Abstract
Agroforestry practice is believed to be an effective means of maintaining and improving soil fertility, and is widely used by farmers around the world. To gain better understanding of the effects of agroforestry practice on soil fertility, the organic carbon content, total nitrogen content, microbial biomass, basal respiration, and activity of soil enzymes at three soil depths (0–10, 10–20, and 20–30 cm) of Ginkgo (Ginkgo biloba L.)–tea (Camellia sinensis (L.) O. Kuntze) agroforestry systems were investigated. Study plots were established in Yushan Farm in Changshu, Jiangsu Province, China. These involved two densities of Ginkgo trees mixed with tea (G1 and G2) and monoculture tea systems (G0). The results showed that C, N, microbial biomass, and enzyme activity were higher in surface soil than in soil from the middle and lower layers whereas pH and metabolic quotient increased with soil depth. pH, microbial biomass C, N, basal respiration, and catalase and invertase activity in the 0–10 cm layer were significantly lower for G0 than for G1 and G2. Polyphenoloxidase activity in the 0–10 cm layer was significantly lower for G2 than for G0 and G1. Metabolic quotient in the 20–30 cm layer was significantly higher for G0 than for G2. The activity of soil enzymes, including catalase, dehydrogenase, urease, protease, and invertase, significantly and positively correlated with soil organic carbon and total nitrogen. The results of this study suggest that growing tea with Ginkgo could be regarded as good agroforestry practice which could enhance accumulation of organic matter in soil, improve the activity of soil enzymes, and maintain soil productivity and sustainability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Use of agroforestry to enhance and maintain long-term soil productivity and sustainability has been well documented. In agroforestry systems, incorporation of trees into farmland can enhance soil chemical and biological properties by adding significant amounts of above and below-ground organic matter and releasing and recycling nutrients (Jose 2009). It is generally agreed that agroforestry systems are more suitable than monoculture for maintenance of soil fertility (Wang and Cao 2011). In southern Jiangsu Province, there is widespread interest in developing Ginkgo (Ginkgo biloba L.)–tea (Camellia sinensis (L.) O. Kuntze) agroforestry systems which are less dependent on external inputs, especially fertilizers, to reduce effects on the environment and to improve soils.
Agroforestry practices are believed to improve soil fertility. It can be speculated that the benefit is also associated with soil microbial properties and enzyme activity. The combination of soil microbial biomass and enzyme activity measurements have been widely used over the last 10 years to study microbiological responses to agriculture management (Wick et al. 2000; Clegg 2006; Udawatta et al. 2009). Differences in litter quality between trees and intercropped vegetation can lead to different enzyme activity and microbial functional diversity compared with rows of trees (Nancy and Peter 2005). Studies show that soil microbial biomass and enzyme activity are greater in agroforestry systems because of different in litter quality and quantity, and root exudates (Myers et al. 2001; Mungai et al. 2005; Udawatta et al. 2009). Previous research suggests that relationships between organic matter, microbial activity, and microbial biomass are good indicators of soil quality (Anderson and Domsch 1990). Although there have been extensive studies on soil microorganisms and soil enzymes (Lizarazo et al. 2005; Mungai et al. 2005; Udawatta et al. 2009; Balota and Chaves 2010; Tian et al. 2010), little has been reported of the effects on these of Ginkgo–tea agroforestry.
Tea is an important economic crop widely planted on acid red soils in the tropical and subtropical regions of China (Xue et al. 2006). In agriculture, tea is managed as a shrub; the leaves are harvested annually, to make tea, and the top twigs and stems are pruned annually to stimulate growth of new shoots. Tea is an evergreen crop and tolerates partial shade, and intercropping with tall trees can improve the quantity and quality of the tea (Costa and Surenthran 2005). Gingko is a traditional deciduous economic tree species in China, usually cultivated in agroforestry systems. The practice of Ginkgo agroforestry has been adopted to improve economic benefits during the initial stages of establishment (Wang and Cao 2011).
Understanding and maintaining soil fertility have become increasingly important issues for researchers, with the objective of resource management (Paudel et al. 2011). The purpose of this research was to compare the effects on soil organic carbon, total nitrogen, microbial biomass, basal respiration, and enzyme activity of Ginkgo–tea agroforestry and tea monoculture.
