Abstract
We measured the trend of cigarette smoking among HIV-seropositive and seronegative men over time from 1984 to 2012. Additionally, we examined the demographic correlates of smoking and smoking consumption. Six thousand and five hundred and seventy seven men who have sex with men (MSM) from the Multicenter AIDS Cohort Study (MACS) were asked detailed information about their smoking history since their visit. Prevalence of smoking and quantity smoked was calculated yearly from 1984 to 2012. Poisson regression with robust error variance was used to estimate prevalence ratios of smoking in univariate and multivariate models. In 2012, 11.8 and 36.9 % of men who were enrolled in the MACS before 2001 or during or after 2001 smoked cigarettes, respectively. In the multivariate analysis, black, non-Hispanic, lower education, enrollment wave, alcohol use, and marijuana use were positively associated with current smoking in MSM. HIV serostatus was not significant in the multivariate analysis. However, HIV variables, such as detectable viral load, were positively associated. Though cigarette smoking has declined over time, the prevalence still remains high among subgroups. There is still a need for tailored smoking cessation programs to decrease the risk of smoking in HIV-seropositive MSM.
Resumen
Un análisis de hombres VIH-sero-positivo y -sero-negativo con fechas de nacimiento entre 1984 y 2012. Inclusivo, estudiamos la demografía relacionada entre fumando cigarrillos y la frecuencia de fumar cigarrillos. Se hizo entrevistas detalladas a 6.577 hombres del estudio Multicenter AIDS Cohort (MACS) que han tenido relaciones sexuales con otros hombres, sobre su frecuencia de fumar cigarrillos desde su última cita. Se calculó anual la frecuencia y cantidad de fumar desde el 1984 a 2012. Se usó la regresión de Poisson con un error de discrepancia conservativo para estimar la proporción de frecuencia de fumar en modelos univariante y multivariante. En 2012, de los hombres que estuvieron matriculados en el estudio de MACS antes del 2001 un 11,8 % fumaban cigarrillos y de los que matricularon en el 2001 o luego un 36,9 %. En el análisis multivariante, personas: africano-americano, anglo, baja nivel de educación, fecha de matriculación al MACS, uso del alcohol y mariguana muestrearon una correlación positiva dentro los hombres fumadores que han tenido relaciones sexuales con otros hombres. VIH-sero-estatus no estuvo significante en el análisis multivariante. Pero, los variables de VIH, como el carga viral, eran asociado positivamente. Aunque el consumo de cigarrillos ha bajado con tiempo, la presencia sigue predominante entre los grupos mencionados. Hay falta de programación que les pueda ayudar en eliminar el uso de cigarrillos para reducir el daño de salud entre hombres VIH-sero-positivo que mantienen relaciones sexuales con otros hombres.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research suggests that the prevalence of smoking in men who have sex with men (MSM) is higher than men in the general population [1–5]. This group has the largest proportion of people living with HIV (PLWH). Because of a history of exclusion and discrimination in other social settings, the social focus for MSM has been gay-identified bars and clubs, where the prevalence of smoking continues to be high [6]. Recently, two studies focusing on HIV-seropositive women and indigent adults reported the prevalence of current smoking to be 39 and 67.3 %, respectively [7, 8]. Further, recent studies have assessed smoking in MSM but have not assessed the overall changing trend [9, 10].
Studies assessing smoking in PLWH support research from the general population—showing that smoking is a risk factor for coronary artery disease, myocardial infarction, and stroke [4, 5, 11–13]. Smoking is also the number one reason for non-AIDS defining cancers in PLWH [13]. Implementing tobacco cessation programs has been challenging in PLWH. Crothers et al. (2007) found that HIV care providers in the Veterans Aging Cohort 5-Site Study are less likely to recognize current smoking as a problem compared with non-HIV care providers [14]. Furthermore, providers who identify current smokers are less likely to make smoking treatment a priority because of other competing health concerns, economic barriers, or limited time for health promotion activities [6, 14].
