Abstract
This study evaluates the efficacy of an intervention for persons living with HIV (PLH) and their family members in Thailand. A randomized controlled trial of 813 PLH and family members was carried out at four district hospitals in Thailand. Participants completed Computer Assisted Personal Interview assessments at baseline, 6, 12, 18, and 24 months. The primary outcome was quality of life (QoL); other measures included depressive symptoms and family functioning. Relative to the standard care condition, the intervention group reported significantly improved QoL at 6 months (P = 0.0014). When the intervention efficacy was stratified by baseline depressive symptoms (low vs. high), intervention efficacy was observed only among those with low depressive symptoms. Study findings suggest that the intervention was more efficacious for participants with less depressive symptoms and better family functioning. Extensive interventions may be optimal for those who have the capacity to learn the tools and skills.
Resumen
Este estudio evalúa la eficacia de una intervención para las personas que viven con el VIH (PLH) y sus familiares en Tailandia. Un ensayo controlado y aleatorio de 813 miembros del PLH y sus familiares se llevó a cabo en cuatro hospitales de distrito en Tailandia. Los participantes completaron las evaluaciones de la Entrevista Personal y Asistida por Computadora (CAPI) al inicio del estudio y también después de 6, 12, 18 y 24 meses. El resultado primario fue la calidad de vida (QoL); otras medidas incluyeron síntomas depresivos y el funcionamiento familiar. Relacionada a la condición de la atención estándar, el grupo de intervención reportó que la calidad de vida había mejorado significativamente a los 6 meses (P = 0,0014). Cuando la eficacia de la intervención se estratificó por los síntomas depresivos de base (bajos v. altos), la eficacia de la intervención se observó sólo entre las personas con síntomas depresivos bajos. Los resultados del estudio sugieren que la intervención fue más eficaz para los participantes con menos síntomas depresivos y el mejor funcionamiento familiar. Las intervenciones extensas pueden ser óptimas para los que tienen la capacidad de aprender las herramientas y las habilidades.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With recent advances in antiretroviral therapy (ART) and increased access to ART, HIV has transitioned globally into a chronic illness. The focus on immediate survival from an acute infection has gradually shifted to an increased awareness for living with a protracted illness, and improvements in the quality of life (QoL). As perceptions and treatment procedures have evolved, HIV-related issues were also becoming a prolonged or lifetime challenge for people living with HIV (PLH) and their family members [1–5]. Recent studies on PLH in Thailand suggested that familial factors, such as family relationships and functioning, influenced QoL [6, 7]. Consequently, HIV has the potential to affect the ongoing and long-term quality of life for both PLH and their families.
There are more than one million PLH in Thailand [8]. As in other countries, families affected by HIV in Thailand provide psychological and economic support to their infected family members [9]. About two-thirds of PLH in Thailand live with or receive support from their family [10, 11]. Historically, medical and psychological interventions in Thailand have focused on individuals who have been consistently employed. Among the studies of PLH in Thailand, we identified only a few that involved couples [12, 13]. There has been only one evaluation of an intervention for HIV-positive mothers in Bangkok [14]. Given the high number of cases in Thailand, a sense of urgency has emerged for the need to develop and implement interventions that could strengthen HIV-affected families, and improve the quality of life for PLH and their family members.
Evidence-based interventions for HIV-affected families have demonstrated efficacy in the past 15 years, yet the diffusion of intervention programs has been limited [15]. Broadly speaking, there has been little empirical research addressing the adaptation of evidence-based interventions to ensure their fit for specific cultures and communities [16]. Family relationships are important and family is a valuable resource of support and care for ill family members [17], especially in Thai culture [18]. Previous studies have identified challenges associated with cultural adaptations of interventions based on Western-based behavioral theories to non-Western contexts, where the former focuses more on individual agency, whereas the latter emphasizes collectivistic notions [19–21]. Cultural differences can affect culturally adapted interventions; therefore, investigating the process of adaption and implementation of an intervention in other cultural settings creates an opportunity to assess their relevancy and sustainability.
