Abstract
Forest harvesting is one of the main economic practices in South Patagonia. The impacts produced by forest harvesting have been studied by numerous investigations. And it is known that forest harvesting affects the decomposition of soil organic matter. However, there is no data about how the harvesting by variable-retentions affect this decomposition. Our objective was to determine how impact variable-retention upon decomposition and nutrient release in Nothofagus pumilio forest soils. We hypothesized that variable-retention accelerate decomposition and nutrient release. We compared primary and harvested forests with two types of retentions (aggregated and dispersed) and two times [1 and 5 years after harvesting (YAH)]. To measure litter decomposition, we used bag technique for to determine organic matter loss. We determined carbon; nitrogen; calcium; potassium; magnesium and lignin concentrations in decomposing material. We analysed the data using linear mixed models ANOVA. Decomposition rates were estimated as derivate of the linear mixed model for the logarithm of the remaining leaf litter weight. We found that dispersed retentions treatment had the highest decomposition rates. Primary forest and aggregated retentions had the smaller slopes of the decomposition model. Dispersed and aggregated retention 5 YAH retained more nitrogen compared to primary forest. Dispersed retention 5 YAH had the lowest C/N ratio. Primary forest had higher Lignin/N ratio at 540 incubation days. Dispersed retention 5 YAH released more phosphorus compared to primary forest. Dispersed and aggregated retention 1 YAH had higher C/P ratio. Dispersed retention 5 YAH presented the most mineralization of potassium in the initial time of decomposition. We conclude that the harvesting by variable-retentions had an immediate negative effect on litter decomposition and the nutrients dynamics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Forests in Tierra del Fuego (Argentina) have been harvested since the beginning of the last century (Alfonso 1942; Gea et al. 2004). This activity is one of the main economic practices in South Patagonia. The forestry companies of South Patagonia have modified their harvesting systems to achieve forest certification [FSC-Forest Stewardship Council] (Martínez Pastur et al. 2007). Currently, approximately 500 ha year−1 of forests in Tierra del Fuego are harvested for the extraction of sawn wood. (Dirección de Bosques TDF 2016). The impacts produced by forest harvesting have been studied by numerous investigations (Deferrari et al. 2001; Spagarino et al. 2001; Martínez Pastur et al. 2002a, b), because the objective of forest harvesting is to be sustainable (Franklin et al. 1997). However, degradation of soils and their recovery over time due to forest management have been little studied in the world. Also, degradation of soils and their recovery have not been considered for the definition of forestry practices in the region.
In forests of temperate-cold climates litter decomposition is particularly important in the functioning and stability of those forests (Barrera et al. 2004). The role of litter decomposition gains special relevance in forests subject to harvesting (Caldentey et al. 2001). Forest harvesting affects litter decomposition and nutrients release. Generally, forest harvesting accelerates litter decomposition and promotes loss of nutrients (Caldentey et al. 2001; Ibarra et al. 2011; Bahamonde et al. 2012).
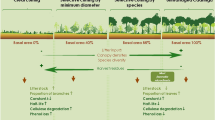
Temperate forests of Tierra del Fuego cover 712.000 ha, around 30% of them are considered timberland (Collado 2001). In these forests, Nothofagus pumilio is currently the only species of economic interest (Martínez Pastur et al. 2000a) because it possess a great forestry potential. Due to this, an intense forest harvesting pressure is threatening the sustainability of Nothofagus pumilio forest in Argentinean Tierra del Fuego since last few decades (Peña-Rodríguez et al. 2013). In the recent years, the harvesting proposals for N. pumilio forests involves complex silvicultural proposals, such as shelterwood or variable-retention (Franklin et al. 1997; Martínez Pastur et al. 2000a). Variable-retention is currently applied in South Patagonia, Canada, and Australia (Beese and Bryant 1999; Mitchell and Beese 2002; Martínez Pastur and Lencinas 2005). Variable-retention involves two forms of retention of native forests: aggregated retention and dispersed retention. Within each 1 ha, aggregated retention (AR) keeps 20–30% of the original forest as an island (30 m radius) and harvests the remainder of the area, leaving it as dispersed retention (DR) of 10–15% of trees as remnant overstory (Martínez Pastur and Lencinas 2005). Thus, biophysical conditions within aggregated retentions would be like the primary forest (PF) (Martínez Pastur et al. 2011, 2013). Thereby, the purpose of variable-retention harvesting is to maintain the sustainability and biodiversity of the original forest as islands (aggregated retentions) (Martínez Pastur et al. 2007, 2009).
To make sustainable management decisions, forestry requires information before, during and after harvesting. Forest regeneration depends on decomposition and nutrients release for its growth (Yoshida et al. 2005). Thereby, decomposition and nutrient release is an important process that must be considered when evaluating the changes produced by forest harvesting (Johnson and Curtis 2001; Idol et al. 2003; Bahamonde et al. 2012). A few studies have examined the influence of forest harvesting upon litter decomposition and nutrient release in Nothofagus forests (Caldentey et al. 2001; Ibarra et al. 2011). However, there are no reports of litter decomposition in Nothofagus pumilio forests under variable-retention harvesting. Within this context, our objective was to determine how variable-retention harvesting impacts upon litter decomposition. To accomplish this, we measured litter decomposition and nutrient release in Nothofagus pumilio forests through 2 years of measurements in harvested and primary forests at different times of harvesting. We hypothesized that the dispersed retentions accelerate the litter decomposition and nutrient release, and aggregates maintain similar litter decomposition and nutrient release to the original forest.
Materials and methods
Study site
This study was carried out in Tierra del Fuego, Patagonia, Argentina. The climate is cold oceanic with strong winds, mainly from the southwest. The mean annual temperature is 5.5 °C (1.6 °C in the coldest and 9.6 °C in the warmest months) and frost may occur at any time of the year. Precipitation is evenly spread over the year, with an annual average of 400–600 mm year−1. Annual average wind speed outside forests is 8 km h−1, reaching up to 100 km h−1 during storms (Tukhanen 1992; Brancaleoni et al. 2003). Forests have soils characterized as shallow, rich in organic matter, acidic pH (3.3–5.2 water pH in organic and 3.2–4.0 in mineral horizons), with a moderate cation exchange capacity, and low saturation of basic cations (Nóvoa-Muñoz et al. 2007; Peña-Rodríguez et al. 2013).