Materials and methods
Study site and soil sampling
The study was conducted in Sanfeng Farm (31° 40′ N, 120° 42′ E), located at the foot of the Yu Mountain in Changshu, Jiangsu Province, China. The area was characterized by a subtropical wet monsoon climate with mean annual temperatures of 15 °C and mean annual rainfall of approximately 1,500 mm, most of the rain falling between April and August.
In 1990 we established three systems for this study: (1) pure tea plantation in rows of hedges 1.5 m wide and 1 m high (G0); (2) tea intercropped with grafted Ginkgo seedlings at a spacing of 10 m × 10 m (G1); and (3) tea intercropped with grafted Ginkgo seedlings at a spacing of 6 m × 6 m (G2). No inorganic nutrient amendments were applied, but herbicide and glyphosate were occasionally used to control vegetation competition.
The experimental plots (3 × 3) were completely randomized in the field with three spatially separated replicates of each treatment. In May 2011, soil samples from 0–10, 10–20, and 20–30 cm depths were collected with a soil auger from 12 randomly selected locations within each plot, and one composite sample from each soil depth was taken for analysis. One half of each soil sample was air-dried, ground, and passed through a 2 mm mesh sieve for chemical analysis; the other half was maintained field moist and stored at 4 °C for analysis of soil enzyme and microbiological properties.
Soil analysis
Soil pH was determined for a soil-to-water ratio of 1:2.5 by use of a combination glass electrode (Xue et al. 2006). Soil organic C was measured by oxidation with potassium dichromate. Total nitrogen was extracted with perchloric–concentrated sulfuric acid and determined by Kjeldahl digestion (Wang and Cao 2011).
Soil microbial biomass C and total N were determined by the chloroform fumigation extraction method with an extraction coefficient of 0.45 and 0.54 for biomass C and total N, respectively (Brookes et al. 1985; Vance et al. 1987). The K2SO4-extracted C of both fumigated and unfumigated samples was analyzed by use of a total organic C analyzer (Elementar Liqui TOC, Germany). Basal respiration was determined by measuring CO2 evolution. Field-moist soil (20 g; oven-dry basis) was incubated in 250 ml airtight glass vessels at 25 °C for 1 day. Vials containing 10 ml 0.1 M NaOH were placed inside the flasks and the CO2 evolved was determined by titration of carbonates with 0.1 M HCl (Hernández et al. 1997; Xue et al. 2006). Microbial quotient was defined as the ratio of microbial biomass C to soil organic C (Xue et al. 2006). The metabolic quotient was defined as the ratio of basal respiration to microbial biomass, i.e., the amount of CO2–C produced per unit of microbial biomass carbon.
Catalase activity was measured by incubating 5 g soil with 5 ml 0.3 % H2O2 for 30 min at 30 °C. The suspension was titrated with 0.1 mol L−1 KMnO4 solution. Activity was expressed as 0.1 mol L−1 KMnO4 ml g−1 soil 30 min−1 (Zhou et al. 2011). Polyphenoloxidase activity was determined as described by Peruccia et al. (2000), and expressed as purpurogallin mg g−1 soil 2 h−1. Dehydrogenase enzyme activity was determined as described by Udawatta et al. (2008). Moist soil samples (6 g) were used in this analysis with two replicates. Soil was incubated with 2,3,5-triphenyltetrazolium chloride at 37 °C for 24 h. After incubation, a previously created regression equation was used to determine the concentration of the triphenylformazan (TPF) product colorimetrically (485 nm) and enzyme activity was expressed in mg TPF released g−1 dry soil h−1. Urease activity was determined as described by Xue et al. (2006); the product, NH4 +, was measured colorimetrically by use of the indophenol blue method (Keeney and Nelson 1982). Sucrase activity was determined with sucrose as substrate; reducing sugars were analyzed as described by Schinner and von Mersi (1990). Protease activity was assayed as described by Hamm and Feger (1996) with calibration by use of tyrosine standards.
