Abstract
Prior research has consistently demonstrated that providers often under recognize symptoms. However, this research was limited by the different ways in which patients and providers were asked about the symptoms patients experience. We sought to (1) describe the prevalence of patient-reported symptoms in the post-cART era; (2) identify those patient-reported symptoms which are most strongly associated with health-related quality of life (HRQoL), hospitalization and mortality; and (3) determine whether primary providers recognize symptoms associated with HRQoL, hospitalization and mortality. We conducted a secondary analysis using baseline survey data from the Veterans Aging Cohort Study and determined which patient-reported symptoms correlated with clinical outcomes using regression analyses. Kappa scores were then calculated. HIV-infected patients suffer from a high burden of symptoms in the post-cART era. Nine out of 20 symptoms correlated with clinical outcomes. Providers universally under recognized symptoms and demonstrated poor agreement beyond chance when patient-report was used as the gold standard.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Through the widespread use of combination antiretroviral therapy (cART), HIV has been transformed into a complex chronic disease as patients are living longer [1] and experiencing non-AIDS comorbid diseases [1–4]. The focus of treatment for HIV is no longer only the avoidance of opportunistic infections and survival, but also symptom management across the disease spectrum [5]. Patients with HIV suffer from a range of symptoms [6, 7] due to HIV, antiretroviral therapy [8, 9], and associated comorbid diseases and their treatment. Symptoms, defined as a “subjective experience reflecting changes in the biopsychosocial functioning, sensations, or cognition of an individual,” are key to understanding the patient’s experience of their illness [10] and to early detection of undiagnosed disease. Symptoms affect medication adherence [11–13], influence sexual behaviors [14], impact health-related quality of life (HRQoL) [15–20], and signal disease progression and drug toxicities. Despite their importance, symptoms are often unrecognized and undertreated by health providers [21].
Research has consistently demonstrated that providers often fail to respond to patient symptoms. Survey data on patients with HIV/AIDS, who were cared for at three different palliative care programs, demonstrated high levels of symptom burden with a substantial proportion of patients perceiving that their symptoms were not being treated [22]. For example, although 29% of patients reported symptoms of diarrhea at a New York City site, only 58% of those with these symptoms believed they were receiving treatment for diarrhea. One likely explanation for the lack of symptom-based treatment is that providers consistently under recognize symptoms [21] because they are not adequately assessing patient symptom experiences.
We previously employed a standard symptom checklist for both patients with HIV and their providers and demonstrated that provider-report had poor sensitivity. Scores on the standard symptom checklist completed by providers correlated less with outcome measures, including health-related quality of life, hospitalization and survival of patients compared with the symptom checklist completed by patients [21]. However, symptom checklist items administered to patients and providers were not identical. They differed in format, in the description of the symptom and in the number of symptoms on the checklists. Furthermore, patients were enrolled in this study from 1991 to 1992, predating the current era of widespread cART availability and its associated change in symptom type and frequency.
Our goal was to build on this previous work by administering a symptom checklist, with identical items and format, at the same clinical encounter to the patient and their designated primary care provider. Specifically, we aimed to:
-
1.
Describe the prevalence of patient-reported symptoms in the post-cART era.
-
2.
Identify patient-reported symptoms most strongly associated with HRQoL, hospitalization and mortality.
-
3.
Determine whether primary providers recognize symptoms associated with HRQoL, hospitalization and mortality.
Methods
Study Overview
The Veterans Aging Cohort Study (VACS) is an ongoing multi-site, cohort study of HIV-infected patients and non-HIV infected patients. For the purposes of this analysis, we performed a cross-sectional secondary analysis of the baseline survey data of the VACS-3 site study [23]. Briefly, VACS-3 consisted of HIV-infected Veterans recruited from Infectious Disease Clinics at Houston, Cleveland and Manhattan. The patient’s primary provider was an Infectious Disease specialist, a general internist, an advanced nurse practitioner or a physician’s assistant. The data were collected using patient and provider-administered questionnaires and retrospective chart review.