Data was obtained in the Cerros Ranch (54°18′S, 67°49′W), from pure old-growth N. pumilio forests. The Cerros Ranch was the first place where variable-retention RV (Fig. 1) was productively implemented in Tierra del Fuego in 2004 (Vukasovic et al. 2004; Martínez Pastur et al. 2007). For this reason, we chose the oldest site of harvesting, 5 years after harvesting (YAH) and the most recent site of harvesting, 1 YAH, available at the beginning of the investigation (October 2009). Thus, we measure the oldest and most recent changes on decomposition and nutrient release produced by variable-retentions at the study site.
Variable-retention scheme with two types of retention (aggregated and dispersed). Black circles represent individual tree’s canopy. Greyish shaded circles represent aggregated retention areas (AR) with 60 m diameter; within a matrix of scattered trees left in the harvested area, i.e., dispersed retention (DR)
Description of experimental areas
Climatic variables of experimental areas (air temperature, soil temperature at 30 cm depth, average maximum wind speed at 2 m height and rainfall reaching the forest floor) were measured by Martínez Pastur et al. (2013) with three weather stations (Davis Weather Wizard III and accessories, USA) for three successive years. Climate was characterized by short, cool summers and long, snowy, and frozen winters. Temperatures below zero occurred all the year round, and the growing season extended for approximately 5 months (November–March). Mean monthly air temperatures were higher in PF than in AR and DR. Soil temperature was always above zero in PF and AR, but soil freezing was observed in DR during July. Wind speed was higher in DR > AR > PF. Rainfall was homogeneously distributed throughout the year, and was higher in DR > AR > PF (Martínez Pastur et al. 2013).
For the edaphic characterization of the experimental area, a description was made in two profiles in undisturbed soils of the experimental area (PF-1 and PF-2). Both soil profiles showed a similar sequence of horizons, the soils had little to moderate degree of evolution (45 cm). Soil classification was endoleptic Cambisols (IUSS 2006). Horizon O was very well represented in N. pumilio soils and showed a high content of organic matter. Horizon A showed a remarkable influence of organic matter contribution, relatively humified, coming from the horizon O. In transition horizon AB exhibited traits attributable to the horizons A and the underlying B horizon, with levels of organic matter lower than the A horizon and structure like B horizon. The Bw horizon presented unambiguous evidence of alteration processes resulting from prolonged weathering activity in the soil starting material, giving rise to the neoformation of clays or the differentiation of the structure with respect to the C horizon. The presence of the 2C horizon in the PF-2 profile indicated the existence of a discontinuity marked by the existence at this depth of gravels of different morphology to those existing in the overlying levels. The texture of the soils was loamy clay in horizons A and AB, and loam-clay-sandy in horizons Bw of both profiles and in horizon C of soil PF-1. The C horizon of the PF-2 profile presented loam-sandy texture (Table 1).
In the experimental area, we obtained data on altitude, slope, depth of the most superficial horizon and, for the case of the areas with DR, the distance to the aggregate (Table 2). According to the Los Cerros Ranch management plan (Vukasovic et al. 2004), experimental areas of this study with variable-retention 5 YAH (DR5 + AR5) had an area of 12 ha, experimental area with variable-retention 1 YAH (DR1 + AR1) had an area of 18 ha, and primary forest (PF) had an area of 15 ha. The distance between the different treatments was approximately 2 km.
Experimental design
The field design consisted in comparing primary forest (PF) and harvested forest with two types of retentions (aggregated and dispersed). In addition, two different times after harvest were selected: 1 and 5 years. As result of type of forest harvesting and the time elapsed since it took place, the following treatments were defined: dispersed retention 1 YAH (DR1); aggregated retention 1 YAH (AR1); dispersed retention 5 YAH (DR5); aggregated retention 5 YAH (AR5). We randomly placed 20 experimental plots (0.5 × 0.5 cm) in each treatment (PF, DR1, AR1, DR5 and AR5) (N = 100; n = 20 experimental plots/treatment) to measure decomposition. Each experimental plot was kept the same orientation, slope, and distance from trees.
The forest structure was characterized in each treatment with five random circular plots of 500 m2, which were measured using a Criterion RD-1000 (K = 6 in aggregated retention and primary forest, and k = 3 in dispersed retention) (Bitterlich 1984). We measured tree dominant height (DH), site quality classes (SQC), basal area (BA) (for equations and methodologies see Martínez Pastur et al. 2000b, 2002a, b), diameter at 1.3 m height, and tree density (BHD) (Table 3). Tree development phase was estimated through the observation of these morphological characteristics of the bark (Schmidt and Urzúa 1982). All treatments presented trees in the aging phase (A), the bark is cracked and forming plates comprising an age range between 140 and 220 years.
Litter decomposition and nutrient release
During the autumn 2009 (March–April), recently senescing leaves of N. pumilio were collected. This study concentrated on the analysis of leaf litter, because leaves are the main contributor to annual litterfall in N. pumilio forests (50–90% of the annual litterfall) (Moretto and Martínez Pastur 2014).
Leaves material were air-dried until constant weight. Initial quality of leaves material sub-samples was evaluated (Table 4). Also, sub-samples were weighed and oven-dried at 60 °C for 48 h to calculate the initial moisture content of the leaf material. Decomposition was analysed using the nylon mesh bag technique (Bocock et al. 1960). A total of 500 litter bags (5 treatments × 5 collection dates × 20 plots) with 3 g of air-dried leaves in each one (15 × 15 cm with 2-mm mesh size) were placed on the forest floor at each treatment. Litter bags were fastened to the forest floor by metal pins to prevent movement and to ensure good contact between the litter bags and the organic layer. One litter bag per plot at each plot was collected after 30, 90, 180, 360 and 540 incubation days.
