Abstract
In this study we compare the richness, abundance and guild composition between two different reforestations in the meridional part of the Amazon. We test the hypothesis that the termite community is affected by the identity of the tree species used in reforestation. More precisely, we test whether the reforestation of a native species of fig (Ficus sp) is more efficient in restoring biodiversity than reforestation of exotic teak trees (Tectona grandis). We sampled the termite community in these reforested areas and three other different “control” areas: active pastures, abandoned pastures (secondary forests) and mature pristine forest. We found that the distance of reforestation from the nearest primary forest had no effect on termite biodiversity, at the scale studied. But, as expected, richness and the abundance were higher in the mature forest, intermediate in reforestation areas, and lower in secondary forest and pastures. In fact, the only studied habitat with biodiversity comparable to the mature forest was the fig plantations. The guild composition in reforested areas was also similar to that of the mature forest. The diversity and abundance of humivorous termites was particularly pronounced in the reforestation areas compared with pasture or secondary forests. The humivorous guild provides important functional services, since its action makes nitrogen and other nutrients available to the plants along ecological succession. Our results show that reforestation is a valuable strategy in restoring termite diversity and recovering the ecosystem services they provide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last decade, the reforestation of degraded areas has gained the attention of public policy aimed at restoring the ecosystem services associated with forests and biodiversity. This reforestation process cannot be restricted to the recovery of the physiognomy of vegetation. In fact, a system under restoration, in order to be effective as an ecosystem services provider, necessarily also needs to recover its biological function. The discussion about the restoration of ecosystems generally addresses complex (and sometimes confusing) terms and concepts, such as “ecosystem health” or “integrity’ (Moulton 2013) or the effects of species richness and the loss of species in the ecosystem function (Chapin et al. 2000; Hooper et al. 2005; Cadotte et al. 2011; Miyashita et al. 2014). However, the “causal relationship” between the loss of species and the ecosystem function is not really clear. In some examples in the literature, an increase in the richness of local species positively increases the complexity of the ecosystem functioning (Bihn et al. 2010), but the occurrence of several redundant species among the pool of local species does not allow the generalization of this pattern. During ecological succession in a given planted forest, the redundancy of functions, a relatively under researched phenomena, can generate scenarios where, even with high species richness, the absence of a species that executes a specific function can keep the entire ecosystem in a low productivity bias (Ehrlich and Ehrlich 1981). So, the recovery of biodiversity should be addressed not just by recovering species diversity, but by using the “Functional Diversity” approach (Diaz and Cabido 2001; Petchey and Gaston 2002; Ren et al. 2007; Aerts and Honnay 2011; Audino et al. 2014) that focuses mainly on the recovery of the resistance and resilience of several ecosystem processes.
The increase in species richness, as well as the increase in functional diversity is usually associated with a higher resource (nutrient) availability along an environmental gradient (Bihn et al. 2010) and the reduction of the probability of alien species invasion (Hooper et al. 2005). Some studies published so far focusing on termites in deforested/degraded landscapes show that this group is a good bioindicator of perturbation and restoration processes (Attignon et al. 2005; e.g. Reis and Cancello 2007; Donovan et al. 2007; Vasconcellos et al. 2010; Cunha and Orlando 2011; Bhavana et al. 2015). Ants and termites in particular are the most abundant arthropods in tropical terrestrial ecosystems (Hölldobler and Wilson 1990) and yet despite this abundance, termites have not been the subject of as many studies as, for example, the ants.
Beyond the importance of termites for soil aeration (Constantino 2005), its association with symbionts in the gut make possible the digestion of cellulose, changing the decomposition process on a regional scale (Higashi et al. 1992). Due to the variability of potential resources they can use, termites can be divided into functional guilds (Lima and Costa-Leonardo 2007). Previous studies have suggested that termites have a positive effect on soil structure and nutrient richness (Jouquet et al. 2011, 2014), especially the humivorous termites (e.g. Ji et al. 2000; Kappler et al. 2000). In fact, previous studies have found more humivorous species in more preserved ecosystems (Bignell and Eggleton 2000).
