Abstract
A pot experiment was carried out to investigate the effect of salinity on growth, leaf anatomy and gas exchange characteristics in two Paulownia hybrid lines (Paulownia tomentosa × fortunei-TF and Paulownia elongata × elongata-T4). The distribution of Mg, Ca, Na, K and Fe between water available fractions from non-saline, saline soils, and different organs showed acropetal concentration gradient for metal accumulation, excepting Ca. Selective uptake of K over Na was enhanced slightly with increasing salinity, where K/Na ratio was reduced in Paulownia elongata × elongata-T4 and rose in Paulownia tomentosa × fortunei-TF. The reduction in the total plant leaf area and in the values of the intrinsic components of photosynthesis-maximal Rubisco catalyzed carboxylation velocity, maximal electron transport rate and triose phosphate utilization rate was observed only in Paulownia tomentosa × fortunei-TF. The CO2 compensation point rose to Paulownia tomentosa × fortunei-TF and declined in Paulownia elongata × elongata-T4, while the stomatal factor values were enhanced in both hybrid lines. Under saline conditions, the stomatal component was more limiting to photosynthesis than its biochemical components. It was concluded that Paulownia elongata × elongata-T4 was more resistant to salinity because its growth and gas exchange characteristics were less affected in comparison with Paulownia tomentosa × fortunei-TF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural or primary salinity is more widespread in arid and semi-arid regions of the world and results from the accumulation of soluble salts in soils or groundwater over long geological periods. Removal of water due to evapotranspiration also leads to increased concentration of salts in the soil bioavailable fraction and excess of sodium ions in the rhizosphere. Sodicity is a secondary consequence of salinity, typical for clay soils, where exchangeable sodium is bound to the negative charges of clay. Besides the naturally-formed saline and sodic soils, the occurrence of so-called secondary salt-affected soils is due to the application of different unsuitable agricultural practices and continues to grow (Munns 2011). According to FAO Land and Plant Nutrition Management Service, the total surface of the saline regions of the world is the almost 397 × 106 ha and 2% of them are secondary salt-affected (Dajic 2006).
Salinity in soil or water is one of the major negative influencing factors of the environment, and especially in arid and semiarid regions, can severely limit crop production. In recent decades, the investigations are focused on biochemical and physiological traits (Munns and Richards 2007; Koyro et al. 2014), identification of key genes and/or discovering of distinctive molecular markers in a great number of agricultural crops (Kosova et al. 2011). Munns (2011) proposed that increased salt tolerance of perennial species (such as woody species) used for fodder or biofuel production is a key component in reducing the spread of secondary salinity, while increased salt tolerance of crops will directly improve production in soils with primary salinity.
Woody species from the genus Paulownia (Paulowniaceae) is native from China. Paulownia tomentosa as high-yielding plants (Woods 2008) can be used for the production of energy, paper pulp, and wooden building materials. Over the last two decades, Paulownia species has been extensively studied due to its ability to uptake heavy metals (Doumett et al. 2008, 2011; Wang et al. 2010; Madejon et al. 2016).
Paulownia tomentosa (Thunb.) Stend, as well as other species, belong to the genus Paulownia (P. elongata, P. fortunei) and hybrid (P. fortunei × tomentosa) are used for afforestation of Eastern Black Sea region of Turkey due to its high adaptive abilities combined with continuous growth and formation of abundant leaf biomass (Yavuzsefik et al. 2001). The combination between the deep root system and high growth rate explain using of Paulownia plants for bioremediation of polluted and degraded soils.
Our preliminary investigations with five selected Paulownia hybrid lines (P. tomentosa × fortunei-TF, P. elongata × fortunei-EF, P. elongata × fortunei × elongata-T2, P. elongata × elongata-T4, P. elongata × kawakamii-EK), grown ex vitro as hydroponic cultures at three levels of salinity—50, 100 and 200 mmol l−1 sodium chloride (NaCl) solution showed that TF and T4 lines are more salt resistant because its net photosynthetic rate (PN) is enhanced, whereas this parameter is reduced in T2 and EF (Ivanova et al. 2014). The study of salinity effect on in vitro and ex vitro growth of Paulownia species is scarce (Ayala-Astorga and Alcaraz-Melendez 2010).
