Abstract
Agroforestry buffers are believed to enhance soil quality parameters in agricultural landscapes. Soil enzyme activities, water stable aggregates (WSA), soil organic carbon (SOC), and total nitrogen (N) have been identified as good indices of soil quality. The objective of this study was to quantify soil quality differences among agroforestry buffer (AGF), grass waterway (GWW), grass buffer (GB), and row crop (RC) areas and distance from the tree base on corn (Zea mays L.)-soybean (Glycine max L.) watershed. Soil samples from AGF at 0, 30, 60, 90 cm distances from the tree base, and from AGF, GWW, GB, and RC areas were collected from summit, shoulder, and foot-slope landscape positions at the paired watershed study near Novelty, MO. Soil enzyme activity, WSA, soil C, and N were determined and data were analyzed statistically. The highest SOC and N percentages were found in AGF and the lowest in RC. β-Glucosidase activity was not significantly different among AGF, GWW, and RC. β-Glucosaminidase and dehydrogenase activities were significantly lower in RC treatment than all other treatments. Fluorescein diacetate (FDA) hydrolase activity was not significant among all treatments. WSA percentage was significantly higher in GWW and AGF as compared to others. Landscape position was not significantly different for all measured soil quality parameters. β-Glucosidase, β-glucosaminidase activities, WSA, and soil N did not differ significantly with distance from the tree base. SOC and FDA hydrolase activity were significantly lower at the tree base. Results imply that permanent vegetation has improved the soil quality by enhancing soil microbial activity and organic matter accumulation, thereby contribute positively to watershed restoration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intensive agricultural practices during last few decades have been recognized as one of the major driving forces of environmental degradation, which has impacted the soil and water resources (Zalidis et al. 2002). Usage of chemicals such as fertilizer, herbicide, and pesticide applications are the causes for increased amounts of nutrients and toxins in ground and surface water resources. This has resulted increased healthcare and water purification costs (Tilman et al. 2002). As an example, soil erosion in the US is responsible for an annual cost of $37.6 billion loss in productivity (Uri 2000). Establishing perennial vegetation that include grass and tree species is a possible solution for restoring degraded landscapes and watersheds (Schultz et al. 2009).
Agroforestry practices have been proven to improve water quality, soil physical and biological properties, and carbon sequestration in ecosystems (Nii-Annang et al. 2009; Udawatta et al. 2009; Paudel et al. 2011; Jose and Bardhan 2012). Furthermore, agroforestry practices such as upland and riparian buffers are effective in reducing non-point source pollution (NPSP) from row crop landscapes by reducing runoff, leaching of chemicals into groundwater and groundwater contamination (Udawatta et al. 2002; Schultz et al. 2009). For example, Dillaha and Inamdar (1996) observed that stiff stemmed grasses in the buffer adjacent to a crop field edge slowed runoff, causing large soil particles to settle. Perennial vegetation enhances nutrient uptake by their roots from varying soil depths (Nair and Graetz 2004; Lovell and Sullivan 2006; Udawatta et al. 2010), increases SOC and total N (Paudel et al. 2011), and reduces soil erosion (Escobar et al. 2002). According to Schultz et al. (2009), the grass buffers convert the concentrated flow from upland to sheet flow. Furthermore, sediment from the sheet flow is subjected to deposition in the grass buffer while water and agrochemicals are infiltrated into the biologically active root zone. Numerous studies have reported effectiveness of perennial vegetative buffers on water quality improvements in agricultural watersheds and nutrient removal efficiencies varying from 20 to 90 % (Lee et al. 2003; Mankin et al. 2007; Schultz et al. 2009).