In the laboratory, litter bags were cleaned to remove any exogenous material and weighed after oven-drying at 60 °C for 48 h. Inorganic contaminants were quantified by the ashing of 0.5 g of leaf litter from each bag (500 °C for 5 h) to correct the obtained decomposition data (Harmon et al. 1999). Organic matter loss was evaluated considering the remaining weight against the initial weight (Suffling and Smith 1974).
Nutrient concentrations were measured in undecomposed material (i.e. 0 days) and in material extracted at each sampling date to estimate nutrient release. Samples were grounded and carbon (C), total nitrogen (N), phosphorus (P), calcium (Ca), potassium (K), magnesium (Mg) and lignin concentrations were determined. C content was estimated as half the organic matter (Gallardo and Merino 1993). N was measured following Kjeldahl’s standard acid digestion. The remaining nutrients (Ca, P, K, Mg) were measured using atomic emission spectrometer (ICP-OES, Shimadzu 9000, Shimadzu Corp., Japan). Lignin was determined with successive extractions with detergents and acids (Van Soest 1963) at 0 and 540 incubation days. Nutrients release was estimated as the remainder percentage of the original content. Furthermore, C/N; C/P; N/P; Lignin/N y Lignin/P ratios were calculated.
Statistical analysis
Remaining leaf litter, decomposition rate, nutrients release and ratios were analysed using linear mixed models ANCOVA given its repeated measures nature (Pinheiro and Bates 2000) because experimental unit evaluated (plot) was the same throughout the study.
We constructed several models to represent the decomposition of remaining leaf litter. The selected model was built based on different adjustments. First, the original data (remaining leaf litter weight dry) was transformed using natural logarithm (LnWD) and incubation time was centred to deal with correlations between slopes and intercepts. Data was adjusted to a linear mixed effects ANCOVA model, with Site and YAH as factors and incubation time a covariate. We choose the best model using AIC criterion and inspection of residuals (Anderson 2008). This model included, in addition to the terms mentioned before, a quadratic term of the incubation time, to deal with non-linearity; a power variance function with incubation time as covariate to model the variance in each Site × YAH combination to deal with the heteroscedasticity (because variation increased with incubation time); and a random effects term with random intercept for each experimental plot.
Daily decomposition rates (DDR) were estimated as derivate of the linear mixed model for the logarithm of the remaining leaf litter weight. The following equation was used:
where a is the intercept, b1 and b2 are the linear and quadratic slope respectively, t1 and t2 are the centred (i.e., time − mean time) time and quadratic time, respectively. The results are then expressed as g g−1 day−1.
Decomposition studies mostly use the constant k (k year−1) and data is analysed by classical ANOVA ignoring the assumption of independence. For this reason, in this study we chose to analyse the data with mixed models and calculate the constants k to be compared with other studies in the discussion. The constant k of decomposition using the exponential model proposed by Olson (1963). The time required for 99% of the litter to decompose (t99, years) was calculated from constant k.
In other to gain more insight from the data, Spearman’s correlation analysis between decomposition rates and nutrients release was performed.
All statistical analyses were done with R (R Development Core Team 2016).
Results
Dispersed retention accelerates litter decomposition
We detected time × treatment interaction (F20,422 = 5.4; p < 0.00001) in the percentage of remaining leaf litter mass. From 180 days onwards, we detected that aggregated retention 1 YAH had higher remaining leaf litter mass (F4,174 = 6.1; p < 0.00001) (Fig. 2). Aggregated retention 1 YAH was significantly higher from variable-retention 5 YAH (aggregated and dispersed) and primary forest at 180 and 540 days, and from all the treatments at 360 days. The total percentage leaf litter loss declined 33–40% from the beginning of the experiment (0 days) towards the end (540 days). We observed significant differences between times from 30 days onwards (0 = 30 > 90 > 180 > 360 > 540 days) (F4,251 = 348; p < 0.00001).
Remnant organic matter of N. pumilio leaves in variable-retention up to 540 days. The remnant organic matter of N. pumilio leaves was higher in aggregated retention 1 YAH. Aggregated retention 1 YAH was significantly higher from variable-retention 5 YAH (aggregated and dispersed) and primary forest at 180 and 540 days, and from all the treatments at 360 days. PF primary forest, AR1 aggregated retention after 1 year after harvesting, AR5 aggregated retention after 5 years after, DR1 dispersed retention after 1 YAH of harvesting, DR5 dispersed retention after 5 YAH of harvesting
At the beginning of the experiment (day 30), the modelled decomposition rates were greater in dispersed retention 5 YAH, and lower in aggregated retention 1 year (Fig. 3). This tendency continued up to 180 days. At 360 days, we observed two groups: dispersed retentions with higher rates of decomposition, and primary forest and aggregated retentions with lower rates of decomposition. Finally, at 540 days the rate of decomposition was lowest in dispersed retentions, especially in dispersed retention 5 YAH. Meanwhile, primary forest and aggregated retentions maintained similar rates. When assessing the slopes of the model, we observed significant differences between the treatments (b1: F4,440 = 14.4, p < 0.0001; b2: F4,440 = 2.6, p = 0.03) with a smaller slope in primary forest and aggregated retentions with regard dispersed retentions (Fig. 3).
Litter decomposition rate in variable-retention up to 540 days. Dispersed retention accelerates litter decomposition rate. The first 180 days the modelled decomposition rates were greater in dispersed retention 5 YAH and lower in aggregated retention 1 YAH. At 540 days the rate of decomposition was lowest in dispersed retentions, especially in dispersed retention 5 YAH. Primary forest and aggregated retentions had the smaller slopes of the decomposition model (b1: F = 14.4; p < 0.0001; b2: F = 2.6 p = 0.03). PF primary forest, AR1 aggregated retention after 1 year after harvesting, AR5 aggregated retention after 5 years after harvesting, DR1 dispersed retention after 1 year after harvesting, DR5 dispersed retention after 5 years of harvesting
The mean values of k at the first month of decomposition ranged between 0.07 and 0.16 g g−1 year−1. At 3 and 6 months of decomposition k increased between 0.54–0.65 and 0.38–0.68 g g−1 year−1 respectively. At longer decomposition times (12 and 18 months), k showed a decrease in the mean values for all sites (0.27–0.42 g g−1 year−1). The time required for 99% organic matter to decompose varied between 13 and 19 years (Table 5).