Statistical analysis
Results obtained from measurement of soil properties at different soil depths and for different agricultural systems were analyzed by one-way ANOVA. Least significant difference tests (Duncan’s LSD) were used for pair-wise comparison of treatment means. Differences were declared significant at the 5 % level of significance (P < 0.05). Pearson correlation analysis was used to determine the significance of relationships between soil organic C, total N, and soil enzyme activity. Statistical analysis was performed by use of SPSS 16.0 software (SPSS Institute 2008).
Results
Soil chemical properties
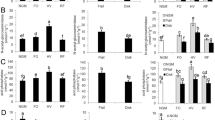
Soil samples from the three agricultural systems had different chemical properties. As shown in Table 1, pH in the 0–10 cm layer was significantly lower for G0 than for G1 and G2 (P = 0.0001); pH increased with soil depths for all three systems. Organic C was different among the three systems. Organic C in the 0–10 cm layer was significantly higher for G2 than for G0 and G1 (Table 1, P = 0.018); in the 10–20 and 20–30 cm layers, organic C was significantly lower for G0 than for G1 and G2 (P = 0.0211). Total N in each soil layer was not significantly different among the three systems. The order of the differences for each soil layer was G2 > G1 > G0. Accumulation of organic C and total N decreased with soil depth (Table 1).
Microbial biomass C and N and microbial quotient
Soil microbial biomass C (Cmic) was lower in the monoculture tea system than in G1 and G2, irrespective of soil layer, and there were significant differences among the three systems in the 0–10 cm layer (P = 0.0002). No significant differences were detected between G1 and G2 in the 10–20 and 20–30 cm layers (Table 2). Microbial biomass N (Nmic) in the 0–10 cm layer was significantly higher in G1 and G2 than in G0 (P = 0.0002) whereas in the 10–20 and 20–30 cm layers Nmic in G2 was significantly higher than in G0 and G1 (P = 0.0021) (Table 2).
Microbial quotient, i.e. the ratio of microbial biomass C to soil organic C (Cmic/Corg), was not significantly different among the three systems in each layer. Microbial quotient in the 0–10 and 10–20 cm layers was highest for G1 whereas in the 20–30 cm layer microbial quotient in G1 was lowest among the three systems (Table 2). Similar to organic C and total N, microbial biomass C and N and microbial quotient decreased with soil depth (Table 2).
Basal respiration and metabolic quotient
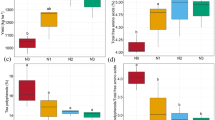
Basal respiration (Rmic) for the three agricultural systems decreased with soil depth (Fig. 1a). Planting system affected basal respiration significantly (P = 0.0001). Significant differences were observed only in the 10–20 cm layer for G1 and G2 (Fig. 1a).
Basal respiration (Rmic; a) and metabolic quotient (qCO2; b) measured at three soil depths for monoculture tea (G0), Ginkgo–tea agroforestry with low Ginkgo density (10 m × 10 m; G1), and Ginkgo–tea agroforestry with high Ginkgo density (6 m × 6 m; G2) in Changshu, Jiangsu Province, China. Different letters indicate significant differences among planting systems at the same soil depth (P < 0.05). Bars represent standard errors
In contrast with basal respiration, metabolic quotient (qCO2) decreased with soil depth. There were no significant differences in the 0–10 and 10–20 cm layers among the three systems. In 20–30 cm layer, however, metabolic quotient for G0 was significantly higher than that for G2 (P = 0.0197) (Fig. 1b).
Enzyme activity
Activity of the six enzymes decreased with soil depth (Fig. 2). Catalase activity in the 0–10 cm layer was significantly higher for G1 and G2 than for G0 (P = 0.0004). In the 10–20 cm layer catalase activity for G1 was 1.1 and 1.13 times that for G0 and G2, respectively. There were no significant differences in the 20–30 cm layer (Fig. 2a). Polyphenoloxidase activity varied significantly among the three systems in the 0–10 cm layer; the order was G1 > G0 > G2. In the 10–20 and 20–30 cm layers, polyphenoloxidase activity was highest for G1, and there was no difference between G0 and G2 (Fig. 2b). In the 0–10 cm layer, dehydrogenase activity was significantly higher for G2 than for G0, with no significant differences between G0 and G1. Dehydrogenase activity was not significantly different in the 10–20 and 20–30 cm layers among the three systems (Fig. 2c).