Agroforestry practices have been shown to improve soil quality parameters as well (Udawatta et al. 2009; Paudel et al. 2011; Udawatta et al. 2014). Soil quality is the capacity of a soil to function within the ecosystem boundaries and has a profound effect on the health and productivity of a given ecosystem and the environments related to it (Doran and Parkin 1994). Soil quality assessment is described as evaluation of the soil functions (Karlen et al. 1997). It is important for assessing the sustainability of agricultural and other land management systems (Doran and Parkin 1994). Soil carbon (C) has been considered as an indicator of soil quality. And, it has been demonstrated that agroforestry practices can sequester greater quantities of soil C compared to traditional agricultural systems (Jose 2009; Udawatta and Jose 2012). Soil enzymes are considered as a biological indicator of soil quality which reflects the changes in land management practices because of their immediate response to changes (Karlen et al. 1997; Gregorich et al. 2006; Acosta-Martínez et al. 2003). Furthermore, soil enzymes such as amylase, arylsulphatases, β-glucosidase, cellulose, chitinase, dehydrogenase, phosphatase, protease and urease regulate soil ecosystem functioning via catalyzing the reactions involved in organic waste decomposition, stabilization of soil structure, organic matter formation and nutrient cycling (Makoi and Ndakidemi 2008). The level of enzymes in soils may vary depending on the amount of organic matter, composition, and activity of its living organisms (Makoi and Ndakidemi 2008). Paudel et al. (2012) reported that, soil enzyme activities and microbial biomass can be improved by establishing perennial vegetation. Udawatta et al. (2009) mentioned that enhanced WSA, SOC, and functional diversity of soil microbial communities, and soil enzyme activities were due to establishment of buffers with trees and grasses. Moreover, agroforestry and grass buffer treatments had greater total porosity, coarse mesoporosity, and lower bulk density values compared with the row crop treatment (Seobi et al. 2005).
Long-term effects of agroforestry practices on soil quality parameters, particularly on soil enzymes, are limited in the literature for the temperate zone. Quantification and evaluation of beneficial effects of agroforestry practices are important for scientists, policymakers, and landowners for making significant decisions for promoting these land use practices while diversifying farm income (Kumar et al. 2010). The objectives of this study were to (1) assess soil enzymatic activities, WSA, SOC, and total nitrogen as soil quality parameters within an agroforestry buffer, grass waterway, grass buffer, and row crop management systems, (2) identify the effects of landscape (summit, mid, and foot slope) on soil quality parameters, and (3) compare the effect of distance from tree base on soil quality parameters with in the AGF.
Materials and methods
Study area
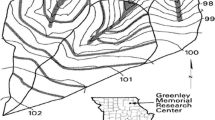
The two experimental watersheds are located at the Greenly Memorial Research Center, Knox County, Missouri, USA (40°01′N, 92°11′W; Fig. 1). They were established in 1991, under a corn (Zea mays L.)–soybean (Glycine max (L.) Merr.) rotation with no-till land preparation. Grass buffers (3–4.5 m wide) containing redtop (Agrostis gigantea Roth), brome grass (Bromus spp.), and birdsfoot trefoil (Lotus corniculatus L.) at 36.5 m apart were established on both watersheds in 1997. In the agroforestry watershed, pin oak (Quercus palustris Muenchh.), swamp white oak (Q. bicolor Willd.), and bur oak (Q. macrocarpa Michx.) trees have been alternately planted at 3 m spacing in 1997 in the middle of the grass strips. Grass waterways on both watersheds consisted of Kentucky 31 tall fescue (Festuca arundinacea var. genuina Schreb.). Putnam silt loam (fine, smectitic, mesic Vertic Albaqualfs) and Kilwinning silt loam (fine, smectitic, mesic Vertic Epiaqualfs) with a minor proportion of Armstrong silt loam (fine, smectitic, mesic Aquertic Hapludalfs) on steeper (5–9 %) slopes are the soils in the watersheds (Udawatta et al. 2009). The presence of an argillic horizon at shallow depths restricts water infiltration in these soils and causes copious surface runoff during the saturation periods i.e. the spring and early summer, and heavy and closely spaced small rainfall events. The region receives about 66 % (600 mm) of the 30-year mean annual precipitation (920 mm) between April and September (Udawatta et al. 2009).