Participants and Procedures
Patients were approached during their clinic visit and asked to self-complete a questionnaire, which included measures of symptom burden and health-related quality of life. Patients who were willing to participate and were able to give informed consent were included in the study; there were no exclusion criteria. Permission to access their medical record was also requested. Patients received $10 compensation for participation in the study. Baseline questionnaire data were collected from June of 1999 to July 2000.
Providers of participating patients were administered the questionnaire on the same day as the patient’s visit and asked to complete it within 1 week. This questionnaire included items about patient demographics, psychiatric comorbidities and symptom burden. Approval was obtained from the Institutional Review Boards from all three sites and Pittsburgh, the study center.
Measures
Symptom Burden
In order to assess symptom burden, participants self-completed the HIV Symptom Index [24]. This widely utilized questionnaire, which has been demonstrated to be valid and reliable, has been translated into over 18 languages (M. Dulac Personal Communication). The questionnaire assesses the frequency and range of discomfort of 20 common symptoms by asking, “The following questions ask about symptoms you might have had during the past four weeks. Please fill in the circle of the one response that best describes how much you have been bothered by each symptom.” If the symptom was present, participants were prompted to indicate the degree of bother, based on a five-point Likert scale. A symptom was reported to be present if the participant stated that he/she had the symptom independently of the degree of bother.
Providers were presented the same HIV Symptom Index and were asked to, “Mark the symptoms this patient has had in the last 4 weeks, and were presented with the options yes, no, or don’t know.” The Provider responses of “no” and “don’t know” were grouped together.
Demographics
Demographic data, including age and race, were obtained through the electronic medical record and verified by patient and provider survey data.
HIV-1 RNA Viral Load and CD4 Count
HIV-1 RNA viral load and CD4 counts were collected from the electronic medical record using results closest to the survey date.
Outcomes
Health-Related Quality of Life
Health-related quality of life was assessed using the short form-12 (SF-12) [25, 26]. This questionnaire has been widely adapted for use with a range of chronic diseases for assessment of patient views about their physical and mental health. For this analysis, we focused on the physical component summary scale (PCS) of the SF-12 as there is overlap between the physical and mental health component summary scales. In addition, more items in the PCS were hypothesized to correlate with measures in the HIV Symptom Index compared with the mental component summary scale. The scores of the PCS range from 0 to 100, with higher scores representing higher functioning.
Hospitalization and Mortality
Data regarding hospitalization and mortality were collected from electronic medical records and the VA Beneficiary Identification Records Locator System (BIRLS) Death File, which is a complete death file of veteran beneficiaries [27].
Data Analyses
Descriptive statistics to determine the frequency of individual patient-reported symptoms were calculated (aim 1). Multivariable linear regression was used to determine the relationship between patient-reported symptoms and the continuous outcome variable, PCS. Multivariable logistic regression was used to assess the relationship between patient-reported symptoms and the binary outcome measures, hospitalization and survival (aim 2). In all models, we adjusted for CD4 count and HIV-1 RNA. The analysis was performed based on both the absence/presence and then degree of bother of symptoms. Then, among those patient-reported symptoms that were found to have a statistically significant correlation with an outcome (P < 0.05), we compared the reliability of provider-recognized symptoms using patient-report as the gold standard. This analysis was completed based only on the absence/presence of patient-reported symptoms as there is no standardized way to weight degree of symptom bother. Measures of agreement and agreement beyond chance (kappa scores) were calculated to compare differences in report of patient symptoms (aim 3). Scores of less than 0.40 were interpreted as poor agreement; a score of 0.40–0.75 fair to good agreement; and a score greater than 0.75 as excellent agreement [28]. Statistical analyses were performed using SAS version 9.1.3 (SAS Institute Inc., North Carolina).