Activity of catalase (a), polyphenoloxidase (b), dehydrogenase (c), urease (d), protease (e), and invertase (f) in soil samples from different depths for monoculture tea (G0), Ginkgo–tea agroforestry with low Ginkgo density (10 m × 10 m; G1), and Ginkgo–tea agroforestry with high Ginkgo density (6 m × 6 m; G2) in Changshu, Jiangsu Province, China. Different letters indicate significant differences among planting systems for the same soil depth (P < 0.05). Bars represented standard errors
Urease activity varied significantly among the three systems in the 0–10 and 10–20 cm layers; the order was G2 > G1 > G0. Differences in the 20–30 cm layers among the three systems were not significant (Fig. 2d). In the 0–10 cm layers protease activity was highest for G2, 1.56 and 1.40 times higher than for G0 and G1, respectively. There were no significant differences in the 10–20 and 20–30 cm layers (Fig. 2e). Invertase activity in the 0–10 cm layer was significantly higher for G1 and G2 than in G0 (P = 0.0021). In the 10–20 cm layers invertase activity was lower for G1 than for G0 and G2. Similar to protease activity, no significant differences were observed for invertase activity in the 10–20 and 20–30 cm layers (Fig. 2f).
Correlations between soil properties and soil enzyme activity
Catalase, dehydrogenase, urease, protease, and invertase activity were significantly correlated with organic C and total N content. Correlation between polyphenoloxidase activity and organic C and total N content was not significant (Table 3).
Discussion
Chemical properties
Agroforestry systems are known to maintain soil organic matter and promote nutrient cycling (Wang and Cao 2011). This study found that soil organic C in Ginkgo–tea agroforestry systems was significantly higher than that in a monoculture tea system. Build-up of soil organic matter and nutrient turnover were affected by input of Ginkgo litter fall. Soil organic matter contained basic cations and was the source of energy for decomposers, which contributed to increasing the supply of nutrients, for example N and K, in soil (Budiadi et al. 2006). The higher organic carbon content of the upper layer in agroforestry systems could because of higher inputs of organic residues from Ginkgo litter fall.
Long-term root exudates and leaf litter can result in a decline of pH and accumulation of Al in soil (Nioh et al. 1993; Pandey and Palni 1996). In this study, soil pH was significantly higher for Ginkgo–tea agroforestry than for tea monoculture, which suggested that Ginkgo–tea agroforestry could improve soil pH, prevent excessive soil acidification, and sustain soil productivity. This may be because of the alkaline matter formed during decomposition of Ginkgo litter.
Microbiological properties
Soil microorganisms, which are important in nutrient cycling and energy flow, are extremely sensitive to environmental changes (Berg et al. 1998). The accumulation of soil organic C enhanced both microbial biomass C and the ratio of microbial biomass C to soil organic C (Xue et al. 2006). Fernandes et al. (2005) found that after four consecutive corn crops in a dark red dystroferric latosol under field conditions in the tropical region in Brazil the C biomass values ranged from 99 to 809 mg kg−1 soil in the top 10 cm and from 71 to 577 mg kg−1 in the 10–20 cm layer, whereas the N biomass values ranged from 11 to 101 mg kg−1 in the 0–10 cm layer and from 8 to 84 mg kg−1 in the 10–20 cm layer. These data indicate that C and N in the microbial biomass were concentrated in the first 10 cm of the soil (Fernandes et al. 2005). Our results provided additional evidence that soil microbial biomass N decreased gradually with soil depth. Different soil microorganisms in the upper layer among different systems could be a result of a combination of a variety of factors, for example root biomass and turnover, lignin content of crop residues, root and litter fall, and the microclimatic environment of the community (Wang et al. 2005). In this study, all tea branches were pruned annually; the branches removed decomposed less readily than Ginkgo leaves. The rapid decomposition of Ginkgo litter fall provided more organic matter for survival of soil microorganisms, eventually resulting in higher soil microbial biomass C and N in agroforestry systems than in the monoculture tea system.