In this study we determined the effect of deforestation and the use of different tree species in reforestation sites in restoring the richness, abundance and functional composition of termites. While the first species (Ficus sp.) is rarely used in reforestation programs, the other (Tectona grandis—teak) is widely used, mainly for timber purposes. However, teak has a damaging effect on plant succession processes, such as allelopathic properties (Healey and Gara 2003) and low attraction of seed dispersers (Tewari 1992). Also, areas reforested by teak and fig species differ in habitat structure and complexity, in ways that fundamentally influence termite diversity along succession (Martius et al. 2004). So, the major goal of our study was to compare the restoration of termite fauna among two reforestation “treatments”: 1-teak monoculture (T. grandis)—employed extensively worldwide with timber harvesting purposes and; 2—the native fig reforestation (Ficus sp), used locally and with high timber production potential, with several “treatment controls”: 3-mature forest; 4—secondary forests and; 5—active pastures. Once reforestation can be seen as a process of rapid increase of the vegetation cover and complexity, affecting the landscape structure, its use will be beneficial in restoring termite fauna. Also, due to differences in the production and in the litter chemical contents between the two species, the effect of using a native fig species in reforested sites should be more effective than the use of teak in the restoration of the patterns observed in mature forests.
Methods
Study area
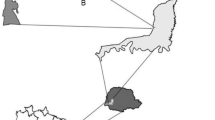
The main study site was São Nicolau Farm, runned by ONF-Peugeot, located in the municipality of Cotriguacu, Mato Grosso state, Brazil (situated between the coordinates 9°49′14.87″S 58°17′44.74″O, 9°49′22.42″S 58°12′32.31″O, 9°52′59.79″S 58°12′49.46″O e 9°52′31.16″S 58°18′0.47″O). This farm is located in the Meridional Amazon region, close to the Juruena River, with a regional climate of the AW type, according to the Köppen classification (warm and humid), and with an average annual temperature of 24 °C, 85 % humidity, and 2300 mm of precipitation (Kottek et al. 2006). This Amazonian region has two well-defined seasons: a dry season (May to October) and a rainy season (November to April) and the terrain is composed of undulations of 30–50 m high.
The São Nicolau farm has about 7000 ha of relatively undisturbed forest and 2500 ha of sites with active pastures and different reforestation planted trees. The most common species used in reforestation are Simarouba amara, Spondias sp., Ficus sp., Tabebuia sp., and teak (Tectona grandis). Reforestation on the farm had two purposes: to test the concept of Forestal carbon sink and recovery of local biodiversity. Pasture had been established across the entire farm and had been active for at least 10 years before the reforestation program was set up, which meant that the seed bank was already depleted at the moment of planting or abandonment. In reforested areas the soil was ploughed and pits were made to receive seedlings between 1999 and 2003. Only a few reforestation forests have spatial replicates and most of them consist of a mix of two or three species. Among the different reforestation stands available on the farm, we chose two because of their importance and because of the number of independent replicates available (spatially independent stands). The studied forests were:
Teak reforestation: Monodominant teak (Tectona grandis) sites with low plant diversity. The understory is extremely open and had abundant leaf litter accumulation on the ground, reaching up to 20 cm. As teak is a deciduous tree, during the wet season incidence of light on the ground is low, while in the dry season it is high. The studied teak plantation is about 10 years old and, after initial clearing in order to plant the seedlings, there has been no other management of the site.
Fig reforestation: the fig studied here were common in the region. The seeds were collected from nature in the mature forest nearby and used to obtain the seedlings. Ficus sp. is an emergent tree that can reach 40 m in natural conditions and is regionally exploited for its timber. Little is know about the natural history of this plant. Fig reforestations show a medium litter layer (c.a. 5 cm), moderate light incidence on the ground all throughout the year and high vegetal diversity in the understory. As with the teak plantation, the fig plantations are about 10 years old and, after initial clearing to plant the seedlings, no other management of the site was carried out.
Secondary forest: these are forests that naturally regenerate on pastures abandoned between 1999 and 2003. These areas were not managed, no trees were planted and the soil was ploughed at the time or after being abandoned (2000). Regeneration occurs without human interference. The understory is very closed, leading to low light incidence on the ground. Plant diversity is high, but the structure of the vegetation is extremely variable, with some areas showing a closed canopy and others without a proper canopy, but with a few trees instead.
Pasture: this is active pasture used by bovine cattle, low diversity and dominated by exotic grasses. There are few trees in this low-density area which are spread across all the sites. The pasture is further characterized by low vegetal diversity and high light incidence on the ground. As the pasture areas in this farm were not sufficient for sampling purposes, we collected additional samples in a nearby farm (Fig. 1), with the same history of deforestation and cattle ranching, but without any recovery program.
Primary forest: this comprises 7000 ha of forest embedded in a much higher matrix of continuous forest. The forest is a typical “terra firme” ombrophilous forest. The understory is relatively open, diverse and dominated by palms. The canopy varies between 30 and 50 m.