Results
Participant Characteristics
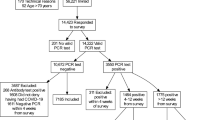
Of 1,038 HIV-infected patients receiving care in the three sites, 881 (85%) were enrolled. The analyses of symptom reports were limited to 807 patients as 71 providers and three patients had missing symptom data. Further analysis assessing symptom report and outcomes was limited to 751 patients as SF-12 data was missing for 51 patients; CD4 count or HIV-1 RNA was missing for an additional five patients. These 56 excluded patients (6% of the total sample) were not demographically different from the remaining group. Provider questionnaires were completed for 92% of enrolled patients.
The majority of patients in the sample were nonwhite (see Table 1, 54% black, 12% Hispanic). The mean age was 49 years, with a range of 28–79 years old, and 41% of patients over 50 years. Sixty-three percent of patients suffered from psychiatric comorbidities, including schizophrenia, depression, bipolar disorder and anxiety. Patients had a mean CD4 count of 331 cells/mm3 and an HIV-1 RNA of 715 copies. The mean score on the PCS of the SF-12 was 40, with an interquartile range of 32–47. More than 85% of primary providers were physicians.
Patient-Reported Symptoms
Patients demonstrated a high symptom burden (see Fig. 1). Nausea/vomiting was least likely to be reported but was endorsed by as many as one-third of patients (see Fig. 1). The symptom most likely to be reported was fatigue (71% of patients). Overall, nine out of 20 patient-reported symptoms were found to be significantly (P < 0.05) associated with at least one outcome, based on the absence/presence of symptoms (see Tables 2, 3, 4). The interpretation and direction of the relationships for these nine symptoms based on absence/presence did not change when the analyses were repeated based on degree of symptom bother.
Patient-Reported Symptoms and Physical Component Summary Score
Seven patient-reported symptoms were associated with the physical summary component score after controlling for CD4 count and HIV-1 RNA (see Table 2). The two strongest symptoms associated with decreasing PCS were muscle aches/joint pains and trouble remembering (β coefficient −2.65 and −2.42, respectively). Problems with weight loss/wasting, coughing/trouble breathing, fatigue/loss of energy, and loss of appetite/food taste were more intermediate in their effects on decreasing PCS (β coefficients ranged from −2.00 to −1.69). In contrast, diarrhea/loose bowels were associated with a statistically significant increase in PCS with an estimated increase of 2.27 (95% confidence interval (CI) 0.87–3.67) points in the PCS when present.
Patient-Reported Symptoms and Hospitalization
Three patient-reported symptoms were significantly associated with hospitalization, including nervous/anxious, problems with weight loss/wasting and hair loss or changes (P < 0.05) after adjusting for CD4 count and HIV-1 RNA (see Table 3).
After adjustment, problems with weight loss/wasting were associated with an increased rate of hospitalization, with a hazard ratio of 1.37 (95% CI 1.10–1.70). Nervous/anxious and hair loss/changes, in contrast, were associated with a decreased rate of hospitalization.
Patient-Reported Symptoms and Survival
One patient-reported symptom was significantly associated with survival after adjusting for CD4 count and HIV-1 RNA (P < 0.05) (see Table 4). Patients who reported diarrhea/loose bowels were significantly less likely to die with a hazard ratio of 0.71 (95% CI 0.51–0.98).
Key Patient-Reported Symptoms and Provider Recognition
Among all nine patient-reported symptoms that were significantly associated with measured outcomes, provider agreement beyond chance was poor (see Table 5). Using patient-report as the gold-standard, kappa scores ranged from 0.03 for hair loss/changes to 0.23 for diarrhea/loose bowels. There was no clear pattern to the distribution of the kappa scores based on the association with different outcomes.
Discussion
The purpose of this study was to characterize patient reported symptoms and identify the subset of symptoms that, after adjustment for CD4 cell count and HIV-1 RNA, predict HRQoL, hospitalization and mortality. Then, we aimed to definitively determine how often providers recognize these symptoms when asked using the same format on the same day. Patients demonstrated a high burden of symptoms. Of the 20 symptoms included in the HIV Symptom Index, nine were associated with important clinical outcomes. Some of these symptoms predicted worse outcomes; other symptoms were associated with improved outcomes. Providers consistently under recognized all of these nine symptoms independently associated with patient outcomes in the post-cART era [21], with kappa scores ranging from 0.03 to 0.23.