Microbial quotient had been used as an indicator of efficiency of conversion of organic C into microbial C and losses of soil C during decomposition. It has also been proposed for use as a soil-quality indicator to enable comparison of soils with different organic matter content (Balota et al. 2003). If a soil is being degraded, the microbial C pools will decline more rapidly than the organic C, thus microbial quotient will also decrease (Balota et al. 2003). Microbial quotient was between 1.12 and 1.71 % in the areas under study. These percentages were in agreement with the results obtained by Wardle (1992), and Anderson and Domsch (1990). In this study, microbial quotient was not significantly different among these systems. The reasons may be that, on the one hand, faster decomposition of litter fall and greater return of organic matter in agroforestry systems resulted in accumulation of inert organic matter. Even total organic C was significantly higher for G2 than for the others; the efficiency of conversion of organic C into microbial C may not be so high. On the other hand, the microbes present in acid soil metabolize very slowly, resulting in extraordinarily low capacity for mineralization of organic C (Xue et al. 2006). Because the three systems were all acid soils the results would be different from normal.
Soil basal respiration is widely used as a measure of microbiological activity and as a sensitive indicator of microbial activity (Wang et al. 2005; Xue et al. 2006). In this study, basal respiration was significantly greater for agroforestry systems than for the monoculture tea system. A higher rate of basal respiration may be because of the existence of a large pool of labile C substrates (Islam and Weil 2000). Metabolic quotient is defined as respiration per unit of microbial biomass, expressed as mg CO2–C h−1 g−1 biomass-C; low values indicate stable and mature systems, because energy optimization occurs as the systems mature (Wang et al. 2005). Our results showed there were no significant differences among the three systems in the 0–10 and 10–20 cm layers, but that metabolic quotient in the 20–30 cm layer was significantly higher for G0 than for G2. Low soil basal respiration could result in highly efficient metabolism and ample available organic C, eventually maintaining soil fertility and sustainability.
Enzyme activity
Soil enzyme activity is critically important for soil quality and could be indicative of changes in metabolic capacity and nutrient cycling because of management practices (Zhou et al. 2011). Enzyme activity is significantly different in soils supporting different types of vegetation (Michel and Matzner 2003). This significant variation of enzyme activity suggests there is a significant difference in functional microbial diversity, because these enzymes are involved in carbon and nitrogen cycling and decomposition of organic matter (Paudel et al. 2011). Numerous studies have reported significantly higher activity of these enzymes in intercropping systems compared with monocultures (Wang et al. 2005; Wang and Cao 2011; Zhou et al. 2011). In our study, significantly higher enzyme activity was observed for Ginkgo–tea agroforestry than for tea monoculture. These enzymes are of crucial importance to biochemical functions in the overall process of material and energy conversion in the soil ecosystem. Better understanding of these six enzymes could lead to more effective ways of elucidating the action of agroforestry systems in improving soil fertility.
Among the oxidoreductase enzyme systems, soil catalase can also be used as a measure of biological activity. This is possible because soil catalase, although acting mainly as an intracellular enzyme, remains active outside microbial cells because of an association with soil organic matter and/or sorption on clay minerals (Perucci et al. 1997). Continuous monoculture can be detrimental to soil enzyme activity, for example, catalase activity decreased significantly in soil used for a continuous cucumber monoculture (Zhou et al. 2011). Our study found that catalase activity was significantly higher in agroforestry systems than in a monoculture tea system, which confirmed results from previous studies. Polyphenoloxidase is an important oxidase in cycling of aromatic compounds and is of crucial importance in the formation of soil organic matter (Gu et al. 2009). It was found that polyphenoloxidase activity for G2 was significantly lower than for the others; this disagrees with results from studies showing greater activity for agroforestry systems (Wang and Cao 2011; Zhou et al. 2011). Zhou (1987) found that polyphenoloxidase activity was negatively related to soil humification, however. It could be hypothesized that humification was highest for the soil in G2. Dehydrogenase activity is regarded as indicative of the total range of oxidative activity of soil microflora and, consequently, may be assumed to be a good indicator of microbiological activity (Perucci et al. 1997). In our study, dehydrogenase activity was higher for G2 than for the monoculture tea system.