Although the overall prevalence of smoking has decreased in the general population [15], it is unclear whether this trend also holds among HIV-seropositive and negative MSM. Compared to the general population, HIV-seropositive and seronegative MSM may have higher rates of other addictive behaviors such as alcohol use and drug use that are likely to increase the risk of cigarette smoke [16]. More importantly, if different trends exist in this subpopulation, then culturally tailored public health messages may be needed to promote health behavior change interventions among HIV-seropositive and negative MSM.
The Multicenter AIDS Cohort Study (MACS) is an ongoing longitudinal study of men who have had sex with men. With nearly 30 years of longitudinal data on almost 7000 HIV-seropositive and HIV-seronegative MSM, the MACS is an ideal cohort to study trends in smoking in MSM over time because of its large sample size, continued enrollment and repeated measures of smoking status.
The aims of this study were [1] to evaluate differences in trends of cigarette smoking and change in daily consumption among HIV-seropositive and seronegative men over time by birth cohort from 1984 to 2012, [2] examine predictors of smoking prevalence and smoking consumption among total MSM and HIV-seropositive men.
Methods
Study Design and Administration
The MACS is an ongoing prospective cohort study of the natural and treated histories of HIV infection among MSM in the United States [17]. The study has been described in detail previously [17]. A total of 6972 men were recruited at four centers: Baltimore/Washington DC, Chicago, Los Angeles, and Pittsburgh. Men were recruited in three waves, 4954 in 1984–1985, 668 in 1987–1991, and 1350 in 2001–2003. Informed consent was obtained from all participants, and the MACS study protocol was approved by the institutional review boards of each of the participating centers.
Participants of the MACS return biannually for detailed interviews, physical examinations, and collection of blood and laboratory testing. At each study visit, the men are asked detailed information about their smoking history since their previous visit. The questionnaires are available online at http://www.statepi.jhsph.edu/macs/forms.html.
This present study utilizes a prospective cohort design to examine the association between demographic characteristics with self-reported smoking. We utilized all data from the three waves since smoking behavior was captured since their initial visit. The study sample included 6577 men who reported their smoking behavior during their initial visit and at least one more visit. The median person-years in the study was 9.6 years (interquartile range 5.4–18.5 years).
Main Outcome Measure
Current smoking was collected based on answers to a detailed interview. Participants were classified as never, former, and current smokers at each visit. These questions include “Did you ever smoke cigarettes?” and “Do you smoke cigarettes now?” Participants who answered yes to both questions were categorized as current smokers. Participants were categorized as former smokers if they answered yes to the first question and no to the second. Never smokers were participants who answered no to both questions.
Quantity of cigarette packs smoked were categorized by the MACS as follows: less than ½ pack per day; at least ½ but less than 1 pack per day; at least 1 but less than 2 packs per day; and 2 or more packs per day. For the current study, the quantity consumed was dichotomized as less than one pack per day, and 1 pack or more a day.
The MACS also assessed the length of time participants had smoked prior to joining the study. Participants were asked at what age they had begun smoking cigarettes and how many packs they had smoked during their heaviest smoking periods. If participants were former smokers, the men were asked for the number of years they had stopped smoking cigarettes. Using this information, we calculated baseline cumulative smoking for each participant, and continued to add onto it while the participants remained in the study.
Independent Variables
Age at the each visit was calculated using self-reported recorded date of birth and was treated as a continuous variable. Self-reported race at enrollment was categorized as follows: White non-Hispanic; White Hispanic; Black non-Hispanic; Black Hispanic; American Indian or Alaskan Native, Asian or Pacific Islander, other, or other Hispanic. Because of the small number of Hispanics (n = 631), American Indian or Alaskan Native (n = 23), Asian or Pacific Islander (n = 32), and other (n = 39) were grouped together with other. Self-reported educational attainment was collected from the most recent semi-annual visit and was categorized as high school diploma or less, some college or college degree, and graduate work or more (reference group).
We dichotomized participants into two groups based on their time of enrollment as either before or after 2001; before 2001 was the reference group. Baseline characteristics differed by time of enrollment. Participants enrolled in 2001 or after were more likely to be younger, HIV-seronegative, black non-Hispanic, Hispanic, express depressive symptoms, have a high school diploma or less, unemployed, and were smokers at baseline.