Variable-retention affected differentially the dynamics of nutrients
We distinguished two groups of nutrients: those which are readily mineralized over time, such as carbon, potassium, magnesium, and phosphorus that quickly decreased over the 30 days of decomposition; and those that are immobilized or did not show a net release over decomposition time, as in calcium and nitrogen (Fig. 4).
Remaining nutrient concentration in variable-retention up to 540 days. Variable-retention affected differentially the dynamics of nutrients. a Carbon was lost gradually over the 540 days of decomposition. Dispersed retention 5 YAH had the lowest carbon content at 180 days. b Nitrogen dynamics were very variable. N was retained at all treatments. Dispersed and aggregated retention 5 YAH retained more nitrogen compared to primary forest (F = 1.8, p = 0.035). At 540 days, there was a slight tendency to release nitrogen in primary forest and aggregated retention 1 YAH. c Phosphorus was quickly released after 30 days of incubation. At 90 days, dispersed retention 5 YAH released less phosphorus compared to primary forest (F = 2.40, p = 0.002). d Calcium was mostly retained in all treatments. Calcium showed a variable trend over the studied time. At 540 days there was a slight tendency to be released in dispersed retention 1 YAH. e Potassium was quickly released. Dispersed retention 5 YAH presented the most mineralization of potassium at the 90 days, time which had steepest release. f Magnesium was quickly released. There were no significant differences between treatments. PF primary forest, AR1 aggregated retention after 1 year of harvesting, AR5 aggregated retention after 5 years after harvesting, DR1 dispersed retention after 1 year of harvesting, DR5 dispersed retention after 5 years of harvesting
We found a treatments x time interaction for carbon, nitrogen, and phosphorus (C: F16,236 = 5.8, p < 0.0001; N: F16,235 = 1.8, p = 0.035; P: F16,229 = 2.40 p = 0.002). Carbon, initial with an initial concentration of 46.47%, was lost gradually over the 540 days of decomposition. Starting at 180 days, we found significant differences between treatments. Dispersed retention 5 YAH had the lowest carbon content at 180 days (73.7% of initial C content), aggregated retention 5 YAH at 360 days (65.9%) and primary forest at 540 days (54.2%). Nitrogen initial concentration was 0.48%, and its dynamics were very variable. We found that nitrogen was retained at all treatments (between 93 and 113.5%). We found significant differences at 90 and 540 days. At 90 days, dispersed and aggregated retention 5 YAH retained more nitrogen compared to primary forest. Towards the end of the experiment (i.e., 540 days), there was a slight tendency to release nitrogen in primary forest and aggregated retention 1 year, both treatments were significantly lower from dispersed retention 5 YAH. Phosphorus, with an initial concentration of 0.24%, was quickly released after 30 days of incubation, at the end of the experiment only remained 18–22.6%. We found significant differences between treatments at 30 and 90 days. Dispersed and aggregated retention 5 YAH, and dispersed retention 1 year released less phosphorus was at 30 days. At 90 days, dispersed retention 5 YAH released less phosphorus compared to primary forest.
Potassium, calcium, and magnesium, with initial concentrations of 0.37, 1.13 and 0.32% respectively, did not show a time x treatment interaction (K: F12,182 = 1.1, p = 0.39; Ca: F16,229 = 0.7, p = 0.74; Mg: F8,51 = 0.5, p = 0.82). Potassium and magnesium released 17.5–91; 32.5–93% respectively; although potassium showed a slight increase at 540 days. Dispersed retention 5 YAH presented the highest mineralization of potassium at day 90, when the steepest release was observed.
Calcium showed a variable trend over the studied time. Calcium was mostly retained in all treatments (between 81 and 121%), and at 540 days there was a slight tendency to be released in dispersed retention 1 year.
Mean initial values for the C/N, C/P and N/P ratios were 96.3, 199 and 2.1 respectively (Fig. 5). C/N ratio decreased over time and had an interaction between treatment and time (F16,239 = 2.2, p = 0.005). Dispersed retention 5 YAH had the lowest C/N ratio in all times except at 30 days, because at 30 days we did not find significant differences among treatments. C/P and N/P ratios changed significantly with time (F4,237 = 93.1, p < 0.00001; F4,237 = 87.2, p < 0.00001 respectively). We found an increase in both ratios until 180 days and then a decrease until the end of the experiment (540 days). Furthermore, C/P ratio changed with treatment (F4,174 = 5.5, p = 0.0003), it was lower in dispersed retention 5 YAH. Lignin/N and Lignin/P mean initial ratios were 35.2 and 70 respectively. Comparing initial against final value (0 vs 540 days), we observed that Lignin/N and Lignin/P ratios were significantly different from the initial value in all treatments (F5,56 = 9.35, p < 0.00001), with exception of Lignin/N in primary forest. When we compared the treatments at 540 days, we only found significant differences in the Lignin/N ratio (F5,57 = 13.7, p = 0.03) where aggregated retention 5 YAH was lower than primary forest (Fig. 6).
Variation of C/N; C/P and NP ratio in variable-retention at 540 days. a C/N ratio decreased over time and had an interaction between treatment and time (F = 2.2, p = 0.005). Dispersed retention 5 YAH had the lowest C/N ratio in all times except at 30 days. b C/P and c N/P ratios changed significantly with time (F = 93.1, p < 0.00001; F = 87.2, p < 0.00001 respectively). We found an increment in both ratios until 180 days and then decreased until the end of the experiment (540 days). Furthermore, C/P ratio changed with treatment (F = 5.5, p = 0.0003), it was lesser in dispersed retention 5 YAH. PF primary forest, AR1 aggregated retention after 1 year of harvesting, AR5 aggregated retention after 5 years after harvesting, DR1 dispersed retention after 1 year after harvesting, DR5 dispersed retention after 5 years of harvesting. Vertical bars represent a confidence interval
a Lignin/N and b Lignin/P ratios comparing the initial with the end value (0 vs 540 days). Lignin/N and Lignin/P ratios were significantly different from the initial value in all treatments (F = 9.35; p < 0.00001), with exception of Lignin/N in primary forest. At 540 days, primary forest had higher Lignin/N ratio (F = 13.7; p = 0.03). Initial initial ratios, PF primary forest, AR1 aggregated retention after 1 year of harvesting, AR5 aggregated retention after 5 years after harvesting, DR1 dispersed retention after 1 year after harvesting, DR5 dispersed retention after 5 years after harvesting. Vertical bars represent a confidence interval. Asterisk indicate significant differences at time 0 (*p < 0.05, **p < 0.01 ***p < 0.001)
Yearly decomposition rate was correlated with all nutrients except C, the correlation strength was moderate at best (Table 6). It was positively correlated to Ca, N, C/P, and N/P ratios. And negatively correlated to K, P, Mg, and C/N ratio.