Topographic map of the grass buffer and the agroforestry watersheds with 0.5 m elevation interval contour lines (black) where agroforestry (grass + trees) buffers (gray lines), and grass waterways (wide black lines) (A). Photographs show agroforestry buffer (B) and grass buffers (c) on the two watersheds in Knox County, Missouri, USA. (Color figure online)
Soil sampling and laboratory analysis
Soil from the surface 0- to 10-cm was sampled in June 2008 using a 5-cm diam. auger from agroforestry buffer (AGF), grass waterway (GWW), grass buffer (GB), row crop (RC) areas. Landscape position was evaluated for the AGF and GB treatments and soil samples were collected from first, third, and fifth buffers (counting from the south; Fig. 1) and these buffers represented summit, mid, and foot slope landscape positions, respectively. The same buffers were sampled from the GB watershed. Three locations (north, south, and middle) of GWW soils were sampled from both watersheds. Within the agroforestry buffer, soils were also collected at 0-, 30-, 60-, 90-cm distances from the tree base at summit, mid, and foot slope positions of the watershed. Soils were placed in labeled zip-lock bags, and transported to the laboratory in a cooler. The samples were stored at 4 °C until the laboratory procedures were conducted. Laboratory analyses for each parameter were carried out using the standard methods (Table 1).
Statistical analysis
The data were analyzed as a completely randomized split block design using Proc GLM in SAS (version 9.4). The measured parameters were analyzed by the four management treatments, by landscape position, and distance from the tree base. Tukey’s range test was used for pairwise comparison of treatment means, at 5 % level of significance (p ≤ 0.05).
Results and discussion
Soil enzyme activities
β-Glucosidase enzyme activity (161 µg p-nitrophenol released g−1 dry soil h−1) was the highest in AGF compared to all other treatments (Fig. 2a). Among four treatments GB area had lowest level of enzyme activity (β-glucosidase) with 43 µg p-nitrophenol released g−1 dry soil h−1. The difference of mean β-glucosidase activity between GWW (93 µg p-nitrophenol released g−1 dry soil h−1) and RC (85 µg p-nitrophenol released g−1 dry soil h−1) was not significant. Similar results have been observed in related studies with permanent vegetation and row crops (Dick et al. 1996; Acosta-Martínez et al. 2003; Kremer and Li 2003; Mungai et al. 2005a, b; Udawatta et al. 2008, 2009; Kremer and Kussman 2011; Paudel et al. 2012). According to the study conducted by Udawatta et al. (2008) on the same watershed, RC had the lowest β-glucosidase activity with an average of 129.1 ± 14.8 µg p-nitrophenol released g−1 dry soil h−1 and GB exhibited the highest value with 228.8 ± 20.6 µg p-nitrophenol released g−1 dry soil h−1. AGF and GWW treatments contained 204.3 ± 19.3 and 199.3 ± 20.6 µg p-nitrophenol released g−1 dry soil h−1 respectively. However, those values are higher than the present study. This difference might be due to the differences in sampling time or the season. Because Udawatta et al. (2008) study was carried out in 2003 where 5 years earlier than current study.