In our sample of patients in which most people are exposed to cART (87%), patients have a high symptom burden and suffer from the full range of symptoms measured by the HIV Symptom Index. This is consistent with previous work that demonstrates high symptom burden in this population [7, 9, 11, 18, 29]. The nature of symptoms parallels that of other progressive chronic diseases, including cancer, chronic obstructive pulmonary disease, end-stage renal disease and heart disease [30]; this is the basis for the WHO’s recommendation for including disease-specific and palliative care for all patients diagnosed with HIV [31].
Nine of the 20 symptoms included in the HIV Symptom Index were independently associated with a clinical outcome. Six of these nine symptoms were associated with a worse outcome. Specifically, fatigue/loss of energy, trouble remembering, coughing/difficulty breathing, loss of appetite/food taste, muscles aches/pains and problems with weight loss/wasting correlated with a decrease in the physical health summary score. This is not surprising as symptoms, specifically symptom bother and number, have consistently been demonstrated to impact health-related quality of life [15–18]. It is generally accepted that a change in three to five points on the MCS or PCS represents a clinically important difference [32]; on average, these six symptoms were associated with a 2.10 point decrease in the PCS score per symptom. Given the frequently modifiable nature of symptoms, this highlights the need for attention to and treatment of symptoms to maximize patient quality of life.
Given the reliance on self-report and the subjective nature of HRQoL, it was expected that symptoms would be commonly associated with HRQoL. Perhaps of even greater interest are the patient reported symptoms which were independently associated with hospitalization and mortality. The relationship of symptoms with hospitalization and mortality, objective, clinically important outcomes measured directly from medical records [21, 33], further highlights the need for provider recognition and attention to symptoms. In addition to its association with a decrease in HRQoL, problems with weight loss/wasting was also associated with an increased likelihood of hospitalization, likely signaling prolonged uncontrolled disease progression. Any potentially modifiable factor that is associated with hospitalization is clinically significant and deserves intervention when possible.
Three of the six symptoms independently associated with clinical outcomes were associated with improved outcomes. Specifically, feeling nervous/anxious, diarrhea/loose bowels, and hair loss/changes, predicted improved PCS or decrease in hospitalization or death. Anxiety may prompt increased health-care utilization, more opportunities for clinical evaluation and thus the protective association on hospitalization. Diarrhea/loose bowels and hair loss likely represent medication side effects [34, 35] supported by their association with increased survival and decreased hospitalization, respectively. Diarrhea/loose bowels were also associated with an improved PCS score. Diarrhea/loose bowels likely specifically reflects the widespread use of nelfinavir and its well documented effects on the gastrointestinal tract [11, 34, 36]. In order to promote continued medication adherence, it is essential that providers address these symptoms and intervene when possible to facilitate improved clinical outcomes.
Regardless of whether the symptoms were associated with an increased or decreased risk of a clinical outcome and even when symptoms were presented in an identical format on the same day, providers consistently under recognized symptoms. This was true for all nine assessed symptoms and consistent with previous data [11, 21, 29, 33]. Thus our findings confirm and enhance earlier studies that did not have as standardized approach to symptom measurement between patient and provider and serve to definitely evaluate provider performance at recognizing patient symptoms. Kilbourne et al. found poor agreement between patient and provider reported of moderate and severe depression [33]. Justice et al. demonstrated that provider-recognized symptoms do not simply represent a clinically relevant subset of symptoms as providers failed to identify approximately one-third of symptoms and patient-reports correlated closely with clinical outcomes [21]. Similarly, work by Duran et al. suggested that reliance of provider recognition of drug toxicities underestimates the patient’s experience [11]. Harding et al. demonstrated that when homosexual men were asked about what is needed to achieve their goal for the future—patients identified better clinical care as a major theme. Specifically, they sought improved recognition and treatment of medication side effects [29]. This previous work, confirmed by our findings, highlights the need for increased attention to patient symptoms, especially those that reflect medication adherence (e.g. diarrhea or hair loss) as these symptoms may subsequently lead to poorer adherence as the patient tires of the side effect [11–13]. In an era when patients live on cART for decades and experience multiple comorbidities, this becomes essential.