Discussion
Dispersed retention accelerates litter decomposition
This study shows that dispersed retentions treatment had the highest decomposition rates at the beginning of the experiment (30 days). We think that the results found in the present study are due to canopy opening for harvesting, generated environmental conditions that increased N. pumilio leaves decomposition. These modifications in the environmental conditions promotes decomposition in cold weather areas (Swift et al. 1979; Yin et al. 1989). Although microclimatic data are not presented in this study, in the same study area Martínez Pastur et al. (2013) found that wind speed and rainfall were higher in DR. Winds and rainfall could cause losses of litter by leaching or physical fragmentation (Brandt et al. 2007). In general, wind speed (of significant importance in Patagonia) is reduced in Nothofagus stands with higher crown cover levels (Bahamonde et al. 2009) and this could have affected organic matter decomposition found in this work and by Bahamonde et al. (2012). In concert with our results, Caldentey et al. (2001) reported higher values of leaf litter decomposition (53% in 360 days) in a N. pumilio forest harvested with shelterwood cuts (50% crown cover) compared to unmanaged forests (80–90% crown cover) in southern Chile. Similarly, Moretto et al. (2004) reported an increased decomposition of leaf litter in N. pumilio forests under silvicultural management compared with unmanaged forests in Tierra del Fuego (Argentina).
The lowest decomposition rates at the end of the study (540 days) in dispersed retentions possibly have different explanations according to the time of harvesting. In the case of dispersed retention 1 YAH, we think that high decomposition was associated to edaphic and microclimatic factors. On edaphic factors, dispersed retention 1-YAH soil had lower pH than the other sites (Oro Castro 2014). Low pH can favour decomposition, both directly, changing changes in the community composition of the organisms involved in breaking down organic matter, and indirectly, changing the solubility and availability of each nutrient (Berg and McClaugherty 2008). On microclimatic factors, the canopy opening allows more solar radiation to reach the soil in dispersed retention harvesting (Martínez Pastur et al. 2013). Thus, increments in soil temperature cause increases in decomposition rate (Bahamonde et al. 2012). In dispersed retention 5 YAH, we believe that the rapid initial loss was related to the understory. This treatment had significantly higher understory cover (> 60%) compared with the other treatments (Martínez Pastur et al. 2002a, b; Oro Castro 2014). An increase in understory cover can change plant-soil relationships and interfere with nutrient cycling (Vivanco and Austin 2008). Differences in nutrient concentrations in plant tissues, size of the plant and N, litter decomposition rates, and litter N dynamics can explain, in part, the changes in the soil (Ehrenfeld et al. 2001).
We expected that primary forest and aggregated retentions have the lowest slopes of decomposition rates compared to dispersed retentions. In this way, we think that aggregated retentions could be retaining some original forest’s characteristics (Sullivan et al. 2001). Some studies claim that microclimatic conditions and forest regeneration inside the aggregated were like the original forest (Martínez Pastur et al. 2011, 2013). However, the microclimatic conditions in aggregate retention sites area not equivalent to the conditions inside the large areas of continuous canopy (Franklin et al. 1997). The decomposition rates decrease was more gradual in primary forest and aggregated retentions perhaps because litter, particularly leaves and branches, usually dominates the coverage in these treatments (Oro Castro 2014). Hence, this coverage can generate stable conditions for microorganisms responsible for decomposition, such as constant humidity levels (Ritter et al. 2005).
We found that the constant k presented values that fluctuated between 0.2 and 0.6 years−1 in PF after 12 months. Other studies found constants k between 0.4 and 0.8 (Godeas et al. 1985; Richter and Frangi 1992; Ibarra et al. 2011). These results concur with the k values of other temperate deciduous forests (0.25–0.7 years−1) (Frangi et al. 2005). The lower rates found in this study could be due to the climatic conditions of the geographical location, mainly due to the low temperatures that determine that many of the ecosystem processes are slower (Vitousek et al. 1994; Hobbie 1996). Likewise, there could be differences in the initial material that could determine a differential decomposition in time, not only considering the chemical characteristics of the litter (Vivanco and Austin 2008), but also the characteristics of initial decomposability.
Variable-retention affected differentially the dynamics of nutrients
Nitrogen from N. pumilio leaves was mainly immobilized in all treatments including primary forest along the entire study. We suggest that the immobilization of N was caused by low soil temperatures during winter that, in general, would slow microbial activities resulting in lower mineralization or immobilization (Swift et al. 1979). However, the primary forest and aggregated retention 1 YAH began to mineralize N at the end of the study (540 days). Differences in N mineralization can be attributed to litter quality of material (i.e., initial N concentration) or environmental factors (temperature, humidity, precipitations) (Prescott 2005) and other factors like understory coverage. Thereby, we think that in dispersed retention 5 YAH and aggregated retention 5 YAH high understory cover (> 60%) played a key role in the N mineralization (Oro Castro 2014). Understory plants could be absorbing N not allowing us to record the mineralized N. Dispersed retention 5 YAH had the smallest C/N ratio at all times (except at 30 days) and C/N ratio was negatively correlated with decomposition rate (Table 6). This smallest ratio is related to higher rates of decomposition that we found in this site because low C/N ratio promotes micro-organisms activity (Bahamonde et al. 2012). Low C/N ratio was also noticed in other species of Nothofagus in Patagonia, where decomposition was related to C quality more than N concentration (Vivanco and Austin 2008). Lignin/N ratio also showed that the primary forest better preserved N than the sites with variable-retention (Fig. 6). We think that the abundance of litter in the primary forest (Oro Castro 2014) can explain why primary forest better preserved N because litter can have secondary recalcitrant metabolites (Gilliam et al. 2001; Ritter et al. 2005).