β—Glucosidase, β—glucosaminidase, and dehydrogenase enzyme activities (A), fluorescein diacetate hydrolase activity (FDA; B), water stable aggregates (WSA; C), and soil organic C and total N contents (D) for agroforestry buffers (AGF), grassed waterways (GWW), grass buffers (GB), and row crop (RC) treatments. Bars with different letters denote significant differences among treatments at p ≤ 0.05
The activity of β-glucosaminidase was significantly different between row crop and permanent vegetative buffers (Fig. 2a). The lowest activity (56 µg p-nitrophenol released g−1 dry soil h−1) was observed in the RC among all four treatments. However, β-glucosaminidase activity was not significantly different among GWW (114 µg p-nitrophenol released g−1 dry soil h−1), AGF (113 µg p-nitrophenol released g−1 dry soil h−1), and GB (99 µg p-nitrophenol released g−1 dry soil h−1) treatments. The lowest activity of β-glucosaminidase in RC treatment can be attributed to the disturbances occurred in conventional tillage practices. This agrees with the findings of research by Ekenler and Tabatabai (2003) and Paudel et al. (2012) where the RC treatment had a significantly lower level compared to perennial vegetation treatments. According to Udawatta et al. (2008), GWW and GB had β-glucosaminidase activity of 135.0 ± 7.80 and 133.8 ± 8.34 µg p-nitrophenol released g−1 dry soil h−1 respectively, while AGF exhibited 124.9 ± 6.65 µg p-nitrophenol released g−1 dry soil h−1 and RC contained the lowest value of 73.5 ± 5.51 µg p-nitrophenol released g−1 dry soil h−1. However, β-glucosaminidase activity of the current study was lower than values obtained by Udawatta et al. (2008).
In this study, greater levels of dehydrogenase activity was exhibited by the AGF and GB with 10 µg p-nitrophenol released g−1 dry soil h−1 and 9 µg p-nitrophenol released g−1 dry soil h−1 respectively, compared to other treatments (Fig. 2a). However, the difference was not significant among AGF, GB, and GWW (7 µg p-nitrophenol released g−1 dry soil h−1). The lowest level of dehydrogenase activity was observed in the RC treatment. In a related study Paudel et al. (2012) have reported similar results. According to Udawatta et al. (2008), dehydrogenase activity was highest in GB (79.1 ± 7.5 µg p-nitrophenol released g−1 dry soil h−1) and lowest in RC treatment (48.0 ± 5.2 µg p-nitrophenol released g−1 dry soil h−1). GWW exhibited 65.5 ± 7.2 µg p-nitrophenol released g−1 dry soil h−1 and AGF contained 51.0 ± 7.5 µg p-nitrophenol released g−1 dry soil h−1 dehydrogenase activity respectively. Although the values in their study were greater than the current study, the pattern of results was similar in both studies.
According to our results, fluorescein diacetate (FDA) hydrolase activity was not significantly different among all four treatments (Fig. 2b). The numerical values of FDA hydrolase activities among the four treatments can be ranked as, AGF > GB > GWW > RC. These results support the findings of a related study that was carried out by Paudel et al. (2012). However, Udawatta et al. (2008) have observed significant differences in FDA hydrolase activity among treatments. RC had the lowest with an average of 8.49 ± 0.61 µg p-nitrophenol released g−1 dry soil h−1 while GB contained the highest (13.55 ± 0.86 µg p-nitrophenol released g−1 dry soil h−1) FDA hydrolase activity in their study.
Water stable aggregates (WSA)
The GWW had the highest percentage of WSA (36 %) and it was not significantly different from AGF (30 % WSA; Fig. 2c). RC and GB treatments had the lowest percentages of WSA (21 and 20 % respectively). The findings are parallel to the results obtained by Udawatta et al. (2009), where GWW had the highest WSA percentage while RC had the lowest.
WSA percentage is a measure of the resistance of soil to breakdown by water and mechanical stress (Paudel et al. 2011). Therefore, these greater soil aggregates in the perennial vegetation areas of the watershed play a significant role for maintaining soil porosity and stabilizing soil against erosion and improving soil quality (Barthès et al. 1999; Lupwayi et al. 2001). In addition, they can reflect the microbial activity as soil microbes improve soil aggregation (Six et al. 2006). Clay and organic matter and bonding of soil particles both chemically and physically by labile organic compounds and fungal hyphae and roots mainly affect stabilization of soil aggregates (Lupwayi et al. 2001).
Improved WSA in these watersheds imply stabilization of C and N facilitated by greater microbial activity and continuous supply of organic matter by both root biomass and aboveground litter in perennial vegetation systems (Ingham 2000; Paudel et al. 2012). In contrast, the negative effects of tillage practices implemented in row crop areas such as disruption of soil aggregates and organic matter depletion in the plow layer may have reduced microbial diversity and activity through compaction, desiccation, mechanical destruction, etc. (Lupwayi et al. 2001; Paudel et al. 2012) and thereby lower WSA percentage. Greater enzyme activities and WSA under the perennial vegetation may also help increase nutrient cycling and reduce runoff losses.