Elaborated on the system of suitable physiological and biochemical markers will help to understand the mechanisms of the salt resistance of Paulownia. The differences in the distribution of Mg2+, Ca2+, Na+, K+ and Fe3+ between water available soil fraction and different organs of two hybrid Paulownia lines (P. tomentosa × fortunei-TF and P. elongata × elongata-T4), grown on two types of salinized soils during two vegetative periods were investigated. The hypothesis that both hybrid lines adapted any sort of avoidance mechanisms towards increasing substrate salinity is tested.
Materials and methods
Plant material
Four-year-old plantlets of Paulownia tomentosa × fortunei-TF and Paulownia elongata × elongata-T4, derived from in vitro micropropagation of seedlings (Miladinova et al. 2013), were cultivated in plastic pots with 2.5 kg soil, collected from two different areas situated in the vicinity of the village Belozem, Bulgaria. A sampling strategy was carried out consisting of the collection of soil aliquots (about 10 kg) from the surface and at depths of 30–60 cm in two different locations of the area. The soil samples were combined (full weight about 60 kg), air dried and sieved through nylon mesh and then mixed with sand in the ratio 3:1. On the basis of agrochemical characteristics the first type of soil was characterized as non-saline and the second type of soil might be determined as middle alkaline (Referative basic data 2009). The second saline soil possessed exchangeable Na content about 5.3 times higher and Sodium Adsorption Ratio about 6 times higher in comparison with the first non-saline soil. The electrical conductivity were: saline soil—14.0 mS/cm, non-saline—6.3 mS/cm (Ivanova et al. 2015). The experiment was set as 4 treatments, with 7 replications and was conducted in a glasshouse (natural sunlight, temperatures 15–35 °C, relative humidity 40–65%) for two vegetation seasons. The pots were irrigated daily with tap water to exceeding the field capacity of the soils.
Growth parameters
The plants were harvested to determine gravimetrically plants dry mass (leaf, petiole, stem) after heating at 60 °C. Leaf area (LA) was calculated using software program SigmaScan Pro 5. The following indexes were calculated: total leaf area/total dry mass (LAR), total leaf area/leaf number (MLA).
Plant and soil metal analysis
The water soluble bioavailable fraction (free metal ions, soluble metal complexes and metals adsorbed to inorganic soil constituents at ion exchange sites—Mg2+, Ca2+, Na+, K+ and Fe3+) from the soils was determined by extraction tests (Doumett et al. 2008). Total metal content in available fractions and plant organ samples was determined by atomic absorption spectrophotometric analysis (Perkin-Elmer 5000, UK), after acidic digestion with Suprapur grade Fluka reagents.
Gas exchange measurements
Assimilation (A, μmol CO2 m−2 s−1) versus sub-stomatal CO2 concentration (Ci, μmol mol−1) response curve (A:Ci) of fully developed attached leaves of P. tomentosa × fortunei and P. elongata × elongata were measured for at least two leaves using portable photosynthesis open system (Li-6400; Licor Inc., Lincoln, Nebraska, USA). The A:Ci response curve was generated after setting the environment inside the leaf chamber: leaf-to-air vapor pressure deficit was 1.5 kPa, leaf temperature was 20 °C, light saturation capacity 1000 μmol m−2 s−1 blue/red light (Li-6400-02-LED light source). CO2 regulator (Li-6400-01) provided different CO2 concentrations (Ca) in the range 1000–50 μmol/mol. The A:Ci measurements started at a CO2 concentration close to ambient (350 μmol/mol) and continued down to 50 μmol/mol in a step-wise fashion, then up again to 1000 μmol/mol through the ambient CO2 concentration. At each step, gas exchange variables were recorded after achieving steady-state conditions. Calculations of gas exchange parameters were performed according to Von Caemmerrer and Farquhar (1981). Data of each A:Ci curve were fitted into a nonrectangular hyperbola equation (Jones 1992). Mechanistic analysis of each A:Ci curve was performed (Farquhar et al. 1980). The model was used to estimate the maximal Rubisco catalyzed carboxylation velocity (Vcmax) and CO2 compensation point (Γ). The relative contribution of stomatal limitation to photosynthesis, maximal electron transport rate (Jmax), triose phosphate utilization rate (VTRU) and CO2 which was the proportional decrease in light-saturated (A) attributed to stomatal component (SL, %) was calculated (Farquhar and Sharkey 1982).
Statistical analysis
Data were expressed as mean ± SE, where n was given into the tables. Comparison of means was performed by the Fisher LSD test (P ≤ 0.05) after performing ANOVA analysis.
