Abstract
It is often claimed that agroforestry could increase the total productivity per land unit compared to monocropping systems. The aim of this study was to evaluate, in a sub-humid Mediterranean climate, the behavior of the yield components, phenology, LAI and NDVI of durum wheat in an alley-cropping system. Our hypothesis was that the microclimate changes in agroforestry could change the devlelopment and yield of cereals. Two different experiments were carried out: in 2015 under 16-year old poplars in East–West lines and in 2016 under 21-year-old ash trees in North–South lines. In each experiment, 12 genotypes of durum wheat were sown. The grain yield was not significantly different in agroforestry and full sun conditions in 2015; however, both systems in this experiment had a particularly low yield (≈ 10% of the historical average yield of the plot). In 2016, the grain yield was significantly lower in agroforestry in comparison with full sun conditions. In both experiments, the most impacted yield component by agroforestry was the number of grains per spike. Similarly, in both experiments, the number of grains per spike was the only yield component impacted by the position within the alley inside agroforestry. Surprisingly, in 2016 the grain yield was higher in the West than in the center position of the alley. In both experiments, agroforestry delayed the maturity of the crop. The use of standard growing degree days was not sufficient to explain the difference in phenology between agroforestry and full sun conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agroforestry, i.e. a land use that combines agriculture and forestry, including the agricultural use of trees (Van Noordwijk et al. 2016), has been said to provide different services at various scales: field (Jose et al. 2004; Simelton et al. 2015), farm (Malézieux et al. 2009; Leakey et al. 2012), landscape (Nair and Graetz 2004; Rockwood et al. 2004), country (Garrity 2004; Jerneck and Olsson 2014) and world (Droppelmann et al. 2000; Stavi and Lal 2013). One of the services is the increased total productivity (i.e. considering both crop and tree production) (Muschler 2015). However, when considering only crop yield, agroforestry usually results in a decrease in crop yield compared to the pure crop because of the competition for resources between the crop and the trees (Cannell et al. 1996; Jose et al. 2004). Agroforestry, by its conception, imposes light reduction to the crop (i.e. shade) which can be a limitation for its productivity (Fischer 1975). Belowground, the competition for nutrients and water could also reduce productivity (Jose et al. 2000a, b). On the other hand, agroforestry can have beneficial effects on crop yield, e.g. by changing the microclimate. On top of the protection that trees can bring against adverse climatic extremes (Lin 2007), agroforestry microclimate could modify not only the thermal time experienced by the crop (Lott et al. 2009) and consequently impact crop phenology (Sudmeyer and Speijers 2007), but also the evapotranspiration rate (Karki and Goodman 2013). Due to the spatio-temporal complexity of both below-ground and above-ground competitions (Talbot and Dupraz 2012), as well as the possible beneficial effects, the net effect of agroforestry on crop productivity is uncertain (Ivezic and Van Der Werf 2016). Often, the balance between a positive or negative tree-crop interaction depends on the pedoclimatic conditions of the system (Mosquera-Losada et al. 2009; Muschler 2015), the management practices (Kohli and Saini 2003; Gill et al. 2009) and the intrinsic characteristics of the crop and the trees (Singh et al. 1993; Manceur et al. 2009).
In order to better understand the effect of different management and/or environmental conditions on crop yield, it is useful to decompose yield into measurable yield components (Kambal 1969). These yield components develop sequentially, with later-developing components under the control of earlier-developing ones and interacting in compensatory patterns, particularly under stressful environments (Simane et al. 1993; Moragues et al. 2006). During a crop cycle, the light requirements (Dong et al. 2014) and the optimal temperatures (Porter and Gawith 1999) vary according to the phenological stage. Thus, considering the sequential formation of the yield components through the phenological development of the crop, the timing of occurrence of a beneficial or detrimental microclimate condition could impact (positively or negatively) a specific yield component.
Crop phenology is a function of accumulated degree days, photoperiod (day length) and vernalization requirements (Brisson et al. 2004). The phenology in cereals has been predicted using these factors (Streck et al. 2003; Mc Master et al. 2008). Specifically, Mc Master et al. (2008) report that the use of vernalization and photoperiod as explicative factors results in accurately simulating anthesis date for a wide range of sowing dates. Slafer and Rawson (1996) reported that development in all phases is modified by photoperiod and air temperature to a different extent depending on the genotype. Also, they found that the ratio between the influence of photoperiod and temperature changes along the cycle. Gouache et al. (2012) agree that photoperiod affects the phenology during the whole cycle. However, they pointed out that vernalization is only relevant to calculate phenology until stem elongation. As agroforestry modifies both air temperature (Peng et al. 2015; Gosme et al. 2016) and, most importantly, radiation under the trees, it is likely that it changes crop temperature and it could have an impact on crop phenology. A delay in phenological development of the crop might allow some sort of compensation for the reduced light under the tree canopy by extending the growing period of the crop. Furthermore, a change in the timing of occurrence of the sensitive stage in relation to an adverse weather event, or a mitigation of the extreme weather events itself, might be beneficial to the crop.
Agroforestry conditions could also modify cereal morphology (Li et al. 2010; Wang et al. 2014), which in turn could change the interaction between the environment and the crop. It has been proved that shade conditions can increase the leaf area index (LAI), improving the capacity of the understory crop to intercept radiation (Li et al. 2010). Other vegetation indices, such as the normalized difference vegetation index (NDVI), can be used as a proxy to estimate photosynthetic area in cereals (Hansen and Schjoerring 2003). NDVI is a classical index of the crop, calculated using the red reflectance and near-infrared reflectance. These changes in the photosynthetic area could compensate to some extent the reduction of light by the tree canopy.