HIV serostatus was assessed using enzyme-linked immunosorbent assay with confirmatory Western blot tests on all MACS participants at each participant’s initial visit and at every semiannual visit for participants who were initially HIV-seronegative. Standardized flow cytometry was used to quantify CD4+ T-lymphocyte subset levels by each MACS site [18, 19]. Through the course of the longitudinal study, 17.3 % of HIV-seronegative MSM were diagnosed with HIV.
Self-reported employment status was dichotomized as employed or unemployed. Self-reported alcohol use was measured using questions about frequency of drinking and average number of drinks the participant consumed since his last visit. Participants were categorized as no drinks since last visit, low–moderate (1–2 drinks per day or 3–4 drinks per day no more than once a month), moderate–heavy (3–4 drinks per day for more than once a month or 5 or more drinks per day for less than once a month), and binge (5 or more drinks for at least once a month) (Substance Abuse and Mental Health Services Administration [20]. Binge drinking: terminology and patterns of use. http://captus.samhsa.gov/access-resources/binge-drinking-terminology-and-patterns-use. Accessed 15 Feb 2014). Marijuana use, hospitalization in the last 6 months, and frequency of depressive symptoms (occasionally or most/all days versus rarely or some days) were dichotomized.
Data Analysis
We first calculated the prevalence of smoking as the number of smokers over the total number of participants for each year. Smoking prevalence was stratified by birth cohort, and HIV serostatus. We used the Cochran–Mantel–Haenszel test to measure the difference between birth cohort and HIV serostatus. We also used the Cochran–Armitage trend test to assess changes by calendar year. We repeated this calculation to assess the prevalence of smoking one pack or more per day. We also stratified by birth cohort and HIV serostatus. Univariate analyses were used to describe characteristics of the population as a function of HIV serostatus.
Poisson regression with robust error variance was used to estimate prevalence ratios for smoking [21]. Univariate and multivariate analysis were first done for all MSM and then HIV-seropositive MSM. We used SAS 9.2 GENMOD procedure (SAS Institute, Cary, North Carolina, USA). We included age, race, education, employment, HIV serostatus, time of enrollment, depressive symptoms, alcohol use, hospitalization, and marijuana use in the univariate models. Covariates with statistical significance at p < 0.05 were entered into an exploratory multivariate model. Missing values for smoking status (n = 113), age (n = 1), race (n = 2), education (n = 50), and alcohol use (n = 147) were imputed with values from the subsequent visit. We tested an interaction term for HIV serostatus and time of enrollment, and then stratified the analysis by HIV+ and HIV− men.
Two sensitivity analyses were conducted to understand the changes in association based on seroconverters and lost to follow up. First, seroconverters were analyzed as HIV-seropositives and were then removed to assess changes in baseline characteristics and longitudinal associations (n = 5865). Additionally, we removed all participants that were lost to follow up to assess the same baseline characteristics and longitudinal associations (n = 1966).
Results
Baseline demographic data for HIV-seropositive and HIV-seronegative men are shown in Table 1. The prevalence of smoking was slightly higher among HIV-seropositive men (44.1 %) compared with HIV-seronegative men (37.9 %). HIV-seropositive men were more likely to be black non-Hispanic (19 % compared with 13.2 %) or other (11.5 % compared with 7.2 %), enrolled in 2001 or after (21.0 % compared with 15.8 %), have a high school diploma or less (19.0 % compared with 14.0 %), have depressive symptoms (25.6 % compared with 21.6 %), and were hospitalized in the past 6 months (6.4 % compared with 4.1 %).
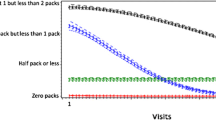
The annual smoking prevalence has declined over time in the MACS. Shown in Fig. 1a, 38.9 % of participants enrolled in the MACS in 1984 smoked. In 2012, the prevalence sharply declined to 11.8 % (test for trend p < 0.0001). Among participants who were enrolled in 2001 or after, the prevalence of smoking in 2002 was 53.9 and 36.9 % in 2012 (test for trend p < 0.0001). Shown in Fig. 1b, differences in prevalence were also observed by birth cohort. Participants in the oldest birth cohort (1914–1934) had the lowest prevalence of smoking while participants in the youngest birth cohort (1960–1969) had the highest (test for trend p < 0.05). However, in participants recruited after 2001, the oldest (1940–1949) and youngest (1970–1992) birth cohorts had a lower prevalence of smoking compared with participants born between 1950 and 1959 (test for trend p < 0.05) (Fig. 1b).