This study was based on a randomized controlled intervention trial conducted in Thailand from 2007 to 2010. The intervention was adapted from previous evidence-based U.S. interventions, using common intervention factors, principles, and processes [22, 23]. In order to adapt the key elements to suit the cultural setting of Thailand, a series of workshops were carried out informing healthcare providers at the provincial and district levels. All participants were called upon to contribute their ideas to ensure the intervention activities fit the Thai context. The three modules of the original intervention (HIV-related Stressors, Improving Health and Mental Health, and Improving Family Adjustments) were adapted into four modules for the Thai intervention (Healthy Mind, Healthy Body, Healthy Family, and Healthy Community). The intervention content and framing were adapted to reflect Buddhist values of “sound body and sound mind,” as well as the cultural importance of individual health and well-being within the family and community. To our knowledge, this is the first large-scale family intervention trial designed to improve the quality of life for both PLH and their family members in Thailand.
Quality of life (QoL) is a multidimensional construct for an individual’s perceived well-being that is centered on physical, mental, and social functioning [24]. In the early 1990 s, QoL assessments were administered to PLH; and they quickly became—and remain—an important outcome in the evaluation of treatment strategies [25–28]. Previous studies have highlighted several factors that can affect QoL, such as treatment adherence, economic hardship, stigma and discrimination, and mental distress and psychiatric morbidity [29–33]. The goal of this article is to examine the efficacy of the intervention with data collected at baseline and during 6-, 12-, 18-, and 24-month follow-up assessments. We also explore how levels of depressive symptoms and family functioning may modify the intervention effects on improving QoL.
Methods
Study Design and Participants
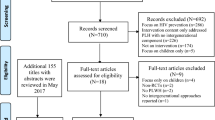
Approval of this study was obtained from the Institutional Review Board (IRB) at the University of California, Los Angeles (UCLA), and the Thailand Ministry of Public Health Ethical Review Committee for Research in Human Subjects. From January 2007 to February 2008, recruiters screened 484 HIV-affected families at four district hospitals in northern and northeastern Thailand. During monthly support group meetings, flyers were handed out to potential participants. Those who were interested in participating in the study were directed to contact a recruiter who screened and determined the eligibility of the participant. The screenings were performed at district hospitals during regular monthly support group meetings. Of the 484 families screened, 431 families (89%) met the eligibility criteria. Eligibility criteria included the following: (1) having at least one PLH in the household; (2) having at least one family member who was aware of the HIV status of the PLH; (3) having at least one school-aged child living in the household; and (4) the ability to provide written informed consent from the PLH and family members. Of the 431 eligible families, 410 consented to participate (refusal rate = 4.9%). Two persons from each family were recruited, a PLH and a family member. For some families, both participants were HIV-positive; therefore, 813 participants were comprised of 505 PLH and 308 HIV-negative family members. All participants received 300 baht (US $10) for each assessment. Intervention participants were compensated with the same amount for transportation to each session.
Services provided for patients in the standard care condition included a regular one-month follow-up treatment with ART for those who needed ART based on Thai national guidelines, prophylaxis of opportunistic infections, and a regular monthly support group for PLH and their family members.
Randomization
Assignment to the study conditions were conducted at the district hospitals and were based on the geographic location of the family’s residence. Each of the four district hospitals in this study accommodated residents from about 6 to 12 sub-districts. Half of the sub-districts served by each district hospital were randomly assigned to the intervention condition and the other half to the standard care condition. All families from the assigned intervention sub-districts were included in the intervention group, as families from the assigned standard care sub-districts were in the standard care condition.
Following informed consent, as shown in Fig. 1, 813 participants from 410 families completed the baseline assessment. Participants in both conditions were followed up and assessed 6, 12, 18, and 24 months after the baseline assessment. There was little attrition and we observed no significant differences in attrition rates between the intervention conditions. Ninety-seven percent of standard care participants and 98.6% of intervention participants completed at least two follow-up assessments.