Soil organic carbon and total nitrogen
SOC percentage was significantly higher at AGF (2.8 %) while it was the lowest at RC (1.8 %) treatment (Fig. 2d). The difference was not significant between GWW (2.6 %) and GB (2.2 %) treatments. Furthermore, these results support the findings of the studies carried out by Udawatta et al. (2009) and Paudel et al. (2011, 2012). These differences in SOC percentages across the treatments imply significant differences in the amounts of C that are being sequestered per unit area of land when AGFs and GBs are incorporated into RC lands. Assuming that each buffer is comprised of 10 % of the total cropped area the amounts of sequestered C per ha can be estimated as 25.3 Mg and 24.5 Mg for AGF and GB incorporated crop land respectively while that of RC land is 24.2 Mg.
Plowing in RC areas lead to significantly lower amounts of SOC as intensive soil disturbance and disruption of soil structure enhances decomposition or mineralization of soil organic matter and soil erosion (Six et al. 2002; Ogle et al. 2003; Paudel et al. 2012). Conversely, under perennial vegetation like AGF and GB such disturbances are not being taken place. Therefore, these conditions lead to enhanced accumulation of carbon and nitrogen compared to the tilled row crop cultivation (Paudel et al. 2012). Furthermore, greater WSA percentage existing in AGF results in greater microbial biomass and mineralizable C within soil aggregates (Lupwayi et al. 2001).
Similar to SOC, highest percentage of TN (0.22 %) was observed in AGF treatment and lowest (0.14 %) was exhibited in the RC treatment (Fig. 2). These results also agree with the findings of related studies by Udawatta et al. (2009), Paudel et al. (2011, 2012). The negative effects of tillage practices used in RC operations also result in lower amounts of TN. In addition, leaching of N also may have contributed to the losses of N in RC treatment. The difference was not significant between GWW (0.2 %) and GB (0.17 %) treatments.
Landscape position on soil quality parameters
Landscape position was also not significantly different for all soil enzyme activities (Table 2). However, numerical values of activity of all the enzymes exhibited same pattern through all the landscape positions as summit > shoulder > foot-slope. WSA, SOC and TN were also not significantly different for landscape position, but it was numerically greatest in the foot-slope and least in the shoulder for SOC and TN.
Similar to these results, a non-significant effect of the landscape position on the measured parameters has been observed in the related previous studies as well (Decker et al. 1999; Mungai et al. 2005a, b; Paudel et al. 2011). As these parameters are affected by the factors such as quality and the quantity of the plant residues and micro-climate, this variation might be due to uniformity of these factors within the buffers (Mungai et al. 2005a, b; Paudel et al. 2011). Furthermore, the soil moisture content at the time of the sampling may have some effect on the insignificant values of the parameters (Udawatta et al. 2008). However, numerically greater values of the enzymatic activities were observed within upper landscape positions (summit and shoulder) compared to the foot-slope. When considering the variation of numerical values of WSA, SOC, and N percentages across three landscape positions, greater values in the foot-slope may be caused by the displacement and deposition of organic matter with the effect of water, gravity, and other displacing forces, and high water content which leads to organic matter build up (Udawatta et al. 2008, 2009).
Distance from tree base and soil quality parameters
Within the AGF, β-glucosidase activity was not significantly different among 0-, 30-, 60-, 90- cm distances from the tree base (Fig. 3a). β-Glucosidase activity is known to be sensitive to residue management (Acosta-Martínez et al. 2003). Since the amount and the type of plant residue accumulated within the AGF do not change significantly, such variation in β-glucosidase activity cannot be expected. Similar to β-Glucosidase, glucosaminidase activity was also not significantly different among four distances from the tree base within the AGF (Fig. 3a). This can be attributed to minimal difference in soil fertility change and soil management within the AGF (Acosta-Martínez et al. 2003). Furthermore, this implies a uniform mineralization of N within these treatments irrespective of the distance from the tree base (Acosta-Martínez et al. 2003). The results of the study also imply that changes in soil within shorter distances from the trees disappear with time as the system matures.