a Annual prevalence of current smoking in the MACS by birth cohort, first wave (1984–2012). b Annual prevalence of current smoking in the MACS by birth cohort, second wave (2002–2012). c Annual prevalence of current smoking in the MACS by serostatus, first wave (1984–2012). d Annual prevalence of current smoking in the MACS by serostatus, second wave (2002–2012)
Among participants who were enrolled before 2001, HIV-seropositive men had a higher prevalence of smoking compared with HIV-seronegative men (Fig. 1c). The rate of decline of smoking was the same among both groups. There were no differences observed among HIV-seropositive and HIV-seronegative men who were enrolled after 2001 (Fig. 1d). Similarly, there were no differences observed in the quantity of daily cigarettes smoked by birth cohort or HIV serostatus (not shown).
In the multivariate analysis using the total sample, black non-Hispanic, lower education, time of enrollment, living in Chicago and Pittsburgh, marijuana use and alcohol use were positively associated with smoking prevalence in MSM (Table 2). To understand the analysis more clearly, among all MSM participants, having less than a high school diploma was associated with a 20 % (95 % CI 1.15–1.25) higher prevalence of smoking compared with MSM who had attended graduate school or more; a 10 % (95 % CI 1.07–1.15) higher prevalence for black, non-Hispanic men compared with white, non-Hispanic men; a 12 % (95 % CI 1.06–1.14) higher prevalence of smoking if enrolled in 2001 or after; a 5 % (95 % CI 1.01–1.08) higher prevalence of smoking if participants lived in Chicago compared with participants living in Baltimore/Washington DC; a 5 % (95 % CI 1.01–1.08) higher prevalence of smoking if participants living in Pittsburgh compared with participants living in Baltimore/Washington DC; an 11 % (95 % CI 1.09–1.14) higher prevalence rate if they were marijuana users; and a 11 % (95 % CI 1.09–1.14) higher prevalence rate if they were binge drinkers. Being HIV-seropositive was positively associated with smoking prevalence in the univariate analysis, but the association was no longer significant after adjusting for covariates.
Although the association with HIV serostatus was no longer significant after adjusting for covariates, we further examined potential predictors of smoking using HIV-related variables among HIV-seropositive men (Table 3). Having less than a high school diploma was associated with a 18 % (95 % CI 1.10–1.27) higher prevalence of smoking compared with MSM who had attended graduate school or more; a 13 % (95 % CI 1.06–1.21) higher prevalence of smoking if enrolled in 2001 or after; a 9 % (95 % CI 1.05–1.13) higher prevalence of smoking if participants used marijuana; a 2 % (95 % CI 1.01–1.04) higher prevalence of smoking if participants exhibited depressive symptoms; and a 14 % (95 % CI 1.08–1.16) higher prevalence of smoking if participants were binge drinkers compared to those who did not drink. CD4+ count and HAART use were not statistically significant associated with smoking prevalence in the multivariate analysis. However, HIV-seropositive men had a 4 % (95 % CI 1.02–1.06) higher prevalence of smoking if they had a detectable viral load.
The same analysis was performed to observe prevalence ratios for smoking one pack or more by among participants who were smokers. Shown in Table 4, among all MSM participants, black, non-Hispanic, and other were less likely to smoke more than one pack a day compared to White non-Hispanics. Having less than a high school diploma was associated with a 10 % (95 % CI 1.07–1.13) higher prevalence of smoking one pack or more per day compared with MSM who attended graduate school or more; a 6 % (95 % CI 1.03–1.09) higher prevalence if they lived in Pittsburgh compared with those who lived in Baltimore/Washington, DC; a 3 % (95 % CI 1.00–1.05) higher prevalence for unemployed MSM compared with those that were employed; a 6 % (95 % CI 1.04–1.09) higher prevalence rate if they were binge drinkers compared with MSM who were non-drinkers; an 11 % (95 % CI 1.08–1.09) higher prevalence in marijuana users compared with non-users; and 3 % (95 % CI 1.00–1.05) higher prevalence in MSM who were HIV-seropositive compared with HIV-seronegative MSM. After further stratifying by HIV serostatus, CD4+ count was still not statistically significant associated with a higher prevalence of amount smoked among HIV-seropositive men (Table 5). HIV-seropositive men had a 1 % (95 % CI 1.00–1.02) higher prevalence of smoking if they had a detectable viral load.