Intervention Methods
The intervention was delivered over 13 weeks (12 sessions and one preparation session). Each session was led by two trained intervention facilitators, and was designed as a group session (8–10 participants per session). Each 90-minute session was participatory and was designed to focus on one or two challenges faced by PLH and their families. Module 1 (Healthy Mind) included four weekly sessions: (1) Emotional regulation while living with HIV; (2) Positive thinking and doing; (3) HIV disclosure; and (4) Stress management. Module 2 (Healthy Body) had three sessions: (1) Medication adherence and access to care; (2) Prevention of HIV transmission to others; and (3) Self care and healthy daily routines. Module 3 (Parenting and Family Relationship) consisted of three sessions: (1) Family roles and relationships; (2) Parenting while ill; and (3) Long-term plans with family members and children. Module 4 (Social and Community Integration) focused on two sessions: (1) Community participation and support; and (2) Employment while ill.
Across all sessions, intervention tools and activities were framed in a culturally relevant way to ensure intervention acceptability. In particular, it was important to consider the significance of Buddhism, which is the religious affiliation of 95% of the Thai population [34]. For the intervention development, some aspects of Buddhism (which played a significant role) proved useful in framing the intervention contents and activities such as its emphasis on personal responsibility, its support of personal betterment in the present time, and its attention to impermanence and change [19, 35]. Under the Buddhist paradigm, the “Feeling Thermometer” was framed as an effective tool to help participants understand their current state (feelings and emotions), thus informing them of their present state (being self-aware). The application of the “Feeling Thermometer” made this concept more tangible and concrete, which helped participants to visualize and express feelings in relation to their thoughts and actions. In addition, the Feel-Think-Do (FTD) model was adapted to promote the positive cycle of cause and effect, a concept closely linked to positive thinking in dealing with HIV-related challenges and the philosophies of Buddhism. The adaptation and tailoring of the intervention activities were essential for ensuring intervention acceptance.
Quality Assurance
Several measures were applied to ensure the fidelity of intervention implementation. Local project investigators from the provincial health department were present at each session to monitor intervention facilitators and provide cues to ensure fidelity to the time allotted for each activity. In addition, all sessions were videotaped. After each session, facilitators participated in a debriefing session to assess issues with implementation and their degree of fidelity to activities as written in the intervention manual. All of the intervention facilitators reported a high degree of fidelity, and there were no significant variations among facilitators in relation to the number of sessions completed.
Data Collection
Initial screenings of PLH were conducted in the district hospitals and performed by healthcare workers and research staff specifically hired for the study. Once the screenings were completed and permission to contact family members was given, written informed consent was obtained from all PLH and family members. A trained interviewer administered assessments using Computer Assisted Personal Interview (CAPI). Participants were asked about their demographic characteristics, HIV status, and questions about their family functioning, depressive symptoms, and QoL. The loss-to-follow-up rate was approximately 5% across the four study sites.
All the scales utilized in this trial were previously used and validated among Thai samples, or were developed locally by the Thai Department of Mental Health. In addition, prior to launching the main trial we conducted a Phase 1 pilot study to test all measures to assure scale reliability and validity.
Primary Outcome Measure
The primary outcome, QoL, was defined by the World Health Organization (WHO) as “an individual’s perception of their position in life in the context of the culture and value system in which they live and in relation to their goals, expectations, standards, and concerns” [36]. In this study, QoL was measured using the Thai version of the WHO Quality of Life (WHOQOL) assessment instrument called the WHOQOL-BREF (Thai) [37]. This was a 26-item questionnaire; a shorter version developed from the original 100-item questionnaire, the WHOQOL-100 [38]. Both the 100-question (WHOQOL-100) and 26-question (WHOQOL-BREF) versions have been validated in different settings across many cultures, including Thailand, and have been shown to have excellent psychometric properties of reliability [39–42]. Each individual item of the WHOQOL-BREF was scored 1 to 5 on a response scale. The score of each subscale was the sum of scores from each item within that subscale. The score of the overall QoL scale was the sum of the scores from the four domains; higher scores indicated better QoL. For this study population, the overall Cronbach’s alpha was 0.84.
Other Outcome Measures
Depressive symptoms were assessed using the Thai 15-item depressive symptomatology screening test which was developed for use in Thailand [43]. Questions were asked about problems that had bothered participants in the past week (e.g., feeling depressed most of the time, feelings of hopelessness or worthlessness, loss of self-confidence), with response categories from 0 (not at all) to 3 (usually [5–7 days a week]). A summative composite scale was developed with a range of 0 to 45, with a Cronbach’s alpha of 0.91, reflecting an “excellent” internal consistency.