Alley cropping is a type of agroforestry system in which parallel tree lines are planted in croplands, the alleys between tree lines are covered by natural or sown herbaceous vegetation and the soil on tree lines is usually not tilled (Cardinael et al. 2015). In alley cropping systems, the environment is not homogeneous across space, depending mainly on the distance to the tree line (Kohli and Saini 2003; Sudmeyer and Speijers 2007).Therefore, trees in alley cropping system affect crop yield differently in the different positions in the alley, as both direct effects (competition for light, water, and nutrients) and indirect effects (modification of microclimate) depend on the distance to the tree as well as the position of the tree’s shade as determined by the system’s architecture, and the combination of latitude and time in the year and during the day. Considering the above, there is a lack of knowledge about the effect of alley cropping system (and within these, the distance with respect to the tree line) in Mediterranean conditions on foliar development, phenology and yield components of durum wheat. The aim of this study was to evaluate the impact of two different alley cropping systems, under typical conditions of the Northern Mediterranean region, on the yield, yield components, phenology, LAI and NDVI of a range of durum wheat genotypes, as well as the possible interactions between these traits that could allow compensation mechanisms to take place.
Materials and methods
Study sites
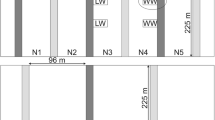
Two experiments were carried out in 2015 and 2016 at two different sites located in the ‘Restinclières Agroforestry Platform (RAP)’ (CIRAD 2017) in Hérault department in the South of France (43°42′N, 3°51′E). The climate is sub-humid Mediterranean and the soil is deep calcareous silty clay. A local farmer rents the land to grow arable crops, but part of the plots can be dedicated to scientific experiments. In the experimental subplot, all cultural practices except sowing and harvesting are done by the farmer. The performance of durum wheat was evaluated in both alley cropping systems, (agroforestry, AF) and full sun (FS) conditions. In order to introduce genetic variability, 12 genotypes were tested in each experiment, among which four were evaluated in both experiments, totaling 20 genotypes. Genotypes were taken from old cultivars kept at INRA’s durum wheat genebank (INRA 2017), as well as commercial cultivars. AF conditions were different in the two experiments. In 2015, wheat was sown in a single alley (13 m wide), with 15-year-old poplars (Populus canadensis CV I214) 30 m of height, planted at six meter distance within the row. The gap fraction of the trees canopy in the alley, measured through hemispherical photographs at harvest, was 67%. The cropped alley was split into 36 microplots (1.55 × 6 m). The 12 genotypes were planted in triplicate in the 36 microplots so that each genotype was present once in the two rows of microplots nearest to the trees on the South side of the alley, once in the microplots in the middle of the alley, and once in the microplots nearest to the trees on the North side of the alley (Fig. 1a). In 2016, wheat was sown in three 13 m wide alleys, with 21-year-old ash trees (Fraxinus angustifolia Vahl) 15 m of height, planted at two meters along the line. The gap fraction of the trees canopy in the alley, measured through hemispherical photographs at harvest, was 65%. Each alley was considered as a block and was split into 12 microplots (1.55 × 6 m), totaling 36 microplots among the three alleys. The 12 genotypes were planted in each block so that each genotype was present once in the microplots nearest to the trees on the West side of the alley, once in the microplots in the middle of the alley, and once in the two microplots nearest to the trees on the West side of the alley (Fig. 1b). In order to assess the effect of the position of the plot in the alley regarding the trees, the single alley in 2015 and the three alleys in 2016, were split into three positions, each one formed by two lines of plots. Therefore, in 2015 the alley was subdivided into North, central and South positions and in 2016 into East, central and West positions. In both experiments, the distance from the tree rows to the first plot in each side was 1.85 m. The same planting pattern was repeated in FS conditions as the AF conditions (Fig. 1).
Management practices
In the ‘2015 experiment’, the soil was prepared by plowing followed by a rotary harrow on January 07, 2015 because of floods that prevented sowing in the previous autumn. Sowing was done on January 12, 2015, at a density of 300 seeds per m2, using a sowing machine. The seeds were pretreated with PREMIS 25FS (active ingredient: triticonazole) in order to prevent fungal infection. Due to the late sowing, the usual treatment calendar of the farmer could not be applied to the experimental subplot, so no fertilizers or pesticides were applied. Harvest was done on June 30, 2015. In the ‘‘2016 experiment’’, the soil was prepared with a rotary harrow on October 23, 2015, and sowing was done on November 02, 2015, with the same methodology and density as in the ‘2015 experiment’. Applications of an herbicide (Athlet®, 3, 6L/ha) and of a fertilizer (180 kg ha−1 ammonium nitrate + 33 units of sulfur), were carried out on November 13, 2015, and December 01, 2015, respectively. Due to a serious weed infestation, two hand weeding were done on 28/01/2016 and 22/03/2016. However, due to the size of the field, it was not possible to clean it all, so the weeds were only removed in a central area of 1 m × 1.55 m in each microplot, and all measurement thereafter were done on this subset of the microplot. Harvest was done at maturity, on June 28, 2016, in FS and July 6, 2016, in AF.
Microclimate conditions
The air temperature was monitored using humidity and temperature probes (HMP155, Campbell Scientific, USA), placed inside a radiation and precipitation shield (DTR500, Vaisala, Finland). The incoming solar radiation under the tree canopy was measured using pyranometers (SP1110, Campbell Scientific, USA). The sensors were installed from stem elongation onwards in 2015 and over the whole cycle in 2016, at locations shown in Fig. 1. Data from a meteorological station located in full sun conditions at 1.3 and 0.8 km from the ‘2015’ and ‘2016’ experiments, respectively, were used to fill in when data from the sensors were missing (e.g. due to battery failure or displaced cable).