Sampling design
The field study was conducted at the beginning of the rainy season, between November and December of 2011. All samples were collected during daylight hours, between 8:00 AM to 16:00 PM. In each of the studied habitats (pastures, secondary forest, teak and fig plantations), we undertook ten replications, being five close (10 m) and five far (300 m) from the preserved forest (Fig. 1). Additionally, inside the forest we delimited five parcels, situated at 500 m from the edge. Each statistical replication consisted of a parcel 52 × 57 m, spaced at a minimum distance of 250 m between them. In each parcel we actively collected termites for one hour per person, registering the presence of termite species. We adapted the protocol proposed by Jones and Eggleton (2000), that consists of a standardized sampling effort, dividing the area of the parcel in five subparcels of 5 × 2 m, with one of the subparcels in the center and the other four at the edge. With this design, each subparcel is more than 30 m distance from one the other in order to guarantee that termites of the same nest were not sampled in different subparcels. All the specimens were determinate to the species level or classified in morphospecies at the—Museu de Zoologia da Universidade de São Paulo (MZUSP) and the samples deposited with MZUSP and in the UFMT entomological collection.
The guild classification follows Abe’s (1987) and Donovan et al.’s (2000) classification system. The respective guild for each species was determined using prior knowledge about each species, including field notes and the worker mandibles/gut anatomy. This is necessary because in some cases species level identification is impossible through taxonomic limitations (for example the Apicotermitinae), and in other cases this information is unknown. The guilds were: (1) Humivorous, termite species that feed on highly decomposed organic matter mixed with higher silicate soil; (2) xylophagous, species that feed on wood, such as trunks or bark; (3) intermediate species that feed upon decomposed wood, roots, barks and twigs in the inner soil; (4) grass/litter feeder, species that feeds upon litter, leaves and grasses in the soil surface.
Statistical analysis
As termites are social insects that live in stationary colonies, considering each individual as an occurrence could lead to misleading and erroneous parameters. In this case, we assume that each register of a species in a subparcel should belong to only one colony. The abundance of termite was considered as the number of colonies in a given parcel (max number = 5). So, we calculated the abundance of termites as the sum total of species registered in all subsamples. The richness was considered as the number of species found in the whole parcel. In order to compare (1) the number of species collected per parcel (richness) and; (2) the abundance of collected termites, we used two analyses of variance (ANOVA two way). In these analyses, habitat and distance from the edge of the mature forest (close or far) were employed as factors. We conducted a posteriori Tukey test in order to determine which groups in the sample differed from one another.
To determine the difference of the guild composition among habitats and the influence of the distance to the edge of the closest mature forest, we used two distinct MANOVA (multivariate analysis of variance). In the first MANOVA we compared the number of species found in the four termite guilds as the response variable, while in the other we employed the abundance of species in each guild. All the statistical analysis was done on Systat (Wilkinson 2010).
Results
We collected 50 termite species (Table 1). The most representative subfamily was Apicotermitinae (23 spp). The most frequent species were Heterotermes tenuis (Hagen, 1858), Cornitermes bequaerti Emerson, 1952, Nasutitermes ephratae (Holmgren, 1910), Dentispicotermes sp. and Nasutitermes corniger (Motschulsky, 1855). The majority of the collected species are humivorous (25 species), followed by Xylophogous (12 spp), grass/litter feeders (7 spp) and intermediates (6 spp).
The fig forest was the habitat with the most number of species (31 spp.), followed by teak reforestation forests (24 spp.), active pastures (22 spp.) and the secondary forests (18 spp.). We found 26 spp. in the mature forest, but in just half of the samples collected in other sites. We did not observe the effect of the distance from the mature forest in the restoration of termite biodiversity in any studied parameter [richness, abundance or guild composition (P > 0.5 in all cases)], and this is therefore omitted in subsequent results.
The abundance of termites in the parcels was different among the studied habitats (ANOVA: F = 5.42, P = 0.001). The secondary forest showed a lower mean abundance among all studied habitats, but very close to the pastures (Fig. 2a). We did not observe differences in the abundance of termites between the reforestation and mature forest (Fig. 2a). However, we observed differences in the total number of species per parcel (richness) among the habitats (ANOVA: F = 9, 15, P < 0.001). The richness did not differ between secondary forests and pastures, but in both we collected less species than in the reforestation forests and the mature forests (Fig. 2b). The number of species collected per parcel was higher in mature forests, but did not differ from the number found in the fig forests.