Family Functioning was assessed with the Thai Family Functioning Scale (TFFS), adapted from the McMaster Model of Family Functioning [44] and previously used by our group in Thailand [11]. The TFFS has three subscales: cohesion, expressiveness of conflict feelings, and problem solving. We utilized the TFFS to assess the interactions among family members during the past three months. There were a total of 30 items, with response categories rating 0 (never) to 3 (always); the Cronbach’s alpha for the overall scale was 0.92.
Demographics included age in years, gender, marital status, income, and years of education. HIV status was also included and used in our data analysis, as presented in Table 1.
Statistical Analysis
An intent-to-treat approach was used to analyze intervention effects. Baseline differences between the intervention group and standard care condition were tested using Chi-square and t tests (or Wilcoxon signed-rank tests) for categorical and continuous variables, respectively. A mixed-effects regression model with family- and participant-level random effects was used to assess the intervention effect on the improvement of QoL. Covariates included age, gender, HIV status, group (standard care vs. intervention), time (baseline, 6-, 12-, 18-, and 24-month follow-ups), and group-by-time interaction. The family- and participant-level random intercepts were included to account for dependence within family and the correlation between repeated observations for each participant, respectively. Multilevel modeling allows separation of the nested sources of variation, which helps to properly estimate the fixed effect variances and increases efficiency in identifying important sources of variation [45].
Next, QoL was expected to be correlated with the other outcome measures, depressive symptoms (risk factor), and family functioning (protective factor). It was of interest to examine the intervention effects separately by the baseline levels of depressive symptoms and family functioning. This part of the analysis was for exploratory purposes. Therefore, the 50 percentile cut-off values (11 and 61, respectively) for baseline depressive symptoms and family functioning were chosen to make sure that the sample sizes remained reasonable. The baseline level of depressive symptoms was defined as low if the baseline depressive symptom score was 11 or lower, and high if the baseline score was 12 or higher. Similarly, the baseline level of family functioning was low if the TFFS score was 61 or lower, and high if the baseline TFFS was 62 or higher. We used the same mixed-effects regression model described above for each of the stratifications. Estimated improvements of QoL with standard errors from these models were also plotted.
All statistical analyses were carried out with SAS 9.2 (for Windows) and all graphs were generated using the publicly available statistical software R [46].
Results
Baseline Characteristics
More than two-thirds of the participants (69%) were women, and 62% of the participants were HIV-positive (Table 1). The average age of the participants was 41, with 66% reporting being married. A majority of the participants (78%) reported having less than a high school education. At baseline, no significant differences were observed for HIV status, gender, age, marital status, education, or annual income. Comparable levels of depressive symptoms and family functioning across the two intervention conditions were observed. Participants in the standard care reported a higher overall QoL score at baseline, compared to those in the intervention group.
Effects of the Intervention
Improvement in QoL—Overall
Table 2 presents the results from the mixed-effects regression model. Younger age and male participants had a significantly higher level of QoL (P < 0.0001). QoL was higher for the participants in the standard care versus intervention groups at baseline (P = 0.0484). Across both groups, we observed a significant increase in QoL over time (P < 0.0001). We also observed a significant interaction effect, intervention-by-time (P = 0.0118), indicating that the improvement of QoL for the intervention group was significantly different from the standard care condition over time. At the 6-month follow-up assessment, the estimated difference in improvement in QoL from baseline was significantly higher for the intervention participants compared to the standard care participants (Estimate = 2.207, SE = 0.688; P = 0.0014). The estimated difference in improvement in QoL remained significant at the 18-month follow-up (Estimate = 1.350, SE = 0.690; P = 0.050). To examine the robustness of the observed intervention effect we also conducted a sensitivity analysis, which adjusted for the individual’s QoL at baseline. The results were similar.
