to have been LTFU had died, one fifth of the patients were still receiving care at the facility and were listed as LTFU in error in the clinic’s records, and thirty percent had transferred to another clinic. Of the 353 patients that the tracer reached over a 4 month span, 20 returned to care at a cost of $432/patient [46]. With less than 6 % of the patients believed to be LTFU returning to the clinic, the intervention did not seem like a cost effective or successful method of reducing LTFU long-term. While the cost-effectiveness of this intervention fails in most models, it is worth noting that LTFU in the first year of treatment reduces life expectancy by an average of 112 months (~9 years) [50]. In these terms, the intervention potentially saved years of life expectancy at less than $50/year.
A program in Côte d’Ivoire conducted a prospective study comparing standard care to an enhanced follow-up program. The enhanced follow-up consisted of a free plasma HIV-1 RNA (viral load) every 6 months plus a dedicated research physician to attend to the patients in the cohort and a research coordinator who tracked study patients by phone or home visit [48]. Patients in the enhanced group had a 46 % decrease in the risk of LFTU compared with the standard care group [48]. Although additional resource allocations in this study yielded better results, there would be concerns about sustaining a program built on extra staff in a resource-constrained environment.
In Uganda, community support agents are volunteers of ART clients that visit the homes of HIV-positive people. In a randomized controlled trial, HIV-positive individuals not yet eligible for ART either continued to receive standard care (test results, cotrimoxazole prophylaxis, and post-test counseling) or an intervention (a monthly 2-hour visit by a community support agent) [44]. After 24 months, individuals who were in the intervention group were almost three times more likely to still be retained in pre-ART care as opposed to those in the standard care group of the study (82.0 and 33.5 %, respectively). In addition to the reduction in LTFU, individuals in the intervention arm were almost two times more likely to disclose their HIV status and consistently use condoms or other method of protected sex compared to those receiving standard care [44].
This intervention was effective in reducing LTFU at the pre-ART stage in the continuum of HIV care, where the majority of patients are lost and were the least amount of research had been conducted. The use of unpaid volunteers in this intervention was effective and led to promising results [44]. Community-based voluntary support could be considered in other interventions. However, the use of unpaid volunteers in resource limited countries does have ethical and sustainability concerns. While volunteers often do so to make a social contribution and for altruistic reasons, in countries with few formal employment opportunities, they may also do so in hopes of future rewards. Because of this, the opportunities (or lack thereof) for future reward should be clearly communicated with the volunteers [51]. For sustainability, programs need to consider the opportunity cost of the volunteers in terms of time and develop a method to calculate those costs into sustaining the program [51].
Using paid community-based adherence support patient advocates (PAs) to visit ART patients’ homes is a strategy that has also worked in South Africa. The PAs supervised the taking of medications and provide one-on-one counseling. The 5 year LTFU for patients receiving visits from PAs was 13.2 % versus a 17.7 % LTFU in the group that did not receive these visits [45]. This study had a global quality rating of weak based on the Effective Public Health Practice Project’s Quality Assessment Tool for Quantitative Studies. The rating was determined primarily due to the limited control of confounders and withdrawal/dropout of participants. This may have resulted in the small difference in LTFU between the intervention and control groups.
Doctors without borders (Medecins Sans Frontieres or MSF) has devised a number of community-supported models of ART delivery. In Malawi, stable adults had clinical assessments every 6 months rather than every 6 weeks. They were also given 3 month ART refills which could be dispensed from a multitude of health centers rather than from just one central location. The 36 month retention of individuals on the standard care was high at 83 %, but the intervention group boasts a 36 month retention rate of 94.3 %. Reducing clinic visits and reducing the amount of time or difficulty in obtaining medications results in increased retention [42].
In South Africa, adherence clubs of up to 30 clinically stable individuals met every 2 months for less than an hour. At these clubs, individuals were weighed and assessed by a peer educator who gave pre-packaged prescriptions to members of the club. LTFU was decreased by 57 % over a 40 month span [42]. In the Democratic Republic of the Congo, the cost of transport to a clinic and a clinical assessment equals about half of the average Congolese monthly salary. However, in the capital of Kinshasa, MSF set up three distribution points where stable individuals received medications every 3 months. The 24 month LTFU in this program was 7.6 % [42].