β—Glucosidase and β—glucosaminidase activities (A), dehydrogenase and fluorescein diacetate activities (B), and soil organic C percentage (C) for different distances from the tree base within the agroforestry buffers. Bars with different letters denote significant differences among treatments at p ≤ 0.05
Within the AGF, the greatest dehydrogenase activity was observed at 90 cm (13 µg p-nitrophenol released g−1 dry soil h−1) and lowest at 0 cm (7 µg p-nitrophenol released g−1 dry soil h−1) from tree base (Fig. 3b). The FDA hydrolase activity was lowest at 0 cm (1.6 µg p-nitrophenol released g−1 dry soil h−1) and the highest at 60 cm (2.7 µg p-nitrophenol released g−1 dry soil h−1) from the tree base (Fig. 3b). This variation in activities of dehydrogenase and FDA hydrolase could be due to fluctuations in the soil microbial activity and substrate availability with response to the distance from tree base and soil landscape position (Makoi and Ndakidemi 2008).
The percentage of WSA was not significant among different distances from the tree base (Data not shown). However, highest SOC percentage was exhibited at 30 cm distance (3.1 %) while lowest was at 0 cm distance (2.1 %) from the tree base within the AGF (Fig. 3c). TN percentage was also not significant among different distance at the AGF (Data not shown). The reasons for being not significant from the tree base at AGF may be due to the uniformity of the quality and the quantity of the litter accumulated and the micro-climatic conditions caused by the long-term (1997–2008) effects of the management.
Conclusions
This study was conducted to evaluate the changes in soil enzyme activities, water stable aggregates, soil organic carbon and total N in permanent vegetative buffers, in comparison to the row crop cultivation. Most of the measured soil quality parameters were significantly higher in permanent vegetation treatments in comparison to the row crop management system. Based on the above observations, continuous disturbance has reduced the soil quality in row crop areas.
The study shows that established agroforestry, grass waterways and grass buffers improved soil quality relative to a continuous row cropping system and thus sustain ecosystems functions. These improvements may in turn help enhance water and soil quality and land productivity. Therefore, the incorporation of perennial buffers into annual cropping systems would help farmers and the landowners to keep their lands productive without compromising the food production objectives.
Further studies are needed to evaluate the spatial and temporal changes in soil physical, biological, and chemical properties according to the weather pattern and type of vegetation. The findings of those studies will be beneficial in making substantial comparisons on the soil quality changes with response to land use management practices.