Measured variables
In both experiments, the phenology was assessed from stem elongation stage to maturation stage (see below), weekly or twice a week depending on the season. The follow-up was done using the Zadoks scale (Zadoks et al. 1974) that describes the phenology of cereals using 10 stages: the code from 0 to 9 correspond to the germination stage, 10–19 to seedling stage, 20–29 to tillering stage, 30–39 to stem elongation stage, 40–49 to booting stage, 50–59 to heading stage, 60–69 to anthesis stage, 70–89 to grain filling stage and 90–99 to maturation stage. In 2015, the monitoring of the phenology was done at the microplot level. Due to the variability observed within the plots in 2015, in 2016, the phenological stage of the plot was determined by recording the Zadok’s stage of 20 individual plants. The LAI was estimated using the LAI-2000® (LI-COR®) and the normalized difference vegetation index (NDVI) with a handheld crop sensor (greenseeker, Trimble®).
The components of yield considered in the analysis were the number of plants per m2, the number of tillers per plant, the percentage of fertile tillers, the number of grains per spike and the weight of grains. The harvest index was calculated as the ratio of the dry weight of grain to the total dry matter harvested (straw and spike). In both years, the number of plants was determined at the end of winter and tillers were counted before heading. The plants and tillers were counted in a line meter in two places of the microplot. The spikes were harvested in quadrats included in the weeded subsets of each microplot, 1 m × 1 m (2015) or 0.78 m × 1 m (2016, the four central sowing rows, to avoid edge effects). The harvested spikes were counted and the fresh and dry (after 3 days in a stove at 60 °C) weight were measured. The spikes were threshed and the grains counted and weighed.
Data management and statistical analysis
Due to the differences in experimental conditions in 2015 and 2016, each year was analyzed separately. The climate data of each hour were classified as day or night according to the sunset and sunrise times of each day, using the ‘R’ package ‘RAtmosphere’. The data concerning temperature and radiation were grouped according to three “growth periods” (germination-stem elongation, stem elongation-anthesis, and anthesis-maturity) defined based on the median stage of all microplots in a given system (FS vs. AF). The thermal time was calculated as the number of growing degree days (GDD), using the daily maximal and minimal temperature (Arnold 1960) and considering a base temperature of 0 °C (Brisson et al. 2008; Richter et al. 2010). Using data from observation dates when at least one phenological change occurred, a cumulative link mixed model (‘Ordinal’ package of R statistical software), was used to estimate the probability of each of the 72 microplots (36 in AF and 36 in FS) to remain in the previous stage, as a function of system (fixed effect) and genotype (random effect). Then, data from AF only were used to test the effect of position in the alley (North, center, South in 2015; East, center, West in 2016) considered as a fixed effect. Similarly, each yield component was analyzed using a mixed effect model (‘lmer’ package of R statistical software), considering the system as a fixed effect and the genotype, the block (only in 2016) and all the first order interactions as random effects. Using first the ‘forward-fit methodology’ for the random effects and the ‘backward-fit methodology’ for the fixed effects the best model was chosen based on the Akaike information criterion (AIC). Then, if the system effect was kept in the model, comparisons between systems were performed with Tukey’s HSD test. The threshold for significance was set at α = 0.05. Then, the effect of position in the alley was analyzed with the same methodology but taking only data from AF. The LAI and NDVI data were analyzed with mixed effect models with the same random effects, but the fixed effect was a factor with four levels: FS and the three positions in the alley in AF.
Results
Yield and yield components
In 2015, the grain yield was not significantly different between FS and AF, with a mean of 45 and 46 g m−2, for FS and AF respectively (Table 1). In 2016, the grain yield was considerably higher in FS than in AF, with 203 and 62 g m−2 respectively. The comparison of yield components between the AF and the FS conditions (Table 1) showed that in 2015 the number of plants per square meter, the weight of grains and the harvest index were significantly higher in AF, whereas the number of grains per spike was significantly lower in AF. In 2016, the number of tillers per plant, the number of grain per spike and the harvest index were significantly lower in AF, while for the other yield components no statistical differences were found.
In both experiments the only yield components impacted by the position in the alley within agroforestry, was the number of grains per spike (Table 2). In 2015, it was higher in the South position of the alley in comparison with the North position; the central position was not statistically different from any of the border positions and in 2016 the West position was higher than the other two (Table 2). In 2016 the final grain yield was also impacted by the position in the alley, being higher in West position than in the central position; the East position was not different from any of the other two positions.
Phenology
In both years, considering the median of all microplots, FS reached maturity first. However, in 2015, the difference between systems was small (about 2 days) with a high variability between genotypes (data not shown). In 2016, wheat ripened 1 week earlier in FS than in AF. In 2015, the probability of remaining in the earliest Zadoks’s stage at the end of a given time period, was higher in AF from anthesis onward. In 2016 this probability was higher in AF whatever the period, but particularly since heading (Fig. 2).
Probability of remaining in the earliest phenological stage at the end of a time period for 2015 (a) and 2016 (b). Only the time periods in which at least one of the plots changed their stage were considered. ste stem elongation stage, bot booting stage, hed heading stage, ant anthesis stage, gaf grain filling stage, mat maturation stage
Changes in temperature and radiation
There were no visible differences in the cumulative air temperature per hour (base 0 °C) between systems during all periods for either year (Fig. 3a). However, AF showed a ‘buffer’ effect, warming the air below the canopy of the trees at night and cooling it during the day, considering hourly temperature. This effect is clearly seen in the period between stem elongation and anthesis (Fig. 3b, c). The mean difference in this period between AF and FS in 2015 was + 1.14 during the night and − 1.13 during the day; in 2016 the difference was + 0.86 during the night and − 1.72 during the day. On hot days in both years, the air temperature could be almost 6 °C lower in AF.