An interaction term was introduced to further assess the association of cigarette smoking on HIV serostatus by time of enrollment. There were no statistically significant interactions for both outcomes. After removing seroconverters from the dataset, we assessed changes in baseline characteristics and longitudinal associations. There were no major differences in baseline characteristics (data not shown). There were no major differences in prevalence ratios for smoking in multivariate analysis except for a statistically significant positive association in participants enrolled after 2001 (data not shown). Additionally, we removed all participants that were lost to follow up in order to assess the same baseline characteristics and longitudinal associations. Because participants who were enrolled before 2001 were more likely to have died or have been lost to follow up, baseline characteristics were similar to the characteristics of participants enrolled after 2001. Compared to the participants analyzed in the study, participants who were not lost to follow-up, were more likely to be Black non-Hispanic, enrolled after 2001, be unemployed, and be current smokers (data not shown). After conducting the same multivariate analysis, the same predictors were positively associated with prevalence of smoking in total MSM, with the exception of hospitalization in the last 6 months. Additionally, being HIV-seropositive was associated with a 4 % (95 % CI 1.01–1.06) higher prevalence of cigarette smoking compared with HIV-seronegative participants (data not shown).
Discussion
In this study, although prevalence of smoking in MSM remain high, there were no differences by HIV serostatus. We found that among all men in the MACS, the prevalence of current smoking has been declining significantly, with a greater likelihood of current smoking among men in certain subgroups. These include black, non-Hispanic men, participants with lower education, those who were enrolled in 2001 and after, binge drinkers, and marijuana users. Multivariate analysis on HIV-seropositve participants showed that, CD4+ cell count and HAART use were not statistically significantly associated with prevalence of smoking, but detectable viral load was. Additionally, we found that the prevalence of smoking one pack or more per day was positively associated among HIV-seropositive men. Lower education, unemployment, alcohol use and detectable viral load were positively associated with prevalence of smoking one pack or more per day among HIV-seropositive men.
Race, alcohol consumption, level of physical activity, depression, and substance abuse have been shown to be associated with smoking among PLWH [5, 14, 16]. The low prevalence of smoking among HIV-seropositive and HIV-seronegative MACS participants enrolled before 2001 may be lower than expected because of loss to follow up or death. Being in a cohort for nearly 30 years may have also modified their smoking behaviors because they are aware of being observed. The 2001 enrollment in the MACS increased the number of minority participants in order to better represent the current HIV-positive MSM in the US. Though there is a decline in prevalence of smoking among this group, they were still more likely to smoke compared with the earlier enrolled group. This can reflect the overall historical shift of smoking prevalence in the population as whole. We found the prevalence of smoking among those enrolled in 2001 and later to be similar to other HIV-subpopulations [5, 7, 22]. This comparison includes a recent study from the Women’s Interagency HIV Study that showed that 39 % of women living with HIV were current smokers in 2011 [7]. The Veterans Aging Cohort found that 45.9 % of HIV seropositive patients were current smokers [5]. Tesoriero et al., reported the prevalence of smoking among PLWH in New York State to be 59 % [12]. Lifson et al., assessed smoking prevalence for 5472 HIV-seropositive men enrolled in 33 countries, and found that 40.5 % were current smokers [23].
Prevalence of smoking in MSM enrolled in 2001 and later was similar to older published studies [1, 2, 24–26]. For the first time in 2013, the National Health Interview Survey (NHIS) established a sexual orientation question for their annual health survey. The landmark addition will enable surveillance and long-term monitoring of the Healthy People 2020 goals to improve the health, safety, and well-being of lesbian, gay, and bisexual populations [27]. In 2013, 27.2 % of gay men between the ages of 16–64 were current smokers compared with 22.3 % of straight men of the same age group. Although the prevalence of smoking differed in our current study, the NHIS did not include a question on HIV serostatus. Additionally, a recent study utilizing data from the National Survey on Drug Use and Health examined the association between sociodemographic characteristics and smoking status among HIV-positive individuals [28]. Among their participants, 40 % of PLWH were smokers, and were more likely to smoke if they were previously married, binge drinkers, and were in lifetime drug and alcohol treatment.