The mean (+SD) of the total number of colonies found in all summed the subparcels (Abundance) (a) and number (+SD) of species per parcel (Richness) of termites (b) in pastures, Teak and fig reforestations, and secondary and primary forests of the Nicolau farm, Cotriguaçu-MT, Brazil. The different letters above the bars represent statistical difference using Tukey´s pos hoc test
The guild composition using the presence-absence data (=richness of species in each guild) was different among the studied habitats (MANOVA: Pillay trace’s P = 0.001), but not for all termite guilds. In posteriori tests, we observed differences in the guild of humivorous (MANOVA: F = 7.288, P = 0.001), intermediate (MANOVA: F = 3.180, P = 0.023) and in the grass/litter feeders (MANOVA: F = 2.692, P = 0.045). In the case of the quantitative composition (abundance of colonies in the guild), we also observed differences among habitats (MANOVA: Pillay trace’s P = 0.001). The difference was restricted to the humivorous termites (MANOVA: F = 7.741, P = 0.001) and intermediate (F = 3.162, P = 0.024). The grass/litter, although not found in secondary and mature forests, is naturally occasional, and did not differ among habitats (F = 2.208, P = 0.085) (Fig. 3b).
Discussion
As expected, the samples from degraded areas, active pastures or sites abandoned to natural succession, presented lower relative richness and abundance than those from mature forests, a pattern also found in other studies (Carrijo et al. 2009; Cunha and Orlando 2011). These results indicate that the termite community is affected by the degradation of the habitat. However, abundance did not differ in undisturbed forest and in both the reforestation treatments, indicating a recovery of the fauna in such environments. So, a treatment where abandoned pastures are being managed, as with the reforestation of the pasture by tree species, can help the termite community’s recovery and, thus, the recover of the functionality of these areas. The reforestation of a given site is basically a faster recomposition of a forest structure than the secondary forest growing in an exotic grass matrix. The small trees undergo rapid growth initially, providing some shade and a leaf litter layer in the soil, recomposing the structure of the habitat. Also, some studies have already demonstrated an increase in termite diversity along succession (Bihn et al. 2010; Cunha and Orlando 2011). In this study we employed just half of the sample effort in mature forests than in any other studied habitat and the fact that the total number of species was even higher in fig reforestations does not indicate a total recovery of diversity in such treatments. But, in this paper we show that the number of species per sample (or the density of species) is higher in mature forests but not different to that found in the fig reforestations, suggesting that this treatment is better for restoring the local richness of termites.
The major difference between the mature forest and the pastures and both secondary forests treatments, is the richness and abundance of humivorous termites. The abundance of humivorous in the agroecosystems is promising in terms of the capacity of agroforests to support soil fertility restoration on degraded pasture lands (Ackerman et al. 2009). The observed restoration of the humivorous on reforested sites is of considerable interest to plant succession, since the humivorous termites can make essential nutrients, particularly nitrogen, available to the plants (e.g. Ji et al. 2000). So, strategies to restore habitats can facilitate the colonization and persistence of humivorous termite populations, which in turn can increase the whole succession process by making the soil more fertile for other tree species. In the teak reforestation sites, the abundance of humivorous species was disproportionately high, being higher even than in the undisturbed forests. This can be explained by the high leaf litter production and because teak is a deciduous species and as such, loses all its leaves during dry periods. This feature, not uncommon among Amazonian trees, is not common where several deciduous trees exist in a single site. In any case, the monodominance of a single deciduous species generates large amounts of litter on the soil, being even more abundant than that found in the mature forest in the study area. The relation with the soil litter layer suggests that humivorous termite colonies are restricted by resources and should be the focus of future studies.
Surprisingly, results in guilds other than the humivorous termites are conflicting. No differences were found in the xylophagous present in the different habitats. A particularity of the study area is that the farmers, after deforestation, did not remove the dead trunks. Instead, they piled the trunks in the area, at regular intervals. Thus, a dead wood shortage is unlikely as a limiting factor to the xylophagous. On the other hand, the intermediate termites show a different pattern to that of all other guilds. Locally, diversity and abundance among intermediate termites was moderate to low, even in the mature forest, when compared with the other guilds collected. We found a higher diversity and abundance in the mature and secondary forests, but a lower diversity in all other studied habitats. A possible explanation is that intermediate termites feed on highly decomposed organic matter in the ground and, as part of the reforestation process, the soil was ploughed up and turned in order to plant the seedlings. Ploughing decreases the variability of the soil profile, decreasing the content of organic matter by favoring lixiviation and fast decomposition (e.g. Balesdent et al. 2000; Lal 2015). So, since intermediate species feed on the organic matter in the soil, ploughing would limit the resources available. Lower abundance and diversity of intermediate termites was also observed in pastures, the habitat with the lowest floristic diversity, no litter and with the highest light incidence in the soil. The microclimatic variance, observed also in the reforested sites, can limit the colonization of well lit sites by intermediate termites (Carrijo et al. 2009).