In the present study we found low N/P ratios. Litter N/P ratios may indicate which of the two elements is more limiting, and the question arises whether a certain ‘critical N/P ratio’ discriminates between N- and P-limited decomposition, similar to the ‘critical N/P ratios’ for biomass production (Koerselman and Meuleman 1996). Several ‘critical N/P ratios for decomposition’ have been suggested in different studies. However, in this study we found values below those studies. Berg and Laskowski (1997) reported that initial low N/P ratio of litter facilitate the release of P. Furthermore, Aerts and Caluwe (1997) found that decomposition during the first 3 months was strongly related to P concentrations, whereas decomposition after 1 year was related more to concentrations of lignin and phenolics. The initial decomposition times (30 and 90 days) were the most sensitive to changes in the dynamics of phosphorus by variable-retentions. At 90 days, more mineralization of P in dispersed retention 5 YAH compared with primary forest is suggesting a more rapid loss of P. Additionally, higher C/P ratio in dispersed and aggregated retention 1 year indicated a loss of P in these treatments. Thereby, we observe that there was an alteration of the P cycle on soil studied (Fig. 5).
Phosphorous was mineralized mainly in the first 90 days in all treatments. The rapid initial losses of P probably reflect the tendency of extra organic P to be stored as inorganic P, which is rapidly leached from the litter early in the decomposition process (Prescott 2005).
In this work, potassium was mineralized in all evaluated situations. This is consistent with other authors who reported for different types of material and environmental conditions a potassium release from litter (i.e., Barrera et al. 2004; Osono and Takeda 2004). Dispersed retention 5 YAH presented the highest value of remaining K at day 90, we believe that this was due to the high cover of Poa pratensis this site (Oro Castro 2014). Also, potassium showed a slight increase at 540 days in all sites. K dynamics in freshly fallen litter depends to a considerable extent on the initial concentration of this element (Laskowski et al. 1995a, b). Although K is a mobile element and is usually leached quickly from decomposing litter at high initial concentrations, at low concentrations, such as those found in this study, its concentration may begin increasing as soon as the litter starts to decay.
The fact that we didn’t found significant differences between treatments in calcium may be because the cycling of these nutrients is relatively slow within an ecosystem, and recycling within the tree is almost non-existent. Furthermore, approximately 50% of calcium plant is as calcium pectate in the cell wall, a compound with very slow decomposition (Fisher and Binkley 2000). We found calcium retained in all sites in this study, which agrees with Osono and Takeda (2004) who reported a response in two phases with increasing calcium concentration (immobilization) up to the first 21 months, and then a decay in calcium concentration (mineralization) 3 years later. Neither we found significant differences between treatments in magnesium. However, magnesium showed high loss (60–70% after 1 year of decomposition). We speculate that its cycling may have been even faster but the lack of data between day 30 and day 360 does not allow for a more complete analysis.
Conclusions
Our results suggest an immediate negative effect of variable-retention on litter decomposition and the nutrients dynamics. We observed that variable-retention had immediate and short-term effects on initial decomposition rates. Specifically, dispersed retention showed the highest decomposition rates at the beginning of the experiment (30 days). Furthermore, the aggregated retention showed similar decomposition rates to the primary forests, and both declined gradually in contrast to dispersed retention. Also, we found that dispersed retention had changed nutrients release, mainly in the initial stages of decomposition. We believe that these changes in nutrient dynamics during the initial stages of decomposition might have a negative effect for the establishment of forest regeneration and affect the future wood yield.
To our best knowledge, this is the first study about litter decomposition under variable-retentions systems in Patagonia. Thereby, we recommend that degradation of soils and their recovery are considered for the definition of forestry practices in the region. We suggest that decomposition and release nutrient should be evaluated before, during and after variable-retentions systems (and other forestry practices in the region) because the negative impacts of this harvesting on decomposition and release nutrient could be mitigated and/or remedied earlier taking measures to protect soil.
References
Aerts R, Caluwe HD (1997) Nutritional and plant-mediated controls on leaf litter decomposition of Carex species. Ecology 78:244–260
Alfonso JL (1942) Los bosques de Tierra del Fuego. Rev Suelo Argent 1:47–51
Anderson DR (2008) Model based inference in the life sciences: a primer on evidence. Springer, New York, p 184
Bahamonde HA, Peri PL, Martínez Pastur G, Lencinas V (2009) Variaciones microclimáticas en bosques primarios y bajo uso silvopastoril de Nothofagus antarctica en dos Clases de Sitio en Patagonia Sur
Bahamonde HA, Peri PL, Alvarez R, Barneix A, Moretto A, Martínez Pastur G (2012) Litter decomposition and nutrients dynamics in Nothofagus antarctica forests under silvopastoral use in Southern Patagonia. Agrofor Syst 84:345–360
Barrera MD, Frangi JL, Ferrando JJ, Goya JF (2004) Descomposición del mantillo y liberación foliar neta de nutrientes de Austrocedrus chilensis (D. Don) Pic. Serm. Et Bizzarri en El Bolsón, Río Negro. Ecol Austral 14:99–112