Results and discussion
Effect of soil salinity on the distribution of metals
The water bioavailable amount of target alkaline and alkaline earth metals present in the fractions of the soils before planting showed that Ca2+ and Na+ prevailed in saline soil (Table 1). Higher Mg2+, Fe3+ and especially Na+ and K+ contents are established in the fractions from the non-saline soil after harvesting of both plants. The obtained ratio K+/Na+ in these fractions is reduced about 3 times after harvesting of T4 (0.32) in comparison with TF (0.98). The pH values decreased too from alkaline to middle acidic. The accumulation of Na+ and K+ prevailed in the roots of both hybrid lines grown on non-saline soil (Table 2). The differences in the content of these elements in the roots of both plants grown on saline soil are insignificant. Nevertheless, the obtained ratio K+/Na+ in T4 is 1.2 time higher (13.93) than that in TF (12.00). The results showed that both hybrid plants grown on non-saline soil avoid stress by excreting excess contents of alkaline metals and Fe3+ in the rhizosphere zone. The strategy of salt exclusion relies on the selective release of Na+ into the xylem and its resorption from the xylem stream. Net accumulation of the Na+ in the plants is dependent on the balance between passive influx and active efflux. Salt exclusion operates at the cellular and whole plant level (Munns et al. 1983) and is to a great extent related to regulation of K+/Na+ selectivity (Jeschke and Hartung 2000). The salt tolerance in species that exclude salts is achieved by changes between Na+ and Ca2+, rather than changes in osmotic potential since adsorption of Ca2+ on membranes of root cells leads to reduced penetration of monovalent cations (Munns et al. 1983). This is demonstrated for wheat where inhibition of non-directional Na+ influx occurred following the addition of external Ca2+ (Reid and Smith 2000). Salt exclusion is a very efficient but complex way of preventing massive ion uptake in the root zone, enabling a lower uptake and accumulation of salts in the upper parts of the plant, especially in the transpiring organs. Salt sensitive plants, such as beans and maize are the most prominent Na+ excluders (Jacoby 1994; Bayuelo-Jimenez et al. 2003). The accumulation of Ca2+ in the leaves of TF is 3 times higher than that of T4 grown on non-saline and saline soils at the end of the second vegetative season. Na+ prevailed in the leaves of T4 grown on both soils. The accumulation of Fe3+ is more in the leaves of TF (Table 3). Our results showed acropetal concentration gradient of metals distribution between soil pore water and plant tissues with the exception of Ca2+. The K+ accumulation in the fully developed leaves of both lines is highest as compared to Na+ at the end of second vegetation season. Changes in K+ and Na+ concentrations reflected in the K+/Na+ ratio (Table 2). This ratio is an important selection criterion for salt tolerance (Ashraf and Orooj 2006). The K+/Na+ ratio decreased by increasing salt concentration in safflower cultivars grown in hydroponics, but highest values determined more tolerant to salt stress plant species (Erdal and Çakirlar 2014). The obtained ratio K+/Na+ in T4 is twice lower in comparison with TF has grown on non-saline and saline soils. Irrespective of salt exclusion by root membranes, leaf content of K+ and Na+ remain similar as compared to plants grown on saline soil (Table 2).
Effect of soil salinity on seedlings growth
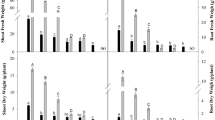
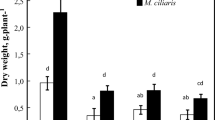
The investigations showed that plant growth is limited under salt stress (Maeda and Nakazawa 2008). TF grown on non-saline soil formed higher fresh and dry biomass, total leaf area and MLA ratio than those of T4. The ratio root/shoot dry biomass is higher in T4 as compared to TF. With increasing soil salinity the enhancement of growth parameters of T4 (stem length, leaf number, total dry biomass, total leaf area) is observed in comparison with TF. Two important parameters characterizing photosynthetic leaf surface—LAR and MLA are enhanced in TF, while in T4 grown on the saline soil it decreased insignificantly. The ratio root/shoot dry biomass is enhanced more in TF than in T4 (Table 3). Besides salt exclusion in the roots, low NaCl levels in leaves can also be achieved by salt retention in the lower plant parts and also through abscission of old leaves once they accumulate large quantities of salts. The mechanism of intra-plant allocation is also characteristic for many halophytes, which, due to the limited transpiration, can keep an excess of salts within their roots and lower parts of the shoot, thus preventing ion accumulation in the photosynthetic tissues (Dajic 2006). We established yellowing and later abscission of older leaves of both hybrid plants during vegetation. The leaf abscission is observed mainly in TF grown on the saline soil as compared to non-saline soil and can be considered as avoidance mechanism by which is preserved younger leaves from the action of high salt concentrations.