Comparison of air temperature between full sun (FS) and agroforestry (AF) systems. Cumulative temperature per hour (base 0 °C) in full sun (FS) and agroforestry (AF) systems in 2015 and 2016 experiments during the periods between phenological stages: ger-ste period from germination until before the stem elongation, ste-ant period from stem elongation until before the anthesis, ant-mat period from anthesis until the end of maturation (harvest) (a). Difference in the temperature measured at 1 m above the soil (TAF–TFS), for each hour of the day in the period between stem elongation and anthesis for 2015 (b) and 2016 (c) experiments. The black line shows the mean values for each hour in the day
There was a large difference between systems in the cumulated radiation that reached the crop in different growth periods for both years (Fig. 4). In 2015, the cumulative radiation received in FS in the periods from stem elongation until before anthesis and from anthesis to harvest was 272 and 471 MJ m−2, respectively; meanwhile, AF received in the same periods 115 and 192 MJ m−2. In 2016, the cumulative radiation received in FS in the periods from emerging until before stem elongation, from stem elongation until before anthesis and from anthesis to harvest was 953, 578 and 586 MJ m−2, respectively; meanwhile, AF received in the same periods, 523, 263 and 303 MJ m−2. In both experiments and in all periods, the reduction rates in received radiation between FS and AF were around 50%. In spite of the fact that the late sowing in 2015 entailed higher instantaneous incident radiation at a given stage in comparison with 2016, the crop received more radiation during the stem elongation-anthesis period in 2016 because the duration of this phenological stage was longer (it lasted 24 days in 2016 and only 14 in 2015). Neither the temperature nor the radiation cumulated during the different periods of growth were statistically different in the different positions of the alley within the agroforestry system.
Cumulative global radiation received in full sun (FS) and agroforestry (AF) systems in 2015 and 2016 experiments during the periods between phenological stages: ger-ste period from germination until before the stem elongation, ste-ant period from stem elongation until before the anthesis, ant-mat period from anthesis until the end of maturation (harvest)
Relationship between phenology and temperature
The number of days after sowing (DAS) needed to achieve the stem elongation, anthesis and maturation stages in 2015 were lower than in 2016 by 56, 59 and 77 days, respectively (Fig. 5a). In general, in 2015, the phenology in both systems was faster than in 2016, reducing the cycle by more than 2 months. This was due to the date of sowing: sowing in January in 2015 produced very different climate conditions, with both fewer days of the crop in winter and warmer temperature and longer photoperiod at a younger stage of the crop. Indeed, considering the thermal time instead of the DAS the differences between both years got shorter, but there were still differences in the number of growing degree days to reach each stage (Fig. 5b). Similarly, using thermal time instead of DAS was not sufficient to explain the lag between FS and AF.
Photosynthetic area
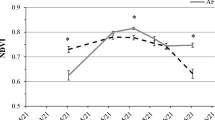
In 2015, the LAI of the crop measured close to anthesis (21/05/2015) was significantly higher in FS than in the center and South position of the alley, but not significantly different from the North position. In turn, the LAI of the crop was significantly higher in the North position than in the South position, and the central position had an intermediate value, not being significantly different from either the North or the South positions (Fig. 6a). In 2016 the LAI of the crop around anthesis (03/05/2016) was significantly higher in FS than in AF, whatever the position in the alley (Fig. 6b). In 2015, the NDVI of the crop measured close to anthesis (13/05/2015) was significantly higher in FS than in AF (whatever the position), and the North position was significantly higher than the center and South positions (Fig. 6c). In 2016, the NDVI of the crop, also measured close to anthesis (06/05/2016), was statistically not different between FS and the West position of the alley, and both were significantly higher than the center and East positions, which were not significantly different one from the other (Fig. 6d).
Boxplots of the crop LAI and NDVI, according to the system and position in the alley within AF. Leaf area index (LAI) of wheat at anthesis in 2015 (a) and 2016 (b) experiments. Normalized difference vegetation index (NDVI) of wheat at anthesis in 2015 (c) and 2016 (d) experiments. In all graphs, the number of microplots was 12 in each AF position and 36 in FS
Discussion
Our results show that the impact of agroforestry on durum wheat yield was different in the two experiments. In 2015, the yield was not statistically different between systems, however, it is important to highlight that the yield obtained in this experiment in both systems was considerably lower than the historical yield of the site (i.e. mean yield was about 10% of the normal yield, which is around 4.5 t ha−1; (Dufour et al. 2012), most likely due to lack of fertilizers and pesticides. Meanwhile, in 2016, the yield in agroforestry was 70% lower than in full sun conditions, and yield in full sun conditions was not too bad, considering that the tested genotypes were mostly old varieties. This is consistent with Malézieux et al. (2009) and Ong et al. (2015), who, in their respective reviews, present a range of situations, in which the results of agroforestry change depending on the conditions of the system. Despite the fact that the experiments in 2015 and 2016 were different in terms of sowing date, understory tree species and tree line orientation, some results are similar. The yield component with the highest negative impact of agroforestry in both years was the number of grains per spike. In the same way, agroforestry always delayed crop maturity by a few days. It is worth mentioning that in both experiments, there was a great variation among genotypes in all the yield components (the genotype effect was always included in the statistical model by the forward-fit procedure for the random effects in the linear mixed models). However, there was never an interaction between the genotype and the system meaning that on average, wheat genotypes were impacted in the same way by agroforestry. In line with this, other authors reported significant variation among genotypes under shade (Lakshmanakumar et al. 2013) and agroforestry conditions (Singh et al. 1993; Gill et al. 2009), even concluding that the success of agroforestry systems depends on the use of shade tolerant genotypes (Barro et al. 2012; Ehret et al. 2015). The comparison between genotypes was beyond the scope of this paper, but further analyses of the performance of the tested genotypes in full sun and agroforestry systems in several sites and years should be performed in the future.