References
Acosta-Martínez V, Zobeck TM, Gill TE, Kennedy AC (2003) Enzyme activities and microbial community structure in semiarid agricultural soils. Biol Fertil Soils 38:216–227
Angers DA, Mehuys GR (1993) Aggregate stability to water. In: Carter MR (ed) Soil sampling and methods of analysis. Canadian Society of Soil Science, Lewis Publishers, Boca Raton, pp 651–657
Barthès B, Albrecht A, Asseline J, De Nonic G, Roosec E (1999) Relationship between soil erodibility and topsoil aggregate stability or carbon content in a cultivated Mediterranean highland (Aveyron, France). Commun Soil Sci Plant Anal 30:1929–1938
Decker KLM, Boerner REJ, Morris S (1999) Scale dependent patterns of soil enzyme activity in a forested landscape. Can J For Res 29:232–241
Dick RP, Breakwell DP, Turco RF (1996) Soil enzyme activities and biodiversity measurements as integrative microbiological indicators. In: Doran JW, Jones AJ (eds) Methods of assessing soil quality, vol 49. SSSA Special Publication, Madison, pp 247–271
Dillaha TA III, Inamdar SP (1996) Buffer zones as sediment traps or sources. In: Haycock NE et al (eds) Buffer zones: their processes and potential in water protection. Quest Environmental, Hertfordshire, pp 7–20
Doran JW, Parkin TB (1994) Defining and assessing soil quality, vol 35. SSSA Special Publication, Madison, pp 3–3
Ekenler M, Tabatabai MA (2003) Tillage and residue management effects on β-glucosidaminase activity in soils. Soil Biol Biochem 35:871–874
Escobar GA, Mackay AD, Hodgson J, Kemp PD (2002) Soil properties of a widely spaced, planted poplar (Populus deltoides)—pasture system in a hill environment. Soil Res 40:873–886
Gregorich EG, Beare MH, McKim UF, Skjemstad JO (2006) Chemical and biological characteristics of physically uncomplexed organic matter. Soil Sci Soc Am J 70:9705–9985. doi:10.2136/sssaj2005.0116
Ingham E (2000) The vermicompost foodweb: effects on plant production. Oral scientific sessions, the vermillenium (conference), Kalamazoo
Jose S (2009) Agroforestry for ecosystem services and environmental benefits: an overview. Agrofor Syst 76:1–10
Jose S, Bardhan S (2012) Agroforestry for biomass production and carbon sequestration. Agrofor Syst 86:105–112
Karlen DL, Mausbach MJ, Doran JW, Cline RG, Harris RF, Schuman GE (1997) Soil quality: a concept, definition, and framework for evaluation (a guest editorial). Soil Sci Soc Am J 61:4–10
Kremer RJ, Kussman RD (2011) Soil quality in a pecan–kura clover alley cropping system in the Midwestern USA. Agrofor Syst 83:213–223
Kremer RJ, Li J (2003) Developing weed-suppressive soils through improved soil quality management. Soil Till Res 72:193–202
Kumar S, Anderson SH, Udawatta RP (2010) Agroforestry and grass buffer influences on macropores measured by computed tomography under grazed pasture systems. Soil Sci Soc Am J 74:203–212
Lee KH, Isenhart TM, Schultz RC (2003) Sediment and nutrient removal in an established multi-species riparian buffer. J Soil Water Conserv 58(1):1–8
Lovell ST, Sullivan WC (2006) Environmental benefits of conservation buffers in the United States: evidence, promise, and open questions. Agric Ecosyst Environ 112:249–260
Lupwayi N, Arshad M, Rice W, Clayton G (2001) Bacterial diversity in water-stable aggregates of soils under conventional and zero tillage management. Appl Soil Ecol 16:251–261. doi:10.1016/S0929-1393(00)00123-2
Makoi JH, Ndakidemi PA (2008) Selected soil enzymes: examples of their potential roles in the ecosystem. Afr J Biotechnol 7:181–191
Mankin KR, Ngandu DM, Barden CJ, Hutchinson SL, Geyer WA (2007) Grass–shrub riparian buffer removal of sediment, phosphorus, and nitrogen from simulated runoff. J Am Water Resour Assoc 43(5):1108–1116
Mungai WN, Motavalli PP, Kremer RJ, Nelson KA (2005a) Spatial variation in soil enzyme activities and microbial functional diversity in temperate alley cropping systems. Biol Fertil Soils 42:129–136
Mungai WN, Motavalli PP, Kremer RJ, Nelson KA (2005b) Spatial variation in soil enzyme activities and microbial functional diversity in temperate alley cropping systems. Biol Fertil Soils 42:129–136