Improvement in QoL—Stratified by Depressive Symptoms
Table 3 shows the regression results for each of the stratifications. For those who had a low level of depressive symptoms at baseline, a significantly higher improvement in QoL for the intervention group was observed at 6 months (P = 0.0035). The intervention effects were observed at 12 and 18 months (P = 0.0205 and 0.0401, respectively). The magnitude of the difference decreased, but remained significant, as shown in Fig. 2a. Conversely, for those who had a high level of depressive symptoms at baseline, the intervention effect was not significant. Figure 2b shows that for the study participants with a high level of depressive symptoms, although they improved over time, no significant differences were observed between the intervention group and standard care condition at any of the follow-up assessments. This stratified analysis suggests that participants with low risk (e.g., lower levels of depressive symptoms) at baseline benefitted more from the intervention.
Improvement in Quality of Life (±SE) at 6, 12, 18, and 24 months for standard care vs. intervention by baseline depressive symptoms and family functioning: a low depressive symptoms at baseline, b high depressive symptoms at baseline, c low family functioning at baseline, and d high family functioning at baseline. The solid line with triangles represents intervention (black), and the dashed line with circles represents standard care (red). Estimated improvements in quality of life were from the mixed-effects models with two-level of random effects, adjusting for age, gender, and HIV status
Improvement in QoL—Stratified by Family Functioning
When examining the intervention effects at different levels of family functioning, we observed that for the participants who had a high level of family functioning at baseline, the estimated improvement in QoL for the intervention group was higher than that for the standard care condition at the 6-month follow-up (P = 0.0076). However, for those with low family functioning at baseline, the intervention effect was not significant (P = 0.0553). This suggests that the participants who had a higher level of family functioning benefitted more from the intervention, even though the intervention effect did not last beyond the 6-month assessment. Figure 2c shows an increase trend in QoL improvement for the intervention participants with low family functioning at baseline. The difference in QoL improvement for those with high family functioning between intervention and standard care reduced over time, as shown in Fig. 2d.
Discussion
Results from this study show that QoL can be improved by an intervention based on a cognitive behavioral framework that is tailored to Thai culture. The efficacy of the intervention may be attributable to a program that targets both PLH and their family members. The inclusion of both PLH and family members can provide a unique and relevant opportunity to stimulate conversations and strengthen interactions within a family. This allows participants to have a chance to see familiar challenges shared by other families, and form a social network for community support. Moreover, in the process of intervention adaptation, it appears essential to have knowledge about the core elements of evidence-based interventions [22, 23] and their related outcome measures. It is important, however, to establish a relationship with local collaborators and cultural experts who can provide informed opinions on whether a given core element is relevant to the target population, and fits the cultural context. This adaptation experience is not unique to HIV-related interventions, but rather represents a general model used in the cultural adaptation of evidence-based interventions and treatments [16].
An exploratory finding from our study was the differential intervention effect by level of depressive symptoms. Although depression has been consistently linked to poor QoL, we examined whether depressive symptoms served as an effect-modifier in relation to improvements in QoL attributable to the family intervention. For participants who started with relatively fewer depression symptoms, the intervention effect was not only greater, but also longer lasting compared to those with relatively more depressive symptoms.
Our findings are somewhat contrary to the expectation that those who are at the greatest risk have the greatest opportunity for improvement from behavioral interventions [15]. This finding, however, is consistent with other literature that indicate those who need services the most are often least likely to benefit from services because of their functioning capacity. For example, there is a body of evidence demonstrating associations between depressive symptoms and cognitive functioning [47–49]. There are also reports that suggest patients with severe depression do not respond as successfully to cognitive behavioral therapy as those with mild depression, and therefore influence its effectiveness [48, 50].
Our intervention was intensive in this study. For the intervention sessions to be successful, participants must be motivated and willing to put in the work (e.g., learning and practicing the skills), both during and outside of the intervention group. In addition, to process the information and skills learned from the intervention sessions required a good degree of cognitive functioning from the participants. When we examined the intervention effect by stratifying at the family level, we found that those participants with higher family functioning benefitted more from the intervention than those with lower family functioning at baseline. It is plausible that participants may have benefited more from the intervention if they had stronger support from their families.