Finally, our results show that actively reforested areas can provide benefits to the recovery of soil termites, particularly in fig plantations. After 10 years, the fig reforestation was not only more abundant but had recovered a similar richness per area found today in mature forests nearby. The most abundant guild found in reforested areas were the humivorous, a group functionally important to the succession and restoration of soil function, and responsible for making nitrogen available to plants. A higher abundance and diversity of humivorous termites, as found in reforested sites, would make the plant restoration process faster, increasing restoration towards a site with a structure and function closer to that of a mature forest. Though not the focus of this study, we suggest that future studies focus the study of restoration on multispecific reforestation programs, since they can encourage other guilds to colonize and persist in reforested sites.
References
Abe T (1987) Evolution of life types in termites. Kawano S, Connel JH e Hidaka T (1987) Evolution and Coadaptation in Biotic Communities. University of Tokyo Press, Tokyo, pp 126–148
Ackerman IL, Constantino R, Gauch HG, Lehmann J, Riha SJ, Fernandes ECM (2009) Termite (Insecta : Isoptera) Species composition in a primary rain forest and agroforests in Central Amazonia. Biotropica 41:226–233
Aerts R, Honnay O (2011) Forest restoration, biodiversity and ecosystem functioning. BMC Ecol 11:29
Attignon SE, Lachat T, Sinsin B, Nagel P, Peveling R (2005) Termite assemblages in a West-African semi-deciduous forest and teak plantations. Agric Ecosyst Environ 110:318–326
Audino LD, Louzada J, Comita L (2014) Dung beetles as indicators of tropical forest restoration success: Is it possible to recover species and functional diversity? Biol Conserv 169:248–257
Balesdent J, Chenu C, Balabane B (2000) Relationship of soil organic matter dynamics to physical protection and tillage. Soil Till Res 53:215–230
Bhavana KV, Poovoli A, Rajmohana K (2015) A comparison on termite assemblages in coffee & teak plantations and semi-evergreen forest—a case study in North Wayanad, Kerala, India. Trop Agric Res 26(3):456–467
Bignell DE, Eggleton P (2000) Termites in ecosystems. In: Abe T, Bignell DE, Higashi M (eds) Termites: evolution, sociality, symbioses, ecology. Kluwer Academic Publishers, Dordrecht, pp 363–387
Bihn JH, Gebauer G, Brandl R (2010) Loss of functional diversity of ant assemblages in secondary tropical forests. Ecology 91:782–792
Cadotte MW, Carscaddem K, Mirotchnick N (2011) Beyond Species: functional diversity and the maintenance of ecological processes and services. J Appl Ecol 48:1079–1087
Carrijo TF, Brandão D, Oliveira DE, Costa DA, Santos T (2009) Effects of pasture implantation on the termite (Isoptera) fauna in the central Brasillian Savanna (Cerrado). J Insect Conserv 13:575–581
Chapin FS, Zavaleta ES et al (2000) Consequences of changing biodiversity. Nature 405:234–242
Constantino R (2005) Padrões de diversidade e endemismo de térmitas no bioma Cerrado. In: Scariot et al. (eds) Biodiversidade, ecologia e conservação do Cerrado. Ministério do Meio Ambiente, Brasília, pp 319–333
Cunha EF, Orlando TYS (2011) Functional composition of termite species in areas of abandonated pasture and in secondary sucession of the Parque Estadual Altamiro de Moura Pacheco, Goiás, Brasil. Biosci J 27:986–992
Diaz S, Cabido M (2001) Vive la difference: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655
Donovan SE, Jones DT, Sands W, Eggleton P (2000) Morphological phylogenetics of termites (Isoptera). Biol J Linn Soc 70:467–513
Donovan SE, Griffiths GJK, Homathevi R, Winder L (2007) The spatial pattern of soil-dwelling termites in primary and logged forest in Sabah, Malaysia. Ecol Entomol 32:1–10
Ehrlich PR, Ehrlich AH (1981) Extinction: the causes and consequences of the disappearance of species. Random House, New York