For both outcomes in this study, site was associated with higher prevalence of smoking in MSM overall. Of the four sites, California’s state smoking laws were enacted in 1994 but allowed for exemptions for smoking in ventilated employee smoking rooms. The exemption still remains in effect [29]. Washington, DC/Baltimore, MD and Chicago, IL have 100 % smoke-free laws in all non-hospitality workplaces, restaurants and bars [29]. Pennsylvania has enacted smoke-free worksites but allow exemptions for smoking in ventilated restaurants. Compared to participants in Washington, DC/Baltimore, MD, MSM living in Chicago, IL and Pittsburgh, PA were more likely to smoke. More importantly, site was not statistically significant associated with smoking prevalence or quantity of cigarettes consumed among HIV-seropositive participants.
Among PLWH, detectable viral load was associated with prevalence of smoking while CD4+ cell count and HAART use were not statistically significant in this study. Previous studies assessing the association of smoking with the progression of HIV disease have yielded inconsistent results. Royce and Winkelstein found that smoking increased CD4+ cell count but the relationship was less pronounced among PLWH while Kabali et al. did not find a statistically significant association between smoking and CD4+ cell count and viral load [3, 30]. It has been suggested that nicotine may alter the metabolism of HAART by increasing clearance and decreasing its efficacy, thus increasing viral load among smokers [31]. This may have been why detectable viral load was associated with smoking prevalence in our study while HAART use remained statistically insignificant.
It has been suggested that PLWH maintain the belief that they will not live long enough to suffer the adverse effects of tobacco use and therefore are not concerned about smoking cessation [32, 33]. However, as the life expectancy of PLWH continues to improve, there is a need to focus on modifiable risk factors such as smoking that will further reduce morbidity and mortality from non-AIDS conditions [9]. Additionally, in one study, 63 % of current smokers reported they were interested in quitting smoking [34], but low-self efficacy (believing that they will not be able to quit) is a strong predictor of non-enrollment in smoking cessation programs [35].
As mentioned before, Crothers et al. (2007) showed that HIV providers were more likely to not identify patients who were current smokers when compared with non-HIV providers. Providers who do identify patients that are current smokers often feel that smoking cessation is low priority because of competing priorities, economic barriers, and that it may impose an additional burden on someone who is living with HIV [11, 36].
A strength of our study is the large, diverse sample of HIV-seropositive and HIV-seronegative MSM representing four different cities with nearly 30 years’ worth of data. We were able to assess the prevalence of smoking over time and determine factors associated with prevalence of smoking. However, our study also has limitations. Like most studies assessing cigarette smoking, we relied on self-reported data and were unable to confirm smoking status with biomarkers such as salivary or blood cotinine. Additionally, as mentioned, the very low reported prevalence rate for men who were enrolled before 2001 could have been because of loss to follow-up, death, or participation bias.
Our study shows that prevalence of smoking remains high among certain subpopulations of MSM including HIV-serpositive MSM. There is need for a continued effort to target MSM and PLWH with evidence-based tobacco cessation treatments. We also documented a strong tendency among men in the MACS to decrease smoking consumption. Other studies can build on this research and identify the predictors of successful smoking cessation. Understanding these predictors of smoking prevalence over time can inform targeted intervention for HIV-seropositive and seronegative MSM to mitigate smoking-associated comorbidities (i.e., heart disease, cancer) among HIV-seropositive men and smoking-related health disparities among MSM in general.
References
Skinner W. The prevalence and demographic predictors of illicit and licit drug use among lesbian and gay men. Am J Public Health. 1994;84:1307–10.
Skinner W, Otis M. Drug and alcohol use among lesbian and gay people in a southern US sample: epidemiological, comparative, and methodological findings from the Trilogy Project. J Homosex. 1996;30(59–91):18.