Beese WJ, Bryant AA (1999) Effect of alternative silvicultural systems on vegetation and bird communities in coastal montane forests of British Columbia, Canada. For Ecol Manage 115:231–242
Berg B, Laskowski R (1997) Changes in nutrient concentrations and nutrient release in decomposing needle litter in monocultural systems of Pinus contorta and Pinus sylvestris: a comparison and synthesis. Scand J For Res 12:113–121
Berg B, McClaugherty C (2008) Plant litter: decomposition, humus formation, carbon sequestration, 2a edn. Springer, Berlin, p 338
Bitterlich W (1984) The relascope idea. Relative measurements in forestry. Commonwealth Agricultural Bureaux, Londres, p 242
Bocock K, Gilbert O, Capstick C, Twinn D, Waid J, Woodman M (1960) Changes in leaf litter when placed on the surface of soils with contrasting humus types. I. Losses in dry weight of ok and ash leaf litter. J Soil Sci 11:1–9
Brancaleoni L, Strelin J, Gerdol R (2003) Relationships between geomorphology and vegetation patterns in subantarctic Andean tundra of Tierra del Fuego. Polar Biol 26(6):404–410
Brandt LA, King JY, Milchunas DG (2007) Effects of ultraviolet radiation on litter decomposition depend on precipitation and litter chemistry in a shortgrass steppe ecosystem. Glob Change Biol 13:2193–2205
Caldentey J, Ibarra M, Hernández J (2001) Litter fluxes and decomposition in Nothofagus pumilio stands in the region of Magallanes, Chile. For Ecol Manage 148:145–157
Collado L (2001) Los bosques de Tierra del Fuego: análisis de su estratificación mediante imágenes satelitales para el inventario forestal de la provincia. Multequina 10:1–15
Deferrari G, Camilion C, Martínez Pastur G, Peri P (2001) Changes in Nothofagus pumilio forest biodiversity during the forest management cycle: 2 birds. Biodiv Conserv 10:2093–2108
Dirección de Bosques TDF (2016) Informe de estadísticas de aprovechamiento forestal correspondiente al período 2015–2016 and análisis comparativo de estadísticas históricas
Ehrenfeld JG, Kourtev P, Huang W (2001) Changes in soil functions following invasions of exotic understory plants in deciduous forests. Ecol Appl 11(5):1287–1300
Fisher RF, Binkley D (2000) Ecology and management of forest soils. Wiley, New York, p 362
Frangi JL, Barrera MD, Richter LL, Lugo AE (2005) Nutrient cycling in Nothofagus pumilio forest along and altitudinal gradient in Tierra del Fuego, Argentina. For Ecol Manage 217:80–94
Franklin JF, Berg DR, Thornburgh DA, Tappeiner JC (1997) Alternative silvicultural approaches to timber harvesting. In: Kohm KA, Franklin JF (eds) Creating a forestry for the 21st century. The science of ecosystem management. Island Press, Washington, DC, pp 111–139
Gallardo A, Merino J (1993) Leaf decomposition in two Mediterranean ecosystems of southwest Spain: influence of substrate quality. Ecology 74:152–161
Gea G, Martínez Pastur G, Cellini JM, Lencinas MV (2004) Forty years of silvicultural management in southern Nothofagus pumilio (Poepp. et Endl.) Krasser primary forests. For Ecol Manage 201(2–3):335–347
Gilliam F, Yurish B, Adams M (2001) Temporal and spatial variation of nitrogen transformation in nitrogen-saturated soil of a central Appalachian hardwood forest. Can J For Res 32:1768–1785
Godeas M, Arambarri A, Gamundi I, Spinedi H (1985) Descomposición de la hojarasca en bosque de Lenga. Ciencias Suelo 3(1–2):68–77
Harmon M, Nadelhoffer K, Blair J (1999) Measuring decomposition, nutrient turnover and stores in plant litter. In: Robertson GP, Coleman DC, Bledsoe CS, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 202–271
Hobbie SE (1996) Temperature and plant species control over litter decomposition in Alaskan tundra. Ecol Monogr 66:503–522
Ibarra M, Caldentey J, Promis A (2011) Descomposición de hojarasca en rodales de Nothofagus pumilio de la región de Magallanes. Bosque 32(3):227–233
Idol TW, Pope PE, Ponder F (2003) N mineralization, nitrification, and N uptake across a 100–year chronosequence of upland hardwood forests. For Ecol Manage 176:509–518
IUSS Working Group WRB (2006) World reference base for soil resources, 2nd edn. World Soil Resources Reports No. 103. FAO, Rome, 133 pp
Johnson DW, Curtis PS (2001) Effects of forest management on soil C and N storage: meta analysis. For Ecol Manage 140:227–238
Koerselman W, Meuleman AFM (1996) The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33:1441–1450
Laskowski R, Berg B, Johansson M, McClaugherty C (1995a) Release pattern for potassium from decomposing forest leaf litter. Long-term decomposition in a Scots pine forest X. Can J Bot 73:2019–2027
Laskowski R, Niklinska M, Maryañski M (1995b) The dynamics of chemical elements in forest litter. Ecology 76:1393–1406
Martinez Pastur G, Lencinas MV (2005) El manejo forestal en los bosques de Nothofagus pumilio en Tierra del Fuego. IDIA XXI 01 5(8):107–110
Martínez Pastur G, Cellini JM, Peri PL, Vukasovic RF, Fernández MC (2000a) Timber production of Nothofagus pumilio forests by a shelterwood system in Tierra del Fuego (Argentina). For Ecol Manage 134:153–162
Martínez Pastur G, Lencinas MV, Vukasovic R, Peri P, Diaz B, Cellini JM (2000a) Turno de corta y posibilidad de los bosques de lenga de Tierra del Fuego (Argentina) considerando la influencia del ganado, el manejo silvícola and la calidad de sitio. Actas Reunión Internacional: Modelos and Métodos estadísticos Aplicados a Bosques Naturales. Valdivia, Chile, pp 20–21
Martínez Pastur G, Lencinas MV, Cellini J, Diaz B, Peri P, Vukasovic R (2002a) Herramientas disponibles para la construcción de un modelo de producción para la lenga (Nothofagus pumilio) bajo manejo en un gradiente de calidades de sitio. Bosque 23(2):69–80
Martínez Pastur G, Peri P, Fernández M, Staffieri G, Lencinas MV (2002b) Changes in understory species diversity during the Nothofagus pumilio forest management cycle. J For Res 7(3):165–174
Martínez Pastur GJ, Lencinas MV, Peri PL, Moretto A, Cellini JM, Vukasovic R (2007) Harvesting adaptation to biodiversity conservation in sawmill industry: technology innovation and monitoring program. Technol Manag Innov 2(3):58–70