Healey SP, Gara SI (2003) The effect of a teak (Tectona grandis) plantation on the establishment of native species in an abandoned pasture in Costa Rica. For Ecol Manag 176(1–3):497–507
Higashi M, Abe T, Burns TP (1992) Carbon–nitrogen balance and termite ecology. Proc R Soc Lond B Biol Sci 249:303–308
Hölldobler B and Wilson EO (1990) The Ants. Cambridge, USA
Hooper DU, Chapin FS et al (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35
Ji J, Kappler A, Brune A (2000) Transformation and mineralization of synthetic 14C-labeled humic model compounds by soil-feeding termites. Soil Biol Biochem 32:1281–1291
Jones DT, Eggleton P (2000) Sampling termite assemblages in tropical forests: testing a rapid biodiversity assessment protocol. J Appl Ecol 37:191–203
Jouquet P, Traoré S, Choosai C, Hartmann C, Bignell D (2011) Influence of termites on ecosystem functioning. Ecosystem services provided by termites. Eur J Soil Biol 47:215–222
Jouquet P, Blanchart E, Capowiezc Y (2014) Utilization of earthworms and termites for the restoration of ecosystem functioning. Appl Soil Ecol 73:34–40
Kappler A, Ji R, Brune A (2000) Synthesis and characterization of specifically 14C-labeled humic model compounds for feeding trials with soil-feeding termites. Soil Biol Biochem 32:1271–1280
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006) World map of the Köppen-Geiger climate classification updated. Meteorol Zeit 15:259–263
Lal R (2015) Restoring soil quality to mitigate soil degradation. Sustainability 7:5875–5895
Lima JT, Costa-Leonardo AM (2007) Recursos alimentares explorados pelos cupins (Insecta: Isoptera). Biota Neotr 7:243–250
Martius C, Höfer H, Garcia MVB, Römbke J, Förster B, Hanagarth W (2004) Microclimate in agroforestry systems in central Amazonia: does canopy closure matter to soil organisms? Agrofor Syst 60:291–304
Miyashita T, Amano T, Yamakita T (2014) Effects of ecosystem diversity on species richness and ecosystem functioning and services: a general conceptualization. In: Nakano S, Yahara T, Nakashizuka T (eds) Integrative observations and assessments. Springer, Tokyo, pp 29–47
Moulton TP (2013) Funcionamento de ecossistema e a interface com a comunidade ecológica In: Nogueira FCB et al (eds) A Teoria ecológica: perspectivas e avanços futuros nos últimos dez anos de pesquisa no Brasil. Fortaleza-CE, pp 77–93
Petchey OL, Gaston KJ (2002) Functional diversity (FD), species richness and community composition. Ecol Lett 5:402–411
Reis YT, Cancello EM (2007) Riqueza de cupins (Insecta, Isoptera) em áreas de Mata Atlântica primária e secundária do sudeste da Bahia. Inheringia Ser Zool 97:229–234
Ren H, Li Z, Shen W, Yu Z, Peng S, Liao C, Ding M, Wu J (2007) Changes in biodiversity and ecosystem function during the restoration of a tropical forest in south China. Sci China Ser C 50(2):277–284
Tewari DN (1992) A monograph on bamboo. International Book Distributors, India
Vasconcellos A, Bandeira AG, Moura FMS, Araújo VFP, Gusmão MAB, Constantino R (2010) Termite assemblages in three habitats under different disturbance regimes in the semi-arida Caatinga of NE Brazil. J Arid Environ 74:298–302
Wilkinson L (2010) SYSTAT. WIREs. Comp Stat 2:256–257
Acknowledgments
We are grateful to Victor Landeiro and Jerry M Penha for their helpful comments in early versions of this manuscript. We thank São Nicolau farm administrators and staff and the Carbon sink project (Pegeout propriety, managed by ONF—Office National des Fôrets) for their logistical and financial support. RMP and TJI are grateful to CAPES—Coordenação de aperfeiçoamento de Pessoal de Nível Superior (TJI proccess BEX 2548/14-3 and 479243/2012-3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Paula, R.C., de Moraes Lima Silveira, R., da Rocha, M.M. et al. The restoration of termite diversity in different reforestated forests. Agroforest Syst 90, 395–404 (2016). https://doi.org/10.1007/s10457-015-9862-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-015-9862-2