Effect of agroforestry on yield components
In 2015, the number of plants per square meter was higher in FS, mainly due to the improvement in germination or winter survival, which might be due to milder temperatures under the trees in winter (unfortunately temperature was not measured before 10/03/2015 in the ‘2015 experiment’). This effect was probably exacerbated by the late sowing in 2015, which was carried out in January while the normal sowing date in the region is November. Microclimate conditions that positively impact yield have been reported for wheat in agroforestry for some (but not all) orientations of the tree line and distances between the crop and the tree line (Kohli and Saini 2003).
In 2016, the yield was strongly reduced in agroforestry, through reduced tillering (31% less tillers per plant) as well as a reduction in floral initiation (25% fewer spikelets per spike, data not shown) and fertility (42% fewer grains per spikelets, data not shown). A negative effect of agroforestry on yield caused by belowground (Zhang et al. 2014) and aboveground (Sudmeyer and Speijers 2007) competitions have been reported. Kohli and Saini (2003) found that agroforestry caused a reduction in quantity and quality of light that resulted in a lower number of tillers per land unit area. Similarly, Gill et al. (2009) and Kaur et al. (2010) reported a declining trend in the number of tillers of wheat in agroforestry with poplars, this effect being greater in systems with older trees. These reductions could be due to the effect of shading at tillering stage (Kemp and Whingwiri 1980; Mc Master et al. 1987). It should be noted that although tree budbreak happened in April 2015 (poplars) and March 2016 (ash trees), radiation interception by trunk and branches was important, due to the large tree size in 2015, to the high branchiness of the ash trees in 2016 and in both cases, to the fact that the path of the sun in the sky in winter was remarkably low: the gap fraction measured from hemispherical photographs before budbreak was 42.8% in 2015 and 62.91% in 2016.
The number of grains per spike was significantly lower in agroforestry than in full sun in both experiments (Table 1). This is in agreement with studies showing that the number of grains is highly affected by shade (Slafer 1995; Abbate et al. 1997; Dufour et al. 2012), especially if the shade occurs during the rapid vegetative growth (Slafer et al. 1994; Arisnabarreta and Miralles 2015). Artru et al. (2017) also found a negative effect of shade in the number of grains per spike, but they have contradictory results about the relationship between the reduction in the number of grains and the quantity and daily dynamics of shade. In our experiments, the radiation that reached the crop was always lower in agroforestry than full sun conditions, from sowing (shade of branches and trunks in winter) to harvest (Fig. 4). However, the reduction in the number of grains per spike was not directly proportional to the reduction in the incident radiation. Indeed, the number of grains per spike was lower in agroforestry than in full sun by 21 and 62% in 2015 and 2016, respectively (Table 1), while radiation received in the period of formation of the number of grains (stem elongation-anthesis) was reduced by 41 and 52% in 2015 and 2016, respectively (Fig. 4). The impact of agroforestry on the harvest index of the crop was not consistent in the two experiments. In 2015, the harvest index was significantly higher in agroforestry, although, neither the weight of aerial dry biomass (straw + spikes) nor the yield of grain showed significant differences according to the system. In 2016, the situation was different, the harvest index was lower in agroforestry because although the dry weight of straw was lower in agroforestry, the grain weight was even more reduced in agroforestry and as a result, the harvest index was significantly lower in agroforestry compared to full sun. In line with these results, the literature shows that the relationship between agroforestry and harvest index is not clear. While some authors have found a negative impact of agroforestry systems on harvest index (Gill et al. 2009), others have found that despite a reduction in grain yield, the harvest index was not statistically different (Dufour et al. 2012). This variation in the results could be related to the conditions of radiation and soil humidity. In a study over several years, Sudmeyer and Hall (2015) determined that the impact of agroforestry on harvest index depended on the rainfall conditions during the crop cycle. Non-agroforestry shade experiments also showed that a reduction in light could produce lower harvest index (Lott et al. 2000; Mu et al. 2010). It is worth mentioning that the low average harvest index from both agroforestry and full sun conditions in our study was likely due to the ancient durum wheat varieties that were included in both experiments.
Effect of position in the alley on yield components
In both years the only yield component impacted by the position in the alley was the number of grains per spike, which was higher in the South position in 2015 and in the West position in 2016 (Table 2). It should be noted that in 2016, the position in the alley with the lowest number of grains per spike (and also lowest yield) was the center position. This is surprising because many authors reported higher reductions in the yield components in the areas closer to the trees (Dong et al. 2014; Yang et al. 2016) due mainly to the reduction in the global incident radiation (Bouttier et al. 2014), daily dynamics of radiation (Ding and Su 2010) but probably also due to belowground competition for water and nutrients. Harvest index showed no difference with respect to the position in the alley, which is consistent with Kohli and Saini (2003) and Sudmeyer and Speijers (2007), who did not find differences between the harvest index and the distance between the plot and the trees in different spatial arrangements. Compensation effects (morphological or physiological changes) may have occurred in our experiments allowing wheat to perform better in some border plots (North in 2015 and West in 2016) than in the center of the alleys. For instance, in the 2016 experiment there was a higher NDVI in the West position of the alley than in the other positions (Fig. 6d), and this is where the number of grains per spike as well as yield were highest. This is in agreement with Mu et al. (2010) and Li et al. (2010), who found a negative correlation between the radiation received and the LAI. Specifically, in agroforestry conditions, Abas et al. (2015) found a reduction in the LAI of the crop which was higher in the treatment with narrower alleys. In a North–South oriented alley of poplar and maize, Ding and Su (2010) found that a zone with intermediate PAR achieved the highest grain yield, due to its high LAI (and still sufficient PAR). Another explanation could be that trees caused a beneficial environment in the border positions, which may have benefited the crop near the trees in 2016 and in the whole alley in 2015 (e.g. less evapotranspiration, buffering of extreme temperatures).