Nair VD, Graetz DA (2004) Agroforestry as an approach to minimizing nutrient loss from heavily fertilized soils: the Florida experience. In: Ramachandran Nair PK, Rao MR, Buck LE (eds) New vistas in agroforestry. Springer, Berlin, pp 269–279
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: Sparks DL (ed) Methods of soil analysis-part 3, chemical methods. Soil Science Society of America Journal, Madison, pp 961–1010
Nii-Annang S, Grünewald H, Freese D, Hüttl RF, Dilly O (2009) Microbial activity, organic C accumulation and 13C abundance in soils under alley cropping systems after 9 years of recultivation of quaternary deposits. Biol Fertil Soils 45:531–538
Ogle SM, Breidt FJ, Eve MD, Paustian K (2003) Uncertainty in estimating land use and management impacts on soil organic carbon storage for US agricultural lands between 1982 and 1997. Glob Change Biol 9:1521–1542
Parham JA, Deng SP (2000) Detection, quantification, and characterization of β-glucosaminadase activity in soils. Soil Biol Biochem 32:1183–1190
Paudel BR, Udawatta RP, Anderson SH (2011) Agroforestry and grass buffer effects on soil quality parameters for grazed pasture and row-crop systems. Appl Soil Ecol 48:125–132
Paudel BR, Udawatta RP, Kremer RJ, Anderson SH (2012) Soil quality indicator responses to row crop, grazed pasture, and agroforestry buffer management. Agrofor Syst 84:311–323
Schultz RC, Isenhart TM, Colletti JP, Simpkins WW, Udawatta RP, Schultz PL (2009) Riparian and upland buffer practices. In: Garrett HE (ed) North American agroforestry: an integrated science and practice, 2nd edn. American Society of Agronomy, Madison, pp 163–213
Seobi T, Anderson SH, Udawatta RP, Gantzer CJ (2005) Influence of grass and agroforestry buffer strips on soil hydraulic properties for an Albaqualf. Soil Sci Soc Am J 69:893–901
Six J, Feller C, Denef K, Ogle SM, Sa JCM, Albrecht A (2002) Soil organic matter, biota and aggregation in temperate and tropical soils—effects of no-tillage. Agronomie 22:755–775
Six J, Frey SD, Thiet RK, Batten KM (2006) Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci Soc Am J 70:555–569
Tabatabai MA (1994) Soil enzymes. In: Weaver RW, Angle JS, Bottomley PS (eds) Methods of soil analysis, microbiological and biochemical properties. SSSA Book, Madison, pp 775–833
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418:671–677
Udawatta RP, Jose S (2012) Agroforestry strategies to sequester carbon in temperate North America. Agrofor Syst 86:225–242
Udawatta RP, Krstansky JJ, Henderson GS, Garrett HE (2002) Agroforestry practices, runoff, and nutrient loss: a paired watershed comparison. J Environ Qual 31:1214–1225
Udawatta RP, Kremer RJ, Adamson BW, Anderson SH (2008) Variations in soil aggregate stability and enzyme activities in a temperate agroforestry practice. Appl Soil Ecol 39:153–160
Udawatta RP, Kremer RJ, Garrett HE, Anderson SH (2009) Soil enzyme activities and physical properties in a watershed managed under agroforestry and row-crop system. Agric Ecosyst Environ 131:98–104
Udawatta RP, Garrett HE, Kallenbach RL (2010) Agroforestry and grass buffer effects on water quality in grazed pastures. Agrofor Syst 79:81–87
Udawatta R, Kremer R, Nelson K, Jose S, Bardhan S (2014) Soil quality indicators of a mature alley cropping agroforestry system in temperate North America. Commun Soil Sci Plant Anal 45:2539–2551
Uri ND (2000) Agriculture and the environment—the problem of soil erosion. J Sustain Agr 16:71–94
Zalidis G, Stamatiadis S, Takavakoglou V, Eskridge K, Misopolinos N (2002) Impacts of agricultural practices on soil and water quality in the Mediterranean region and proposed assessment methodology. Agric Ecosyst Environ 88:137–146
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weerasekara, C., Udawatta, R.P., Jose, S. et al. Soil quality differences in a row-crop watershed with agroforestry and grass buffers. Agroforest Syst 90, 829–838 (2016). https://doi.org/10.1007/s10457-016-9903-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-016-9903-5