Royce R, Winkelstein W. HIV infection, cigarette smoking and CD4+ T-lymphocyte counts: preliminary results from the San Francisco Men’s Health Study. AIDS. 1990;4:327–33.
Lifson A, Neuhaus J, Arribas JR, van den Berg-Wolf M, Labriola AM, Read TR. INSIGHT SMART Study Group: smoking-related health risks among person with HIV in the strategies for management of antiretroviral therapy clinical trial. Am J Public Health. 2010;100:1896–903.
Crothers K, Goulet JL, Rodriguez-Barradas MC, Gilbert CL, Oursler KA, Bidwell Goetz M, Crystal S, Leaf DA, Butt AA, Braithwaite RS, Peck R, Justice AC. Impact of cigarette smoking on mortality in HIV-positive and HIV-negative veterans. AIDS Educ Prev. 2009;21(Suppl 3):40–53.
Reynolds N. Cigarette smoking and HIV: more evidence for action. AIDS Educ Prev. 2009;21(3 suppl):106–21.
Hessol NA, Weber KM, D’Souze G, et al. Smoking cessation and recidivism in the women’s interagency human immunodeficiency virus study. Am J Prev Med. 2014;47(1):53–69.
Vijayaraghavan M, Penko J, Vittinghoff E, et al. Smoking behaviors in a community-based cohort of HIV-infected indigent adults. AIDS Behav. 2014;18:535–43.
Robinson WT, Brown MC, Moody-Thomas S, et al. Smoking and experiences with tobacco cessation among men who have sex with men: New Orleans, 2011. AIDS Behav. 2014;18(Suppl 3):325–32.
Ompand DC, Kingdom M, Kupprat S, et al. Smoking and HIV-related health issues among older HIV-positive gay, bisexual, and other men who have sext with men. Behav Med. 2014;40(99–107):19.
Lifson AR, Lando HA. Smoking and HIV: prevalence, health risks, and cessation strategies. Curr HIV/AIDS Rep. 2012;9:223–30.
Tesoriero JM, Giervic SM, Carrascal A, Lavigne HE. Smoking among HIV-positive New Yorkers: prevalence, frequency, and opportunities for cessation. AIDS Behav. 2010;14:824–35.
Worm SW, Bower M, Reiss P, et al. Non-AIDS defining cancers in the DAD study–time trends and predictors of survival: a cohort study. BMC Infect Dis. 2014;13:471.
Crothers K, Goulet JL, Rodriguez-Barradas MC, et al. Decreased awareness of current smoking among health care providers of HIV-positive compared to HIV-negative veterans. J Gen Intern Med. 2007;22(6):749–54.
Centers for Disease Control and Prevention. Current cigarette smoking among adults—United States, 2005–2012. Morb Mortal Wkly Rep. 2014;63(02):29–34.
Mckirnan DJ, Tolou-Shams M, Turner L, et al. Elevated risk for tobacco use among men who have sex with men is mediated by demographic and psychosocial variables. Subst Use Misuse. 2006;41:1197–208.
Kaslow R, Ostrow D, Detels R, et al. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–8.
Giorgi JV, Cheng H, Margolick JB, et al. Quality controls in the flow cytometric measurement of T-lymphocytes subsets: The Multicenter AIDS Cohort Study experience. Clin Immunol Immunopathol. 1990;55(2):173–86.
Schenker E, Hultin L, Bauer K, et al. Evaluation of a dual-color flow cytometry immunophenotyping panel in a multicenter quality assurance program. Cytometry. 1993;14(3):307–17.
Substance Abuse and Mental Health Services Administration (SAMHSA). Binge drinking: terminology and patterns of use. http://captus.samhsa.gov/access-resources/binge-drinking-terminology-and-patterns-use. Accessed 15 Feb 2014.
Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6.
Agaku IT, King BA, Dube SR. Current tobacco use among adults in the United States, 2005–2012. MMWR. 2014;63:29–34.
Lifson AR, Neuhaus J, Arribas JR, et al. Smoking-related health risks among persons with HIV in the strategies for management of antiretrovirals therapy clinical trial. Am J Public Health. 2010;100:1896–903.