Effect of soil salinity on seedlings photosynthetic gas exchanges
The estimated Vcmax and Jmax on the base of (A:Ci) curves were reduced in TF by 11 and 20%, respectively. The same parameters increased in T4 by 20 and 14%, respectively. The value of VTPU declined in TF by 5%, while in T4 increased by 15%, respectively. The CO2 compensation point (Γ) is significantly higher in TF, but in T4 decreased slightly. The SL values are enhanced in both hybrid lines (Table 4). Salinity reduced net-photosynthesis (PN) and stomatal conductivity (Gs) in most plant species (Munns and Tester 2008). The results showed that balance between water loss and CO2 uptake helps to find a weak spot in the mechanism of adjustment of photosynthesis to high salinity. Biomass production of plants depended mainly on the ability to keep a high net-photosynthesis, minimum transpiration, low stomatal conductivity, and minimum internal CO2 concentration at their threshold salinity tolerance. Analysis of (A:Ci) curves indicated that only in the leaves of TF, salinity induced decline in the carboxylation efficiency of Rubisco enzyme, reduced the rate of electron supply by the electron transport system for ribulose-1,5-bisphosphate regeneration and limited the available inorganic phosphorus for Calvin cycle (expressed by the decrease in Vcmax, Jmax, and VTPU). CO2 compensation point (Γ) increased in TF by 25%, while in T4 decreased by 13%, respectively (Table 4). The values for SL increased more significantly in TF than in T4 under salinity (Table 4). That is why the reduction of net-photosynthesis is attributed to both stomatal closures and to the biochemical components of the photosynthetic process. Under saline conditions, the stomatal component played a more dominant role over biochemical components in limiting the photosynthetic gas exchange. Our preliminary results showed that PN, Gs and WUE are more reduced in TF than in T4 grown on saline soil, while transpiration is enhanced insignificantly (Ivanova et al. 2015). Declined stomatal conductance may provide a down regulatory mechanism for controlling salt loading in plants with limited salt compartment capacities (Robinson et al. 1997). The established morphological, biochemical and stomatal responses to salinity are more pronounced in TF than in T4 and have contributed to the overall reduction in carbon gain and then a reduction in plant growth.
In conclusion, determination of the Paulownia hybrid lines responses to soil salinity and development of more tolerant line is important. The results of this study showed that salt stress more negatively affected growth and photosynthetic gas exchange in TF than in T4. The counted parameters may be used as reliable indicators for the investigation tolerance mechanisms in these lines.
References
Ashraf M, Orooj A (2006) Salt stress effects on growth, ion accumulation and seed oil concentration in an arid zone traditional medicinal plant ajwain (Truchyspermum ammi [L.] Spraque). J Arid Environ 64:209–220
Ayala-Astorga GI, Alcaraz-Melendez L (2010) Salinity effects on protein content, lipid peroxidation, pigments, and proline in Paulownia imperialis (Siebold and Zuccarini) and Paulownia fortunei (Seemann and Hemsley) grown in vitro. Electr J Biotechnol 13(5):1–15
Bayuelo-Jimenez JS, Debouck DG, Lynch JP (2003) Growth, gas exchange, water relations and ion composition of Phaseolus species grown under saline conditions. Field Crops Res 80:207–222
Dajic Z (2006) Salt stress. In: Rao KVM, Raghavendra AS, Reddy KJ (eds) Physiology and molecular biology of stress tolerance in plants, chapter 3. Springer, The Netherlands, pp 41–99
Doumett S, Lamperi L, Checchini L, Azzarello E, Mugnai S, Mancuso S (2008) Heavy metal distribution between contaminated soil and Paulownia tomentosa in a pilot-scale assisted phytoremediation study: influence of different complexing agents. Chemosphere 72:1481–1490