Effect of agroforestry on crop phenology
In 2015, the crop reached maturity 149 days after sowing, meanwhile, in 2016 the maturity was reached 226 days after sowing (Fig. 5a); this was due to the date of sowing which imposed completely different climatic conditions to the system. The difference in phenology between systems was clear in both years, in 2015 from the anthesis onward and in 2016 from stem elongation onward (Fig. 2). According to Mc Master et al. (2008), the phenology of wheat is a linear function of the thermal time and responds to the photoperiod and the vernalization. Considering that photoperiod was the same in both systems (i.e. they were located at the same latitude) and that durum wheat has no requirements for vernalization, the differences in the phenology between systems must have been due to thermal time. The use of growing degree days instead of the days after sowing shortened the gap in the phenology between years, but it was not sufficient to explain all the variation in the development (Fig. 5b), nor the difference in phenology between systems since there were practically no differences in thermal time between agroforestry and full sun using the standard way of computing growing degree days. The traditional method uses the maximum and minimum daily air temperature, which is inaccurate in agroforestry due to two phenomena: (a) the fact that in agroforestry systems air temperature is not a good proxy to estimate the crop temperature, which is the variable that actually drives the phenology and (b) the buffering effect of agroforestry on the daily temperature cycle (Fig. 3). Further studies are thus necessary to better understand the complex relationships between agroforestry microclimate and crop phenology. This is all the more important since the delay in phenological development could partially compensate the reduction in incident light under the canopy, i.e. less light but for a longer period.
References
Abas EL, Corpuz OS, Cabilo LD (2015) Light regime under Eucalyptus deglupta as hedgerows and its effect on intercropped Zea mays. Am J Agric For 3:19–29. https://doi.org/10.11648/j.ajaf.s.2015030601.15
Abbate PE, Andrade FH, Culot JP, Bindraban PS (1997) Grain yield in wheat: effects of radiation during spike growth period. F Crop Res 54:245–257
Arisnabarreta S, Miralles DJ (2015) Grain number determination under contrasting radiation and nitrogen conditions in 2-row and 6-row barleys. Crop Pasture Sci 66:456–465. https://doi.org/10.1071/CP14208
Arnold CY (1960) Maximum-minimum temperatures as a basis for computing heat units. Proc Am Soc Hortic Sci 76:682–692
Artru S, Garré S, Dupraz C et al (2017) Impact of spatio-temporal shade dynamics on wheat growth and yield, perspectives for temperate agroforestry. Eur J Agron 82:60–70. https://doi.org/10.1016/j.eja.2016.10.004
Barro RS, Varella AC, Lemaire G et al (2012) Forage yield and nitrogen nutrition dynamics of warm-season native forage genotypes under two shading levels and in full sunlight. Rev Bras Zootec 41:1589–1597
Bouttier L, Paquette A, Messier C et al (2014) Vertical root separation and light interception in a temperate tree-based intercropping system of Eastern Canada. Agrofor Syst 88:693–706. https://doi.org/10.1007/s10457-014-9721-6
Brisson N, Bussì F, Ozier-Lafontaine H et al (2004) Adaptation of the crop model STICS to intercropping. Theoretical basis and parameterisation. Agron EDP Sci. https://doi.org/10.1051/agro:2004031>
Brisson N, Launay M, Mary B, Beaudoin N (2008) Conceptual basis, formalisations and parameterisation of the STICS crop model, 1st edn. Quae, Versailles
Cannell MGR, Van Noordwijk M, Ong CK (1996) The central agroforestry hypothesis: the trees must acquire resources that the crop would not otherwise acquire. Agrofor Syst 34:27–31
Cardinael R, Chevallier T, Barthès BG et al (2015) Impact of alley cropping agroforestry on stocks, forms and spatial distribution of soil organic carbon—A case study in a Mediterranean context. Geoderma 259–260:288–299. https://doi.org/10.1016/j.geoderma.2015.06.015
CIRAD (2017) UMR System. https://umr-system.cirad.fr/en/the-unit/research-and-training-platform-in-partnership/restinclieres-agroforestery-platform-rap
Ding S, Su P (2010) Effects of tree shading on maize crop within a Poplar-maize compound system in Hexi Corridor oasis, northwestern China. Agrofor Syst 80:117–129. https://doi.org/10.1007/s10457-010-9287-x
Dong C, Fu Y, Liu G, Liu H (2014) Low light intensity effects on the growth, photosynthetic characteristics, antioxidant capacity, yield and quality of wheat (Triticum aestivum L.) at different growth stages in BLSS. Adv Sp Res 53:1557–1566. https://doi.org/10.1016/j.asr.2014.02.004
Droppelmann KJ, Lehmann J, Ephrath JE, Berliner PR (2000) Water use efficiency and uptake patterns in a runoff agroforestry system in an arid environment. Agrofor Syst 49:223–243
Dufour L, Metay A, Talbot G, Dupraz C (2012) Assessing light competition for cereal production in temperate agroforestry systems using experimentation and crop modelling. J Agron Crop Sci 199:217–227. https://doi.org/10.1111/jac.12008
Ehret M, Graß R, Wachendorf M (2015) The effect of shade and shade material on white clover/perennial ryegrass mixtures for temperate agroforestry systems. Agrofor Syst 89:557–570. https://doi.org/10.1007/s10457-015-9791-0
Fischer RA (1975) Yield potential in a dwarf spring wheat and the effect of shading. Crop Sci 15:607–613
Garrity DP (2004) Agroforestry and the achievement of the Millennium Development Goals. Agrofor Syst 61:5–17
Gill RIS, Singh B, Kaur N (2009) Productivity and nutrient uptake of newly released wheat varieties at different sowing times under poplar plantation in north-western India. Agrofor Syst 76:579–590. https://doi.org/10.1007/s10457-009-9223-0
Gosme M, Inurreta-Aguirre HD, Dupraz C (2016) Microclimatic effect of agroforestry on diurnal temperature cycle. In: Amaral Paulo J, Borek R, Burgess P et al (eds) 3rd European Agroforestry Conference. European Agroforestry Federation, Montpellier, pp 183–186
Gouache D, Le Bris X, Bogard M et al (2012) Evaluating agronomic adaptation options to increasing heat stress under climate change during wheat grain filling in France. Eur J Agron 39:62–70. https://doi.org/10.1016/j.eja.2012.01.009