Our findings support the use of tools to improve symptom recognition and management [37] and symptom-based frameworks for clinical care [38]. Provider understanding and internalization of the patient’s experience is essential for providing quality clinical care, particularly if we seek to provide integrated palliative and disease-specific care [5]. Relying on the provider alone fails to capture the patient’s experience [11]. Symptom recognition is not only essential for optimizing patient care but is also a safety issue. Consistent with the Institute of Medicine’s report, patients should be more involved in reporting of adverse drug events in order to expand upon data that include mild to moderate symptoms, rather than on focusing only on severe adverse drug events [39]. This becomes only increasingly important as the guidelines for cART change to earlier initiation at higher CD4 counts [40, 41].
There are several strengths to our study worth highlighting. First, patients and their designated primary care providers were administered exactly the same HIV Symptom Index at the same clinical encounter (including by one of the authors—KG) to definitively answer how well providers perform at recognizing patient symptoms. Moreover, our study is based on findings from three different sites within the single system that provides care for the greatest number of HIV-infected patients. When the three sites were compared, there were no statistically significant differences in the kappa scores between patient and provider report for almost all of the symptoms; there were significant differences in kappa scores for coughing/trouble breathing, diarrhea and muscle aches/joint pain (data not otherwise shown). In addition, the average age of our population was 49 years old. Given the aging nature of the population living with HIV, with their increasing number of comorbidities and potential for medication side effects and toxicities, recognition of symptoms becomes more critical.
Our study has some limitations. First, our population consisted of only HIV-infected patients, which may limit the generalizability to other patient populations. HIV-infected patients, however, have been shown to have a similar symptom profile to patients with other diseases including cancer, heart disease, kidney disease and chronic obstructive disease demonstrating that the findings of this work may extend to other groups [30]. Second, our sample was limited to a Veteran population and, therefore, was almost entirely male. Recent analyses demonstrate that this group does not differ significantly from others based on clinically relevant variables [40, 41]. Further, a head to head comparison of symptom reporting among veterans and participants’ in the widely representative HIV Cost and Service Utilization Study demonstrated very similar patterns of symptom reporting [42]. Thirdly, the study data were collected at the beginning of the cART era, potentially limiting our data, as the symptoms experienced in more recent years may have changed. Nonetheless, our findings, showing that providers appear unaware of clinically important symptoms likely remain true. We are unable to distinguish whether providers did not know about a particular symptom because patients did not report it or because providers never asked. Our assumption was that because providers failed to ask, patients failed to report the symptom. In order to address this problem, we suggest the use of a standardized instrument to assess symptoms. A pilot intervention involving a clinical support tool to improve uniform detection of symptoms, demonstrated that providers were more likely to be ‘very aware’ of the patients’ symptoms when the tool was used in comparison to standard care [37]. Finally, our findings represent a conservative estimate of the degree of provider awareness because providers may have asked more frequently about symptoms their patients experience in the context of this study than typically done in routine clinical care.
In summary, our findings serve to support and substantially extend existing data by demonstrating that providers consistently under recognize patient symptoms when asked in a consistent way on the same day and restricted to a clinically relevant subset. Future work will seek to understand and characterize the reasons for this discrepancy, identify major contributing causes of the symptoms and the most effective treatments to operationalize tools to improve patient-centered care.
References
Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146(2):87–95.
Currier JS, Havlir DV. Complications of HIV disease and antiretroviral therapy. Top HIV Med. 2009;17(2):57–67.
Palella FJ Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43(1):27–34.
Braithwaite RS, Justice AC, Chang CC, Fusco JS, Raffanti SR, Wong JB, et al. Estimating the proportion of patients infected with HIV who will die of comorbid diseases. Am J Med. 2005;118(8):890–8.