In Rwanda, adding a community-based support to the national model for ART delivery resulted in better outcomes and higher retention in comparison to a clinic-model alone [43]. The community-based accompaniment model in Rwanda consists of a community health worker who visited patients once per day for directly observed therapy of all medications and social support. The community health worker also accompanied patients to their clinic visits for the first 4 months. Monthly food rations for a family of four and transportation vouchers for getting to and from the clinic were provided by the government. At 1 year, retention in the community-based accompaniment program was 92 % compared to 87 % for individuals in the clinic-based model [43].
Cost is a barrier to heath care and can be a major reason for LTFU. In late 2009, the government of Chad was able to implement free and continuous access to highly active ART. By removing the barrier of cost, the rates of LTFU for patients on ART decreased from 70 % in 2009 to 20 % in 2010 and less than 5 % in 2011 [47]. In Kenya, analysis of over 5000 ART-ineligible patients showed that LTFU was reduced by the implementation of a free cotrimoxazole prophylaxis program. In this program, 12 month retention for patients enrolled in care after the introduction of the free prophylaxis was 84 % which was higher than the 63 % 12 month retention of patients enrolled prior to the implementation of this program [29].
Limitations
As with any research study, there are limitations to this review. Access to EMBASE and other international databases to which the University of Nevada, Las Vegas does not subscribe might result in more records being found that were relevant to the topic. Searching conferences for a listing of relevant abstracts could reduce publication bias. The specificity of interventions designed to reduce LTFU eliminated many studies and reframing the question to look at all manner of attrition could be beneficial. Despite these limitations, this review does provide important information.
Conclusions
A recurring theme was that retention in care is higher when individuals receive community support. Whether one feels less stigmatized or less isolated by these interactions, the result is that an individual is more likely to be linked to care, engaged in care, and retained in care in areas where a community-support model of care is available. The most effective interventions feature a social component. Use of community-supported programs can reduce the burdens on clinical staff while simultaneously improving patient outcomes as patients remain more linked, engaged, and retained in care in comparison to standard models of care. Additionally, removing barriers to care such as providing point of care CD4 testing, decentralization of ART or providing free ART, decreased LTFU. There are few interventions directed at LTFU and none that span across the entire continuum of HIV care. Further research could focus on devising programs that contain a series of interventions that will be effective through the whole cascade.
References
Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800.
Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8(7):e1001056.
Mugavero MJ, Norton WE, Saag MS. Health care system and policy factors influencing engagement in HIV medical care: piecing together the fragments of a fractured health care delivery system. Clin Infect Dis. 2011;15(52 Suppl 2):S238–46.
Ulett KB, Willig JH, Lin H, Routman JS, Abroms S, Allison J, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDS. 2009;23(1):41–9.
Mugavero MJ, Lin HY, Willig JH, Westfall AO, Ulett KB, Routman JS, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48(2):248–56.
Giordano TP, Gifford AL, White AC Jr, Suarez-Almazor ME, Rabeneck L, Hartman C, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44(11):1493–9.
Bradley H, Hall HI, Wolitski RJ, Van Handel MM, Stone AE, LaFlam M, et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV—United States, 2011. Morb Mortal Wkly Rep. 2014;63(47):1113–7.
Genberg BL, Naanyu V, Wachira J, Hogan JW, Sang E, Nyambura M, et al. Linkage to and engagement in HIV care in western Kenya: an observational study using population-based estimates from home-based counselling and testing. Lancet HIV. 2015;2(1):e20–6.
Jani IV, Sitoe NE, Alfai ER, Chongo PL, Quevedo JI, Rocha BM, et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. The Lancet. 2011;378(9802):1572–9.
Assefa Y, Damme WV, Mariam DH, Kloos H. Toward universal access to HIV counseling and testing and antiretroviral treatment in Ethiopia: looking beyond HIV testing and ART initiation. AIDS Patient Care STDS. 2010;24(8):521–5.
Udeagu CC, Webster TR, Bocour A, Michel P, Shepard CW. Lost or just not following up: public health effort to re-engage HIV-infected persons lost to follow-up into HIV medical care. AIDS. 2013;27(14):2271–9.
Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31(2):143.
Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4(6):e5790.
Chi BH, Musonda P, Lembalemba MK, Chintu NT, Gartland MG, Mulenga SN, et al. Universal combination antiretroviral regimens to prevent mother-to-child transmission of HIV in rural Zambia: a two-round cross-sectional study. Bull World Health Organ. 2014;92(8):582–92.
Lessells RJ, Mutevedzi PC, Cooke GS, Newell ML. Retention in HIV care for individuals not yet eligible for antiretroviral therapy: rural KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2011;56(3):e79–86.