Martínez Pastur G, Cellini JM, Peri P, Lencinas MV, Gallo E, Soler Esteban R (2009) Alternative silviculture with variable retention in timber management of South Patagonia. For Ecol Manage 258:436–443
Martínez Pastur G, Cellini JM, Lencinas MV, Barrera M, Peri P (2011) Environmental variables influencing regeneration of Nothofagus pumilio in a system with combined aggregated and dispersed retention. For Ecol Manage 261:178–186
Martínez Pastur G, Soler Esteban R, Pulido F, Lencinas MV (2013) Variable retention harvesting influences biotic and abiotic drivers along the reproductive cycle in southern Patagonian forests. For Ecol Manage 289(1):106–114
Mitchell SJ, Beese WJ (2002) The retention system: reconciling variable retention with the principles of silvicultural systems. For Chron 78(3):397–403
Moretto A, Martínez Pastur G (2014) Litterfall and leaf decomposition in Nothofagus pumilio forests along an altitudinal gradient in Tierra del Fuego, Argentina. J For Sci 60(12):500–510
Moretto A, Martínez Pastur G, Peri P (2004) Producción de hojarasca en diferentes sistemas de regeneración con retención dispersa y agregada en bosques de Nothofagus pumilio. Actas del Segundo Congreso Chileno de Ciencias Forestales. Valdivia, Chile, 7 pp
Nóvoa Muñoz JC, Pontevedra Pombal X, Moretto A, Martínez Cortizas A, García-Rodeja Gayoso E (2007) Caracterización geoquímica de suelos forestales de Nothofagus pumilio (lenga) en un gradiente altitudinal en Tierra del Fuego, Argentina. In: Bellinfante N, Jordán A (eds) Tendencias Actuales de la Ciencia del Suelo. Actas del II Congreso Ibérico de Ciencia del Suelo, pp 689–696
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:331–332
Oro Castro, N. 2014. ¿Cómo varían los ciclos biogeoquímicos debido al aprovechamiento forestal en bosques de Nothofagus pumilio de Tierra del Fuego? Tesis de Doctorado en Biología. Departamento de Biología, Bioquímica y Farmacia, Universidad Nacional del Sur, Bahía Blanca, Argentina. p 137
Osono T, Takeda H (2004) Potassium, calcium and magnesium dynamics during litter decomposition in a cool temperate forest. J For Res 9:23–31
Peña-Rodríguez S, Moretto A, Pontevedra-Pombal X, Oro N, García-Rodeja Gayoso E, Rodríguez-Salgado I, Rodríguez-Racedo J, Escobar J, Nóvoa-Muñoz JC (2013) Trends in nutrient reservoirs stored in uppermost soil horizons of subantarctic forests differing in their structure. Agrofor Syst 87(6):1273–1281
Pinheiro J, Bates DM (2000) Mixed-effects models in S and S-PLUS, 1st edn. Springer, New York, p 530
Prescott CE (2005) Decomposition and mineralization of nutrients from litter and humus. In: Nutrient acquisition by plants. Springer, Berlin, pp 15–41
R Development Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0. http://www.R-project.org/
Richter L, Frangi J (1992) Bases ecológicas para el manejo del bosque de Nothofagus pumilio de Tierra del Fuego. Revista de la Facultad de Agronomía, La Plata. 68: 35–52
Ritter E, Starr M, Vesterdal L (2005) Losses of nitrate from gaps of different sizes in a managed beech (Fagus sylvatica) forest. Can J For Res 35:308–319
Schmidt H, Urzúa A (1982) Transformación y manejo de los bosques de Lenga en Magallanes. Universidad de Chile. Ciencias Agrícolas 11. 62 pp
Spagarino C, Martínez Pastur G, Peri P (2001) Changes in Nothofagus pumilio forest biodiversity during the forest management cycle: insects. Biodivers Conserv 10:2077–2092
Suffling R, Smith D (1974) Litter decomposition studies using mesh bags: spillage inaccuracies and the effects of repeated artificial drying. Can J Bot 52:2157–2163
Sullivan TP, Sullivan DS, Lindgren PMF (2001) Stand structure and small mammals in young lodgepole pine forest: 10-year results after thinning. Ecol Appl 11:1151–1173
Swift M, Heal O, Anderson J (1979) Decomposition in terrestrial ecosystems. University of California Press, Berkeley, p 372
Tukhanen S (1992) The climate of Tierra del Fuego from a vegetation geographical point of view and its ecoclimatic counterparts elsewhere. Acta Bot Fenn 145:1–64
Van Soest PJ (1963) Use of detergents in analysis of fibrous feeds II: a rapid method for the determination of fiber and lignin. Ann Chem 46:829–835
Vitousek PM, Turner DR, Parton WJ, Sanford RL (1994) Litter decomposition on the Mauna-Loa environmental matrix, Hawaii—patterns, mechanisms, and models. Ecology 72:418–429
Vivanco L, Austin AT (2008) Tree species identity alters forest litter decomposition through long-term plant and soil interactions in Patagonia, Argentina. J Ecol 96:727–736
Vukasovic R, Martínez Pastur G, Cellini JM (2004) Plan de manejo forestal Los Cerros. Solicitante Aserradero Kareken (PRODIN SRL)
Yin X, Perry JA, Dixon RK (1989) Influence of canopy removal on oak forest floor decomposition. Can J For Res 19:204–214
Yoshida T, Iga Y, Ozawa M, Noguchi M, Shibata H (2005) Factors influencing early vegetation establishment following soil scarification in a mixed forest in northern Japan. Can J For Res 35:175–188
Acknowledgements
We thank Sr. Roberto Fernández and Eng. Ricardo Vukasovic for their logistic assistance and Dr. Guillermo Martínez Pastur for his scientific and technical advice. We also like to thank the constructive criticism of an anonymous reviewer that greatly improved this manuscript. This study was funded by the Agencia Nacional de Promoción Científica y Tecnológica (PAV2004-22428 and PICTO FORESTAL-36861). N. Oro Castro and L. J. Selzer were recipient of a CONICET doctoral scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oro Castro, N., Moretto, A., Selzer, L.J. et al. Effects of alternative silvicultural systems on litter decomposition and nutrients dynamics in sub-Antarctic forests. Agroforest Syst 93, 885–899 (2019). https://doi.org/10.1007/s10457-018-0183-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-018-0183-0