Hansen PM, Schjoerring JK (2003) Reflectance measurement of canopy biomass and nitrogen status in wheat crops using normalized difference vegetation indices and partial least squares regression. Remote Sens Environ 86:542–553. https://doi.org/10.1016/S0034-4257(03)00131-7
INRA (2017) DIASCOPE. https://www6.montpellier.inra.fr/diascope
Ivezic V, Van Der Werf W (2016) Relative crop yields of European silvoarable agroforestry systems. In: Gosme M (ed) Relative crop yields of European silvoarable agroforestry systems. EURAF, Montpellier, pp 291–293
Jerneck A, Olsson L (2014) Food first! Theorising assets and actors in agroforestry: risk evaders, opportunity seekers and “the food imperative” in sub-Saharan Africa. Int J Agric Sustain 12:1–22. https://doi.org/10.1080/14735903.2012.751714
Jose S, Gillespie AR, Seifert JR et al (2000a) Defining competition vectors in a temperate alley cropping system in the midwestern USA: 3. Competition for nitrogen and litter decomposition dynamics. Agrofor Syst 48:61–77
Jose S, Gillespie AR, Seifert JR, Biehle DJ (2000b) Defining competition vectors in a temperate alley cropping system in the midwestern USA: 2. Competition for water. Agrofor Syst 48:41–59
Jose S, Gillespie AR, Pallardy SG (2004) Interspecific interactions in temperate agroforestry. Agrofor Syst 61:237–255. https://doi.org/10.1023/B:AGFO.0000029002.85273.9b
Kambal AE (1969) Components of yield in field beans, Vicia faba L. J Agric Sci 72:359–363
Karki U, Goodman MS (2013) Microclimatic differences between young longleaf-pine silvopasture and open-pasture. Agrofor Syst 87:303–310. https://doi.org/10.1007/s10457-012-9551-3
Kaur N, Singh B, Gill RIS (2010) Agro-techniques for increasing productivity of wheat (Triticum aestivum) under poplar (Populus deltoides) plantation. Indian J Agron 55:68–74
Kemp DR, Whingwiri EE (1980) Effect of tiller removal and shading on spikelet development and yield components of the main shoot of wheat and on the sugar concentration of the ear and flag leaf. Aust J Plant Physiol 7:501–510
Kohli A, Saini BC (2003) Microclimate modification and response of wheat planted under trees in a fan design in northern India. Agrofor Syst 58:109–118. https://doi.org/10.1023/A:1026090918747
Lakshmanakumar P, Bana OPS, Guru SK (2013) Physiological basis of yield variability in wheat (Triticum aestivum L.) under varying degree of shades. Indian J Plant Physiol 18:164–168. https://doi.org/10.1007/s40502-013-0028-9
Leakey RRB, Weber JC, Page T et al (2012) Tree domestication in agroforestry: progress in the second decade (2003–2012). Adv Agrofor 9:145–173. https://doi.org/10.1007/978-94-007-4676-3
Li H, Jiang D, Wollenweber B et al (2010) Effects of shading on morphology, physiology and grain yield of winter wheat. Eur J Agron 33:267–275. https://doi.org/10.1016/j.eja.2010.07.002
Lin BB (2007) Agroforestry management as an adaptive strategy against potential microclimate extremes in coffee agriculture. Agric For Meteorol 144:85–94. https://doi.org/10.1016/j.agrformet.2006.12.009
Lott JE, Howard SB, Ong CK, Black CR (2000) Long-term productivity of a Grevillea robusta based overstory agroforestry system in semi-arid Kenya II. Crop growth and system performance. For Ecol Manage 139:187–201
Lott JE, Ong CK, Black CR (2009) Understorey microclimate and crop performance in a Grevillea robusta-based agroforestry system in semi-arid Kenya. Agric For Meteorol 149:1140–1151. https://doi.org/10.1016/j.agrformet.2009.02.002
Malézieux E, Crozat Y, Dupraz C et al (2009) Mixing plant species in cropping systems: concepts, tools and models. A review. Agron Sustain Dev 29:43–62
Manceur AM, Boland GJ, Thevathasan NV, Gordon AM (2009) Dry matter partitions and specific leaf weight of soybean change with tree competition in an intercropping system. Agrofor Syst 76:295–301. https://doi.org/10.1007/s10457-008-9181-y
Mc Master GS, Morgan JA, Willis WO (1987) Effects of shading on winter wheat yield, spike characteristics and carbohydrate allocation. Crop Sci 27:967–973
Mc Master GS, White JW, Hunt LA et al (2008) Simulating the influence of vernalization, photoperiod and optimum temperature on wheat developmental rates. Ann Bot 102:561–569. https://doi.org/10.1093/aob/mcn115
Moragues M, García Del Moral LF, Moralejo M, Royo C (2006) Yield formation strategies of durum wheat landraces with distinct pattern of dispersal within the Mediterranean basin I: yield components. F Crop Res 95:194–205. https://doi.org/10.1016/j.fcr.2005.02.009
Mosquera-Losada MR, McAdam JH, Romero-Franco R, et al (2009) Definitions and components of agroforestry practices in Europe. In: Rigueiro-Rodríguez A, McAdam J, Mosquera-Losada MR (eds) Agroforestry in Europe: current status and future prospects. p 454
Mu H, Jiang D, Wollenweber B et al (2010) Long-term low radiation decreases leaf photosynthesis, photochemical efficiency and grain yield in winter wheat. J Agron Crop Sci Crop Sci 196:38–47. https://doi.org/10.1111/j.1439-037X.2009.00394.x
Muschler RG (2015) Agroforestry: essential for sustainable and climate-smart land use? In: Pancel L, Kohl M (eds) Tropical Forestry Handbook, 2nd edn. Springer-Verlag, Berlin Heidelberg, pp 2013–2116
Nair VD, Graetz DA (2004) Agroforestry as an approach to minimizing nutrient loss from heavily fertilized soils: the Florida experience. Agrofor Syst 61:269–279
Ong CK, Black CR, Wilson J (2015) Tree-crop interactions agroforestry in a changing climate, 2°. CABI, Oxfordshire
Peng X, Thevathasan NV, Gordon AM et al (2015) Photosynthetic response of soybean to microclimate in 26-year-old tree-based intercropping systems in southern Ontario, Canada. PLoS ONE 10:1–10. https://doi.org/10.1371/journal.pone.0129467
Porter JR, Gawith M (1999) Temperatures and the growth and development of wheat: a review. Eur J Agron 10:23–36
Richter GM, Acutis M, Trevisiol P et al (2010) Sensitivity analysis for a complex crop model applied to Durum wheat in the Mediterranean. Eur J Agron 32:127–136. https://doi.org/10.1016/j.eja.2009.09.002
Rockwood DL, Naidu CV, Carter DR et al (2004) Short-rotation woody crops and phytoremediation: opportunities for agroforestry? Agrofor Syst 61:51–63.