Ingle SM, May M, Uebel K, Timmerman V, Kotze E, Bachmann M, et al. Outcomes in patients waiting for antiretroviral treatment in the Free State Province, South Africa: prospective linkage study. AIDS. 2010;24(17):2717–25.
Larson BA, Brennan A, McNamara L, Long L, Rosen S, Sanne I, et al. Early loss to follow up after enrolment in pre-ART care at a large public clinic in Johannesburg, South Africa. Trop Med Int Health. 2010;15(s1):43–7.
Kranzer K, Zeinecker J, Ginsberg P, Orrell C, Kalawe NN, Lawn SD, et al. Linkage to HIV care and antiretroviral therapy in Cape Town, South Africa. PLoS One. 2010;5(11):e13801.
McGuire M, Munyenyembe T, Szumilin E, Heinzelmann A, Le Paih M, Bouithy N, et al. Vital status of pre-ART and ART patients defaulting from care in rural Malawi. Trop Med Int Health. 2010;15(s1):55–62.
Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15(s1):1–15.
Wang B, Losina E, Stark R, Munro A, Walensky RP, Wilke M, et al. Loss to follow-up in a community clinic in South Africa: roles of gender, pregnancy and CD4 count. SAMJ S Afr Med J. 2011;101(4):253–7.
Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39(8):II-46–54.
Bemelmans M, Van Den Akker T, Ford N, Philips M, Zachariah R, Harries A, et al. Providing universal access to antiretroviral therapy in Thyolo, Malawi through task shifting and decentralization of HIV/AIDS care. Trop Med Int Health. 2010;15(12):1413–20.
McCollum ED, Preidis GA, Kabue MM, Singogo EB, Mwansambo C, Kazembe PN, et al. Task shifting routine inpatient pediatric HIV testing improves program outcomes in urban Malawi: a retrospective observational study. PLoS One. 2010;5(3):e9626.
Silvestri DM, Modjarrad K, Blevins ML, Halale E, Vermund SH, McKinzie JP. A comparison of HIV detection rates using routine opt-out provider-initiated HIV testing and counseling versus a standard of care approach in a rural African setting. J Acquir Immune Defic Syndr. 2011;56(1):e9–32.
Thornton RL. The Demand for, and Impact of, Learning HIV Status. Am Econ Rev. 2008;98(5):1829–63.
Faal M, Naidoo N, Glencross DK, Venter WD, Osih R. Providing immediate CD4 count results at HIV testing improves ART initiation. J Acquir Immune Defic Syndr. 2011;58(3):e54–9.
Nsigaye R, Wringe A, Roura M, Kalluvya S, Urassa M, Busza J, et al. From HIV diagnosis to treatment: evaluation of a referral system to promote and monitor access to antiretroviral therapy in rural Tanzania. J Int AIDS Soc. 2009;12(1):1.
Kohler PK, Chung MH, McGrath CJ, Benki-Nugent SF, Thiga JW, John-Stewart GC. Implementation of free cotrimoxazole prophylaxis improves clinic retention among antiretroviral therapy-ineligible clients in Kenya. AIDS. 2011;25(13):1657–61.
Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. The Lancet. 2010;376(9755):1838–45.
Pop-Eleches C, Thirumurthy H, Habyarimana JP, Zivin JG, Goldstein MP, de Walque D, et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS. 2011;25(6):825–34.
Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012;26(16):2059–67.
Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2012;15(2):1–15.
Simoni JM, Amico KR, Smith L, Nelson K. Antiretroviral adherence interventions: translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010;7(1):44–51.
Boland A, Cherry MG, Dickson R, editors. Doing a systematic review: a student’s guide. Los Angeles, CA: Sage; 2013.
The World Bank. Country and Lending Groups. 2016. http://data.worldbank.org/about/country-and-lending-groups. Accessed 16 June 2016.
Effective Public Health Practice Project (EPHPP). Quality assessment tool for quantitative studies. 2010. http://www.ephpp.ca/tools.html. Accessed 8 June 2016.
Larson BA, Schnippel K, Ndibongo B, Xulu T, Brennan A, Long L, et al. Rapid point-of-care CD4 testing at mobile HIV testing sites to increase linkage to care: an evaluation of a pilot program in South Africa. J Acquir Immune Defic Syndr. 2012;61(2):e13–7.