We also identified in our study several methodological limitations. First of all, given the fact the randomization resulted in a balanced group, we still found that baseline participants in the standard care condition reported a slightly higher overall QoL score compared to those in the intervention condition. One of the explanations could be that our randomization was based on geographic location—predetermined sub-districts—to avoid potential contaminations; this could have led to sample imbalance due to chance alone. To address this issue, we conducted a sensitivity analysis to adjust for the baseline difference in QoL. The second limitation concerned eligibility criteria. To be eligible for the study, PLH had to have at least one family member who knew about their HIV status. It was possible that perceptions of QoL among those who had not disclosed their status differed from those who participated in our study. Therefore, the selection criteria limited the generalization of the study findings to those whose HIV status was not disclosed. Third, the outcome measures used in this study were based on self-reports and might be susceptible to information bias. In order to minimize potential social desirability bias, we strictly used separate intervention and assessment teams during project implementation. Fourth, the randomization was conducted at the sub-district level based on the assumption that participant characteristics were comparable across the sub-districts. Data collection at the participating district hospitals did not retain the participant information on sub-districts for the purpose of preventing interviewers from identifying participants in the intervention group to reduce potential interviewer bias. However, it would have been beneficial to adjust for potential variability across sub-districts in data analysis. Fifth, we used QoL as the main outcome indicator in this study; however, it was possible that the intervention might have had an impact on depressive symptoms or family functioning, which might be related to the improved quality of life. Future studies should continue to explore intervention effects on multiple outcome indicators and the relationships among them.
It is important to note that our trial findings did not make any definitive conclusions on the clinical significance of outcome. The scales used in our study were useful in describing the characteristics of participants in the trial and how the intervention relates to the changes in their characteristics over a period of 24 months. For instance, the scale for depression was used to screen for depressive symptomatology, not clinical depression. Therefore, the high-low cut-off points used in our analyses did not relate to clinical depression cut-off, but pertained to levels of depressive symptomatology. The goal of our intervention was not to improve the quality of life of PLH and family members who were clinically depressed or were suffering from other psychiatric conditions. Such illnesses require services and care from professional psychiatrists. Our intervention focused on providing skills and tools to PLH and family members with depressive symptoms (but not suffering from clinical depression) so that they could effectively cope with their illness before it became more serious.
Despite these cautionary notes, this study has important implications for current and future programs. First, it was encouraging to see that this study provided evidence for the efficacy of a behavioral intervention designed for both PLH and family members. Although PLH and family members had to deal with their specific issues (e.g., treatment adherence for PLH, caregiver burden for family members), facing these challenges as a family has been a powerful message from the intervention. This study makes a contribution to the current field of family interventions in demonstrating not only the feasibility, but also the efficacy of such an intervention model that has the potential to be adapted to different cultures with a family-oriented tradition. We also learned that there were certain characteristics of participants that put them on a “better platform” to benefit from the intervention. Although the depression scale used in the study was for screening purposes, our findings evince depression screening may be necessary for potential intervention participants; and that additional activities (and potentially treatment for those with clinical depression) may also be necessary to prepare such participants for behavioral interventions. In addition, based on our results, developers of intervention programs might want to explicitly focus on particular moderators, such as depressive symptoms as a target of change. More research would be needed to tease out effective intervention components and moderators to refine interventions for particular populations.
In conclusion, this intervention trial demonstrates that QoL improvement can be attainable with a culturally sensitive family intervention. Our intervention is much more efficacious for those with fewer depressive symptoms (and likewise for those with the general capacity to learn the emphasized tools and skills). In order for participants to fully benefit from a behavioral intervention and for broader scale-up and dissemination, providers must ensure that participants have sufficient capacity and support.
References
Bor R, Miller R, Goldman E. HIV/AIDS and the family: a review of research in the first decade. J Fam Ther. 1993;15(2):187–204.
Pequegnat W, Bauman LJ, Bray JH. Measurement of the role of families in prevention and adaptation to HIV/AIDS. AIDS Behav. 2001;5(1):1–19.
Murphy DA, Marelich WD, Dello Stritto ME, et al. Mothers living with HIV/AIDS: mental, physical, and family functioning. AIDS Care. 2002;14(5):633–44.
Rotheram-Borus MJ, Flannery D, Rice E, et al. Families living with HIV. AIDS Care. 2005;17(8):978–87.
Schuster MA, Kanouse DE, Morton SC, et al. HIV-infected parents and their children in the United States. Am J Public Health. 2000;90(7):1074–81.