Selwyn PA. Palliative care for patient with human immunodeficiency virus/acquired immune deficiency syndrome. J Palliat Med. 2005;8(6):1248–68.
Mathews WC, McCutchan JA, Asch S, Turner BJ, Gifford AL, Kuromiya K, et al. National estimates of HIV-related symptom prevalence from the HIV Cost, Services Utilization Study. Med Care. 2000;38(7):750–62.
Willard S, Holzemer WL, Wantland DJ, Cuca YP, Kirksey KM, Portillo CJ, et al. Does “asymptomatic” mean without symptoms for those living with HIV infection? AIDS Care. 2009;21(3):322–8.
Harding R, Molloy T, Easterbrook P, Frame K, Higginson IJ. Is antiretroviral therapy associated with symptom prevalence and burden? Int J STD AIDS. 2006;17(6):400–5.
Silverberg MJ, Gore ME, French AL, Gandhi M, Glesby MJ, Kovacs A, et al. Prevalence of clinical symptoms associated with highly active antiretroviral therapy in the Women’s Interagency HIV Study. Clin Infect Dis. 2004;39(5):717–24.
Dodd M, Janson S, Facione N, Faucett J, Froelicher ES, Humphreys J, et al. Advancing the science of symptom management. J Adv Nurs. 2001;33(5):668–76.
Duran S, Spire B, Raffi F, Walter V, Bouhour D, Journot V, et al. Self-reported symptoms after initiation of a protease inhibitor in HIV-infected patients, their impact on adherence to HAART. HIV Clin Trials. 2001;2(1):38–45.
Johnson MO, Charlebois E, Morin SF, Catz SL, Goldstein RB, Remien RH, et al. Perceived adverse effects of antiretroviral therapy. J Pain Symptom Manage. 2005;29(2):193–205.
Ammassari A, Murri R, Pezzotti P, Trotta MP, Ravasio L, De Longis P, et al. Self-reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. J Acquir Immune Defic Syndr. 2001;28(5):445–9.
Vincent E, Bouhnik AD, Carrieri MP, Rey D, Dujardin P, Granier F, et al. Impact of HAART-related side effects on unsafe sexual behaviours in HIV-infected injecting drug users: 7-year follow up. AIDS. 2004;18(9):1321–5.
Lorenz KA, Cunningham WE, Spritzer KL, Hays RD. Changes in symptoms and health-related quality of life in a nationally representative sample of adults in treatment for HIV. Qual Life Res. 2006;15(6):951–8.
Mrus JM, Leonard AC, Yi MS, Sherman SN, Fultz SL, Justice AC, et al. Health-related quality of life in veterans and nonveterans with HIV/AIDS. J Gen Intern Med. 2006;21(Suppl 5):S39–47.
Burgoyne RW, Rourke SB, Behrens DM, Salit IE. Long-term quality-of-life outcomes among adults living with HIV in the HAART era: the interplay of changes in clinical factors and symptom profile. AIDS Behav. 2004;8(2):151–63.
Mannheimer SB, Wold N, Gardner EM, Telzak EE, Huppler Hullsiek K, Chesney M, et al. Mild-to-moderate symptoms during the first year of antiretroviral therapy worsen quality of life in HIV-infected individuals. Clin Infect Dis. 2008;46(6):941–5.
Carrieri P, Spire B, Duran S, Katlama C, Peyramond D, Francois C, et al. Health-related quality of life after 1 year of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2003;32(1):38–47.
Burgoyne R, Collins E, Wagner C, Abbey S, Halman M, Nur M, et al. The relationship between lipodystrophy-associated body changes, measures of quality of life, mental health for HIV-positive adults. Qual Life Res. 2005;14(4):981–90.
Justice AC, Chang CH, Rabeneck L, Zackin R. Clinical importance of provider-reported HIV symptoms compared with patient-report. Med Care. 2001;39(4):397–408.
Karus D, Raveis VH, Alexander C, Hanna B, Selwyn P, Marconi K, et al. Patient reports of symptoms, their treatment at three palliative care projects servicing individuals with HIV/AIDS. J Pain Symptom Manage. 2005;30(5):408–17.