Ryan H, Wortley PM, Easton A, et al. Smoking among lesbians, gays, and bisexuals: a review of the literature. Am J Prev Med. 2001;21:142–9.
Stall RD, Greenwood GL, Acree M, et al. Cigarette smoking among gays and bisexual men. Am J Public Health. 1999;89:1875–8.
Greenwood GL, Paul JP, Pollack JP, et al. Tobacco use and cessation among a household-based sample of US urban men who have sex with men. Am J Public Health. 2005;95:145–51.
Ward B, Dahlhamer JM, Galinsky A, et al. Sexual orientation and health among US adults: National health interview survey, 2013. National Health Statistics Report. 2013;77. http://www.cdc.gov/nchs/data/nhsr/nhsr077.pdf. Accessed 1 Sep 2014.
Pacek LR, Harrell PT, Martins SS. Cigarette smoking and drug use among a nationally representative sample of HIV-positive individuals. Am J Addict. 2014;23:582–90.
CDC. State smoke-free laws for worksites, restaurants, and bars—United States, 2000–2010. MMWR. 2011;60(15):472–5.
Kabali C, Cheng DM, Brooks D, et al. Recent cigarette smoking and HIV disease progression: no evidence of an association. AIDS Care. 2011;23(8):947–56.
Wojna V, Robles L, Skolasky RL, et al. Associations of cigarette smoking with viral immune and cognitive function in human immunodeficiency virus-seropositive women. J Neurovirol. 2007;13(6):561–8.
US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2020. Washington DC. http://www.healthypeople.gov/2020/default.aspx Accessed 1 Sep 2014.
Burkhalter JE, Springer CM, Chhabra R, et al. Tobacco use and readiness to quit smoking in low-income HIV-infected persons. Nicotine Tob Res. 2005;7(4):511–22.
Reynolds NR, Neidig JL, Wewers ME. Illness representation and smoking behavior: a focus group study of HIV-positive men. J Assoc Nurses AIDS Care. 2004;15(4):37–47.
Lloyd-Richardson EE, Stanton CA, Papandonatos GD, et al. HIV-positive smokers considering quitting: differences by race/ethnicity. Am J Health Behav. 2008;32(1):3–15.
Mamary EM, Bahrs D, Martinez S. Cigaretts smoking and the desire to quit among individuals living with HIV. AIDS Patient Care STDs. 2002;16(1):39–42.
Acknowledgments
We thank Tariq Syed for his help in translating our abstract. Steve Shoptaw's work was also supported by the Center for HIV Identification, Prevention, and Treatment (CHIPTS) NIMH Grant MH58107; the UCLA Center for AIDS Research (CFAR) NIH/NIAID AI028697; and the National Center for Advancing Translational Sciences through UCLA CSTI Grant UL1TR000124. Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers at Baltimore (U01-AI35042): The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Barbara Crain, Adrian Dobs, Homayoon Farzadegan, Joel Gallant, Lisette Johnson-Hill, Cynthia Munro, Michael W. Plankey, Ned Sacktor, James Shepard, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), John P. Phair, Sheila Badri, Maurice O’Gorman, David Ostrow, Frank Palella, Ann Ragin; Los Angeles (U01-AI35040): University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (Co-PI), Aaron Aronow, Robert Bolan, Elizabeth Breen, Anthony Butch, Beth Jamieson, Eric N. Miller, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-AI35041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), Lawrence A. Kingsley (Co-PI), James T. Becker, Ross D. Cranston, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; and the Data Coordinating Center (UM1-AI35043): The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Alvaro Munoz (Co-PI), Alison, Abraham, Keri Althoff, Christopher Cox, Jennifer Deal, Gypsyamber D’Souza, Priya Duggal, Janet Schollenberger, Eric C. Seaberg, Sol Su, Pamela Surkan. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR000424 (JHU CTSA). Website located at http://www.statepi.jhsph.edu/macs/macs.html. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akhtar-Khaleel, W.Z., Cook, R.L., Shoptaw, S. et al. Trends and Predictors of Cigarette Smoking Among HIV Seropositive and Seronegative Men: The Multicenter Aids Cohort Study. AIDS Behav 20, 622–632 (2016). https://doi.org/10.1007/s10461-015-1099-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-015-1099-6