Simane B, Struik PC, Nachit MM, Peacock JM (1993) Ontogenetic analysis of yield components and yield stability of durum wheat in water-limited environments. Euphytica 71:211–219. https://doi.org/10.1007/BF00040410
Simelton E, Viet Dam B, Catacutan D (2015) Trees and agroforestry for coping with extreme weather events: experiences from northern and central Viet Nam. Agrofor Syst 89:1065–1082. https://doi.org/10.1007/s10457-015-9835-5
Singh A, Dhanda RS, Ralhan PK (1993) Performance of wheat varieties under poplar (Populus deltoides Bartr.) plantations in Punjab (India). Agrofor Syst 22:83–86
Slafer GA (1995) Wheat development as affected by radiation at two temperatures. J Agron Crop Sci 175:249–263
Slafer GA, Rawson HM (1996) Responses to photoperiod change with phenophase and temperature during wheat development. F Crop Res 46:1–13
Slafer GA, Calderini DF, Miralles DJ, Dreccer MF (1994) Preanthesis shading effects on the number of grains of three bread wheat cultivars of different potential number of grains. F Crop Res 36:31–39
Stavi I, Lal R (2013) Agroforestry and biochar to offset climate change: a review. Agron Sustain Dev 33:81–96. https://doi.org/10.1007/s13593-012-0081-1
Streck NA, Weiss A, Xue Q, Baenziger PS (2003) Improving predictions of developmental stages in winter wheat: a modified Wang and Engel model. Agric For Meteorol 115:139–150. https://doi.org/10.1016/S0168-1923(02)00228-9
Sudmeyer RA, Hall DJM (2015) Competition for water between annual crops and short rotation mallee in dry climate agroforestry: the case for crop segregation rather than integration. Biomass Bioenerg 73:195–208. https://doi.org/10.1016/j.biombioe.2014.12.018
Sudmeyer RA, Speijers J (2007) Influence of windbreak orientation, shade and rainfall interception on wheat and lupin growth in the absence of below-ground competition. Agrofor Syst 71:201–214. https://doi.org/10.1007/s10457-007-9070-9
Talbot G, Dupraz C (2012) Simple models for light competition within agroforestry discontinuous tree stands: are leaf clumpiness and light interception by woody parts relevant factors? Agrofor Syst 84:101–116. https://doi.org/10.1007/s10457-011-9418-z
Van Noordwijk M, Coe R, Sinclair F (2016) Central hypotheses for the third agroforestry paradigm within a common definition. Bogor, Indonesia
Wang BJ, Zhang W, Ahanbieke P et al (2014) Interspecific interactions alter root length density, root diameter and specific root length in jujube/wheat agroforestry systems. Agrofor Syst 88:835–850. https://doi.org/10.1007/s10457-014-9729-y
Yang L, Ding X, Liu X et al (2016) Impacts of long-term jujube tree/winter wheat-summer maize intercropping on soil fertility and economic efficiency–A case study in the lower North China Plain. Eur J Agron 75:105–117. https://doi.org/10.1016/j.eja.2016.01.008
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421. https://doi.org/10.1111/j.1365-3180.1974.tb01084.x
Zhang W, Ahanbieke P, Wang BJ et al (2014) Temporal and spatial distribution of roots as affected by interspecific interactions in a young walnut/wheat alley cropping system in northwest China. Agrofor Syst 89:327–343. https://doi.org/10.1007/s10457-014-9770-x
Acknowledgements
This research was partly funded by the Conseil Départemental de l’HERAULT (PIRAT project) and the European AGFORWARD project (Grant Agreement N° 613520), within the 7th Framework Programme of RTD, Theme 2–Biotechnologies, Agriculture & Food. The views and opinions expressed in this paper are purely those of the writers and may not in any circumstances be regarded as stating an official position of the European Commission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare explicitly that they have no conflict of interest. All data presented in this article are results of original research conducted by the authors. This manuscript has not been submitted to any other journal and has not been published before, in a partial or complete manner.
Human and animal rights
This research did not include any animal or human participant as objects of experimentation.
Informed consent
All co-authors have been actively involved in this research and have given their consent for publication.
Rights and permissions
About this article
Cite this article
Inurreta-Aguirre, H.D., Lauri, PÉ., Dupraz, C. et al. Yield components and phenology of durum wheat in a Mediterranean alley-cropping system. Agroforest Syst 92, 961–974 (2018). https://doi.org/10.1007/s10457-018-0201-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-018-0201-2