Smola S, Justice AC, Wagner J, Rabeneck L, Weissman S, Rodriguez-Barradas M. Veterans aging cohort three-site study (VACS 3): overview and description. J Clin Epidemiol. 2001;54(Suppl 1):S61–76.
Justice AC, Holmes W, Gifford AL, Rabeneck L, Zackin R, Sinclair G, et al. Development and validation of a self-completed HIV symptom index. J Clin Epidemiol. 2001;54(Suppl 1):S77–90.
Ware JE. SF-36 Health Survey Update. Cited 2010 February 28; Available from: http://www.sf-36.org/tools/sf36.shtml.
Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales, preliminary tests of reliability, validity. Med Care. 1996;34(3):220–33.
Fisher SG, Weber L, Goldberg J, Davis F. Mortality ascertainment in the veteran population: alternatives to the National Death Index. Am J Epidemiol. 1995;141(3):242–50.
Fleiss JLB, Paik M. Statistical methods for rates and proportions. Hoboken: Wiley; 2003.
Harding R, Molloy T. Positive futures? The impact of HIV infection on achieving health, wealth and future planning. AIDS Care. 2008;20(5):565–70.
Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31(1):58–69.
WHO. Palliative care. WHO; 2002.
Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics. 1999;15(2):141–55.
Kilbourne AM, Justice AC, Rollman BL, McGinnis KA, Rabeneck L, Weissman S, et al. Clinical importance of HIV, depressive symptoms among veterans with HIV infection. J Gen Intern Med. 2002;17(7):512–20.
Hill A, Balkin A. Risk factors for gastrointestinal adverse events in HIV treated and untreated patients. AIDS Rev. 2009;11(1):30–8.
Luther J, Glesby MJ. Dermatologic adverse effects of antiretroviral therapy: recognition and management. Am J Clin Dermatol. 2007;8(4):221–33.
Walmsley S, Bernstein B, King M, Arribas J, Beall G, Ruane P, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346(26):2039–46.
Nader CM, Tsevat J, Justice AC, Mrus JM, Levin F, Kozal MJ, et al. Development of an electronic medical record-based clinical decision support tool to improve HIV symptom management. AIDS Patient Care STDS. 2009;23(7):521–9.
Asch SM, Fremont AM, Turner BJ, Gifford A, McCutchan JA, Mathews WM, et al. Symptom-based framework for assessing quality of HIV care. Int J Qual Health Care. 2004;16(1):41–50.
IOM. Adverse drug event reporting: the roles of consumers and health-care professionals, workshop summary. Washington, DC: The National Academy Press; 2007.
Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360(18):1815–26.
Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, Harris R, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373(9672):1352–63.
Zingmond DS, Kilbourne AM, Justice AC, Wenger NS, Rodriguez-Barradas M, Rabeneck L, et al. Differences in symptom expression in older HIV-positive patients: The Veterans Aging Cohort 3 Site Study and HIV Cost and Service Utilization Study experience. J Acquir Immune Defic Syndr. 2003;33(Suppl 2):S84–92.
Acknowledgements
We would like to gratefully acknowledge Joseph Goulet for sharing his expertise and input in preparing this manuscript. This work was funded by The Veterans Health Administration, Robert Wood Johnson Clinical Scholars Program, Robert Wood Johnson Generalist Faculty Scholars Program, National Institute on Alcohol Abuse and Alcoholism (U10 AA 13566) and VHA Public Health Strategic Health Core Group, and National Institute on Aging.
Conflicts of Interest Statement
No known conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was presented as a poster at the National Meeting of the Society of General Internal Medicine, Minneapolis, MN on April 30th, 2010.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Rights and permissions
About this article
Cite this article
Edelman, E.J., Gordon, K. & Justice, A.C. Patient and Provider-Reported Symptoms in the Post-cART Era. AIDS Behav 15, 853–861 (2011). https://doi.org/10.1007/s10461-010-9706-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-010-9706-z