Doumett S, Fibbi D, Azzarello E, Mancuso S, Mugnai S, Petruzzelli G, DelBubba M (2011) Influence of the application renewal of glutamate and tartarate on Cd, Cu, Pb and Zn distribution between contaminated soil and Paulownia tomentosa in a pilot-scale assisted phytoremediation study. Int J Phytorem 13:1–17
Erdal SÇ, Çakirlar H (2014) Impact of salt stress on photosystem II efficiency and antioxidant enzyme activities of safflower (Carthamus tinctorius L.) cultivars. Tur J Biol 38:549–560
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Ann Rev Plant Physiol 33:317–345
Farquhar G, Von Caemmerrer S, Berry J (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Ivanova K, Tsvetkova N, Georgieva T, Markovska Y (2014) Photosynthesis and growth responses of five Paulownia lines to salt stress. Compt Rend Acad Bulg Sci 67:1101–1106
Ivanova K, Dimitrova V, Georgieva T, Markovska Y (2015) Effect of soil salinity on growth, gas exchange and antioxidant defence of two Paulownia lines. Genet Plant Physiol 4:163–173
Jacoby B (1994) Mechanisms involved in salt tolerance of plants. In: Pessarakli M (ed) Handbook of plant and crop stress. Marcel Dekker, New York, pp 97–123
Jeschke WD, Hartung W (2000) Root-shoot interactions in mineral nutrition. Plant Soil 226:57–69
Jones HG (1992) Plants and microclimate. Cambridge University Press, New York
Kosova K, Vitamivas P, Prasil IT, Renaut J (2011) Plant proteome changes under abiotic stress—contribution of proteomics studies to understanding plant stress response. J Proteom 74:1301–1322
Koyro H-W, Huchzermeyer B, Zrb CH (2014) Effects of hyperosmotic salinity on protein patterns and enzyme activities. In: Pessarakli M (Ed) Handbook of plant and crop physiology, Taylor @ Francis Group LLC, CRC Press, pp 487–507
Madejon P, Dominguez MT, Diaz MJ, Madejon E (2016) Improving sustainability in the remediation of contaminated soils by the use of compost and energy valorization by Paulownia fortunei. Sci Total Environ 539:401–409
Maeda Y, Nakazawa R (2008) Effects of the timing of calcium application on the alleviation of salt stress in the maize, tall fescue, and reed canary grass seedlings. Biol Plant 52:153–156
Miladinova K, Georgieva T, Ivanova K, Geneva M, Markovska Y (2013) The salinity effect on morphology and pigments content in three Paulownia clones grown ex vitro. Bulg J Agric Sci 19:52–56
Munns R (2011) Plant adaptations to salt and water stress: differences and commonalities. Adv Bot Res 57:1–32
Munns R, Richards RA (2007) Recent advances in breeding wheat for drought and salt stresses. In: Jenks MA, Hasegawa PM, Jain SM (eds) Advances in molecular breeding towards salinity and drought tolerance. Springer, New York, pp 565–585
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Ann Rev Plant Biol 59:651–681
Munns R, Greenway H, Kirst GO (1983) Halotolerant eukaryotes. In: Lange OL, Nobel PS, Osmond CB, Ziegler HH (eds) Encyclopedia of plant physiology. Springer, Berlin, pp 59–135
Referative basic data for the soils in Bulgaria (2009) Academy of Agriculture, Sofia
Reid RJ, Smith FA (2000) The limits of sodium/calcium interactions in plant growth. J Plant Physiol 27:709–715
Robinson MF, Very A, Sanders D, Mansfield TA (1997) How can stomata contribute to salt tolerance? Ann Bot 80:387–393
Von Caemmerrer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387
Wang J, Li W, Zhang C, Ke S (2010) Physiological responses and detoxific mechanisms to Pb, Zn, Cu and Cd in young seedlings of Paulownia fortunei. J Environ Sci 22:1916–1922
Woods VB (2008) Paulownia as a novel biomass crop for Northern Ireland? A review of current knowledge. Global research unit, AFBI Hillsborough, Occasional publication No 7: pp. 1–47
Yavuzsefik Y, Cicek E, Cetin B (2001) Discussion of foreign species in Turkey and early results of Paulownia seedlings in Duzce. Turk J For Eng 4:5–10
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ivanova, K., Geneva, M., Anev, S. et al. Effect of soil salinity on morphology and gas exchange of two Paulownia hybrids. Agroforest Syst 93, 929–935 (2019). https://doi.org/10.1007/s10457-018-0186-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-018-0186-x