Leon N, Mathews C, Lewin S, Osler M, Boulle A, Lombard C. A comparison of linkage to HIV care after provider-initiated HIV testing and counselling (PITC) versus voluntary HIV counselling and testing (VCT) for patients with sexually transmitted infections in Cape Town, South Africa. BMC Health Serv Res. 2014;14(1):1.
Wanyenze RK, Hahn JA, Liechty CA, Ragland K, Ronald A, Mayanja-Kizza H, et al. Linkage to HIV care and survival following inpatient HIV counseling and testing. AIDS Behav. 2011;15(4):751–60.
Wanyenze RK, Kamya MR, Fatch R, Mayanja-Kizza H, Baveewo S, Szekeres G, et al. Abbreviated HIV counselling and testing and enhanced referral to care in Uganda: a factorial randomised controlled trial. Lancet Glob Health. 2013;1(3):e137–45.
Bemelmans M, Baert S, Goemaere E, Wilkinson L, Vandendyck M, Cutsem G, et al. Community-supported models of care for people on HIV treatment in sub-Saharan Africa. Trop Med Int Health. 2014;19(8):968–77.
Franke MF, Kaigamba F, Socci AR, Hakizamungu M, Patel A, Bagiruwigize E, et al. Improved retention associated with community-based accompaniment for antiretroviral therapy delivery in rural Rwanda. Clin Infect Dis. 2013;56(9):1319–26.
Lubega M, Tumwesigye NM, Kadobera D, Marrone G, Wabwire-Mangen F, Peterson S, et al. Effect of community support agents on retention of people living with HIV in pre-antiretroviral care: a randomized controlled trial in Eastern Uganda. J Acquir Immune Defic Syndr. 2015;70(2):e36–43.
Fatti G, Meintjes G, Shea J, Eley B, Grimwood A. Improved survival and antiretroviral treatment outcomes in adults receiving community-based adherence support: 5-year results from a multicentre cohort study in South Africa. J Acquir Immune Defic Syndr. 2012;61(4):e50–8.
Rosen S, Ketlhapile M. Cost of using a patient tracer to reduce loss to follow-up and ascertain patient status in a large antiretroviral therapy program in Johannesburg, South Africa. Trop Med Int Health. 2010;15(s1):98–104.
Djarma O, Nguyen Y, Renois F, Djimassal A, Banisadr F, Andreoletti L. Continuous free access to HAART could be one of the potential factors impacting on loss to follow-up in HAART-eligible patients living in a resource-limited setting: N’djamena, Chad. Trans R Soc Trop Med Hyg. 2014;108(11):735–8.
Messou E, Kouakou M, Gabillard D, Gouesse P, Kone M, Tchehy A, et al. Medication possession ratio: predicting and decreasing loss to follow-up in antiretroviral treatment programs in Cote d’Ivoire. J Acquir Immune Defic Syndr. 2011;1(57 Suppl 1):S34–9.
Wynberg E, Cooke G, Shroufi A, Reid SD, Ford N. Impact of point-of-care CD4 testing on linkage to HIV care: a systematic review. J Int AIDS Soc. 2014;17(1):18809.
Losina E, Touré H, Uhler LM, Anglaret X, Paltiel AD, Balestre E, et al. Cost-effectiveness of preventing loss to follow-up in HIV treatment programs: a Cote d’Ivoire appraisal. PLoS Med. 2009;6(10):e1000173.
Kasteng F, Settumba S, Kallander K, Vassall A, inSCALE Study Group. Valuing the work of unpaid community health workers and exploring the incentives to volunteering in rural Africa. Health Policy Plan. 2016;31(2):205–16.
Acknowledgments
For her help in suggesting search terminology and more efficient ways to search databases, the authors wish to acknowledge Xan Goodman.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was partly funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Institute of Mental Health (NIMH), the President’s Emergency Plan for AIDS Relief (PEPFAR) under award number R01HD075050 and R01HD087994.
Conflict of Interest
The authors state that there are no competing interests to declare.
Research Involving Human Subjects or Animals
This research is a systematic review of published literature and deemed excluded by the UNLV IRB.
Informed Consent
Informed consent was not obtained as this was a systematic review of published literature.
Rights and permissions
About this article
Cite this article
Keane, J., Pharr, J.R., Buttner, M.P. et al. Interventions to Reduce Loss to Follow-up During All Stages of the HIV Care Continuum in Sub-Saharan Africa: A Systematic Review. AIDS Behav 21, 1745–1754 (2017). https://doi.org/10.1007/s10461-016-1532-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-016-1532-5