Jongudomkarn D, Camfield L. Exploring the quality of life of people in northeastern and southern Thailand. Soc Indicators Res. 2006;78(3):489–530.
Rotheram-Borus MJ, Stein JA, Jiraphongsa C, et al. Benefits of family and social relationships for Thai parents living with HIV. Prev Sci. 2010;11(3):298–307.
National AIDS Prevention and Alleviation Committee. UNGASS country progress report, Thailand. Nonthaburi: Office of Technical Development to Support HIV/AIDS Responses, Ministry of Public Health; 2008.
Manopaiboon C, Shaffer N, Clark L, et al. Impact of HIV on families of HIV-infected women who have recently given birth, Bangkok, Thailand. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(1):54–63.
Knodel J, Im-em W. The economic consequences for parents of losing an adult child to AIDS: evidence from Thailand. Soc Sci Med. 2004;59(5):987–1001.
Knodel J, VanLandingham M. The impact of the AIDS epidemic on older persons. AIDS. 2002;16(S4):S77–83.
Tangmunkongvorakul A, Celentano DD, Burke JG, de Boer MA, Wongpan P, Suriyanon V. Factors influencing marital stability among HIV discordant couples in northern Thailand. AIDS Care. 1999;11(5):511–24.
McGrath JW, Celentano DD, Chard SE, et al. A group-based intervention to increase condom use among HIV serodiscordant couples in India, Thailand, and Uganda. AIDS Care. 2007;19(3):418–24.
Jirapaet V. Effects of an empowerment program on coping, quality of life, and the maternal role adaptation of Thai HIV-infected mothers. J Assoc Nurses. 2000;11(4):34–45.
Rotheram-Borus MJ, Swendeman D, Lee S-J, Li L, Amani B, Nartey M. Interventions for families affected by HIV. Transl Behav Med. 2011;1(2):313–26.
Lau AS. Making the case for selective and directed cultural adaptations of evidence-based treatments: examples from parent training. Clin Psychol: Sci Pract. 2006;13(4):295–310.
Wright LM, Bell JM. Beliefs and illness: a model for healing. Calgary: 4th Floor Press; 2009.
Wacharasin C. Families suffering with HIV/AIDS: what family nursing interventions are useful to promote healing? J Fam Nurs. 2010;16(3):302–21.
Rongkavilit C, Naar-King S, Kaljee LM, et al. Applying the information–motivation–behavioral skills model in medication adherence among Thai youth living with HIV: a qualitative study. AIDS Patient Care ST. 2010;24(12):787–94.
Camfield L, Ruta D. Translation is not enough: using the global person generated index (GPGI) to assess individual quality of life in Bangladesh, Thailand, and Ethiopia. Qual Life Res. 2007;16(6):1039–51.
Gelfand MJ, Bhawuk DPS, Nishii LH, Bechtold DJ. Individualism and collectivism. In: House RJ, Hanges PJ, Javidan M, Dorfman PW, Gupta V, editors. Culture, leadership, and organization: the GLOBE study of 62 societies. Thousand Oaks: Sage Publications; 2004.
Rotheram-Borus MJ, Ingram BL, Swendeman D, et al. Common principles embedded in effective adolescent HIV prevention programs. AIDS Behav. 2009;13(3):387–98.
Rotheram-Borus MJ, Lee MB, Gwadz M, et al. An intervention for parents with AIDS and their adolescent children. Am J Public Health. 2001;91(8):1294–302.
Smith KW, Avis NE, Assmann SF. Distinguishing between quality of life and health status in quality of life research: a meta-analysis. Qual Life Res. 1999;8(5):447–59.
Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: a conceptual model of patient outcomes. JAMA. 1999;273(1):59–65.
Bozzette SA, Kanouse DE, Berry S, et al. Health status and function with zidovudine or zalcitabine as initial therapy for AIDS: a randomized controlled trial. Roche 3300/ACTG 114 Study Group. JAMA. 1995;273(4):295–301.
Wu AW, Mathews WC, Brysk LT. Quality of life in a placebo-controlled trial of zidovudine in patients with AIDS and AIDS-related complex in AIDS clinical trials. J Acquir Immune Defic Syndr. 1990;3(7):683–90.