Urease catalyzes hydrolysis of urea to CO2 and NH3, a vital process in the regulation of N supply to plants after urea fertilization (Balota and Chaves 2010). The results of this study were therefore consistent with other studies reporting that urease activity was significantly affected by different soil-management systems (Klose and Tabatabai 2000; Longo and de Melo 2005). Understanding of urease activity dynamics could reveal more effective ways of managing N fertilizers (Balota and Chaves 2010). Reductions in urease activity for G0 might negatively affect tea growth and yield. Protease and urease are involved in the N cycle (Wang et al. 2005; Xue et al. 2006). Hydrolysis of proteins in the soil mainly depends on protease; its hydrolysis products are a source of nitrogen for higher plants (Zhou 1987). Protease in G2 was significantly higher than in the other systems, showing that G2 systems might have a higher N-mineralization activity. Soil invertase catalyzes hydrolysis of sucrose to glucose and fructose, and is linked with soil microbial biomass (Gu et al. 2009). Invertase activity affects accumulation of soil organic carbon and its transformation by decomposition. Invertase activity was enhanced in Ginkgo–tea agroforestry systems, leading to accumulation of more organic matter in the soil.
Relationships between soil properties and soil enzyme activity
In this study, enzyme activity was closely related to the distribution of soil carbon and nitrogen among the systems. Organic C and total N content were highly correlated with catalase, dehydrogenase, urease, protease, and invertase activity. The greater correlations between enzyme activity and organic carbon were consistent with previously published results (Myers et al. 2001; Kremer and Li 2003; Mungai et al. 2005; Udawatta et al. 2008). Increased enzyme activity was because of increased organic matter and litter quality and quantity and improvement of the physical properties of the soil. Increased enzyme activity is proportionally linked to microbial function, leading to improved nutrient cycling and availability, which favors root growth, promotes beneficial plant-microbial interactions, and eventually increases the total carbon pool of soil (Udawatta et al. 2009).
Depth effects
Soil organic C, total N, microbial biomass, and enzyme activity were greater in the surface soil than in the sub-surface soil, in agreement with published results (Shamir and Steinberger 2007; Paudel et al. 2011). These differences were attributed to greater accumulation of organic matter, and favorable moisture and temperature in the surface soil compared with sub-surface soil (Paudel et al. 2011). In addition, in this study, metabolic quotient in the upper layer was significantly lower than in the middle and lower layers; this might be because of the relatively high level of organic C on the soil surface.
Conclusions
In this study, three soil depths (0–10, 10–20, and 20–30 cm) were investigated for two densities of Ginkgo trees mixed with tea and a monoculture tea system. The results showed that adoption of a Ginkgo–tea combination could lead to increased long-term sustainability of soil fertility, although the benefits to some soil properties may not be apparent immediately. All the soil properties measured decreased gradually with soil depths, except metabolic quotient. Soil organic C and total N content, microbial biomass, and enzyme activity were significantly higher in Ginkgo–tea agroforestry systems than in the monoculture tea system. Soil enzyme (i.e., catalase, dehydrogenase, urease, protease, and invertase) activity was highly correlated with soil organic C and total N. Higher soil enzyme activity and microbial biomass were enhanced by agroforestry; this may lead to increases of other indicators of soil quality, for example organic matter content, and soil sustainability and productivity, so that soil and ecosystem function would be improved.