Wu AW. Quality of life assessment comes of age in the era of highly active antiretroviral therapy. AIDS. 2000;14(10):1449–51.
Sowell RL, Seals BF, Moneyham L, et al. Quality of life in HIV-infected women in the southeastern United States. AIDS Care. 1997;9(5):501–12.
Bennetts A, Shaffer N, Manopaiboon C, et al. Determinants of depression and HIV-related worry among HIV-positive women who have recently given birth, Bangkok, Thailand. Soc Sci Med. 1999;49(6):737–49.
Mannheimer SB, Matts J, Telzak E, et al. Quality of life in HIV-infected individuals receiving antiretroviral therapy is related to adherence. AIDS Care. 2005;17(1):10–22.
Geurtsen B. Quality of life and living with HIV/AIDS in Cambodia. J Transcult Nurs. 2005;16(1):41–9.
Li L, Lee S-J, Thammawijaya P, et al. Stigma, social support, and depression among people living with HIV in Thailand. AIDS Care. 2009;21(8):1007–13.
Jenkins RA, Kim B. Cultural norms and risk: lessons learned from HIV in Thailand. J Prim Prev. 2004;52(1):17–40.
Christopher MS, Charoensuk S. Mindfulness in Thailand and the United States: a case of apples versus oranges? J Clin Psychol. 2009;56(6):590–612.
World Health Organization. WHOQOL-100 facet definitions and questions. Geneva: World Health Organization; 1995.
World Health Organization. The World Health Organization Quality of Life-BREF (Thai). 2004. Available at: http://www.who.int/substance_abuse/research_tools/en/thai_whoqol.pdf. Accessed 31 Aug 2010.
WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998;28(3):551–8.
Kuyken W, Orley J, Hudelson P, et al. Quality of life assessment across cultures. Int J Ment Health. 1994;23(3):5–27.
Saxena S, Carlson D, Billington R, et al. The WHO quality of life assessment instrument (WHOQOL-BREF): the importance of its items for cross-cultural research. Qual Life Res. 2001;10(8):711–21.
Fang CT, Hsiung PC, Yu CF, et al. Validation of the World Health Organization quality of life instrument in patients with HIV infection. Qual Life Res. 2002;11(8):753–62.
Skevington SM, Lotfy M, O’Connell KA, et al. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. Qual Life Res. 2004;13(2):299–310.
Thai Department of Mental Health. Depression screening test, Ministry of Public Health, Thailand. Available at: http://www.dmh.go.th/test/depress/asheet.asp?qid=1. Accessed 10 Jun 2010.
Epstein N, Baldwin L, Bishop D. The McMaster family assessment device. J Marital Fam Ther. 1983;9(2):171–80.
Snijders TAB, Bosker RJ. Multilevel analysis: an introduction to basic and advanced multilevel modeling. Thousand Oaks: Sage Publications; 1999.
R Development Core Team. An introduction to R: notes on R, a programming environment for data analysis and graphics. Electronic edition. 2008. Also by Venables WN, Smith DM.
Gorwood P, Corruble E, Falissard B, et al. Toxic effects of depression on brain function: impairment of delayed recall and the cumulative length of depressive disorder in a large sample of depressed outpatients. Am J Psychiatry. 2008;165(6):731–9.
McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119(1):1–8.
Nakasujja N, Skolasky RL, Musisi S, et al. Depression symptoms and cognitive function among individuals with advanced HIV infection initiating HAART in Uganda. BMC Psychiatry. 2010;10:44.
Haby MM, Donnelly M, Corry J, et al. Cognitive behavioral therapy for depression, panic disorder and generalized anxiety disorder: a meta-regression of factors that may predict outcome. Aust N Z J Psychiatry. 2006;40(1):9–19.
Acknowledgment
This paper was completed with the support of the National Institute of Nursing Research at the National Institute of Health (R01-NR009922) and the National Institute of Mental Health (NIMH K01MH085503).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, L., Liang, LJ., Lee, SJ. et al. Efficacy of an Intervention for Families Living with HIV in Thailand: A Randomized Controlled Trial. AIDS Behav 16, 1276–1285 (2012). https://doi.org/10.1007/s10461-011-0077-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-011-0077-x