References
Anderson TH, Domsch KH (1990) Application of eco-physiological quotients (qCO2 and qD) on microbial biomasses from soils of different cropping histories. Soil Biol Biochem 22:251–255
Balota EL, Chaves JC (2010) Enzymatic activity and mineralization of carbon and nitrogen in soil cultivated with coffee and green manures. Rev Bras Cienc Solo 34:1573–1583
Balota EL, Colozzi A, Andrade DS, Dick RP (2003) Microbial biomass in soils under different tillage and crop rotation systems. Biol Fert Soils 38:15–20
Berg MP, Kniese JP, Verhoef HA (1998) Dynamics and stratification of bacteria and fungi in the organic layers of a Scots pine forest soil. Biol Fert Soils 26:313–322
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Budiadi, Ishii HT, Sabarnurdin MS, Suryanto P, Kanazawa Y (2006) Biomass cycling and soil properties in an agroforestry-based plantation system of kayu putih (Melaleuca leucadendron LINN) in East Java, Indonesia. Agrofor Syst 67:135–145
Clegg CD (2006) Impact of cattle grazing and inorganic fertiliser additions to managed grasslands on the microbial community composition of soils. Appl Soil Ecol 31:73–82
De Costa WAJM, Surenthran P (2005) Tree-crop interactions in hedgerow intercropping with different tree species and tea in Sri Lanka: 1. Production and resource competition. Agrofor Forum 63:199–209
Fernandes SAP, Bettiol W, Cerri CC (2005) Effect of sewage sludge on microbial biomass, basal respiration, metabolic quotient and soil enzymatic activity. Appl Soil Ecol 30:65–77
Gu Y, Wang P, Kong CH (2009) Urease, invertase, dehydrogenase and polyphenoloxidase activities in paddy soil influenced by allelopathic rice variety. Eur J Soil Biol 45:436–441
Hamm D, Feger KH (1996) An optimized method for the determination of protease activity in acid forest soils. Z Pflanzenernähr Bodenkd 159:37–39
Hernández T, García C, Reinhardt I (1997) Short-term effect of wildfire on the chemical, biochemical and microbiological properties of Mediterranean pine forest soils. Biol Fert Soils 25:109–116
Islam KR, Weil RR (2000) Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agr Ecosyst Environ 79:9–16
Jose S (2009) Agroforestry for ecosystem services and environmental benefits: an overview. Agrofor Syst 76:1–10
Keeney DR, Nelson DW (1982) Nitrogen-inorganic forms. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. American Society of Agronomy, Madison, pp 643–698
Klose S, Tabatabai MA (2000) Urease activity of microbial biomass in soils as affected by cropping systems. Biol Fert Soils 31:191–199
Kremer RJ, Li J (2003) Developing weed-suppressive soils through improved soil quality management. Soil Till Res 72:193–202
Lizarazo L, Jordá J, Juárez M, Sánchez-Andreu J (2005) Effect of humic amendments on inorganic N, dehydrogenase and alkaline phosphatase activities of a Mediterranean soil. Biol Fert Soils 42:172–177
Longo RM, de Melo WJ (2005) Urease activity in oxisols as influenced by vegetation cover and sampling time. Rev Bras Cienc Solo 29:645–650
Michel K, Matzner E (2003) Response of enzyme activities to nitrogen addition in forest floors of different C-to-N ratios. Biol Fert Soils 38:102–109
Mungai NW, Motavalli PP, Kremer RJ, Nelson KA (2005) Spatial variation of soil enzyme activities and microbial functional diversity in temperate alley cropping systems. Biol Fert Soils 42:129–136
Myers RT, Zak DR, White DC, Peacock A (2001) Landscape-level patterns of microbial community composition and substrate use in upland forest ecosystems. Soil Sci Soc Am J 65:359–367
Nancy WM, Peter PM (2005) Spatial variation of soil enzyme activities and microbial functional diversity in temperate alley cropping systems. Biol Fert Soils 42:129–136
Nioh I, Isobe T, Osada M (1993) Microbial biomass and some biochemical characteristics of a strongly acid tea field soil. Soil Sci Plant Nutr 39:617–626
Pandey A, Palni LMS (1996) The rhizosphere effect of tea on soil microbes in a Himalayan monsoonal location. Biol Fert Soils 21:131–137
Paudel BR, Udawatta RP, Anderson SH (2011) Agroforestry and grass buffer effects on soil quality parameters for grazed pasture and row-crop systems. Appl Soil Ecol 48:125–132
Perucci P, Bonciarelli U, Santilocchi R, Bianchi AA (1997) Effect of rotation, nitrogen fertilization and management of crop residues on some chemical, microbiological and biochemical properties of soil. Biol Fert Soils 24:311–316
Perucci P, Casucci C, Dumontet S (2000) An improved method to evaluate the o-diphenol oxidase activity of soil. Soil Biol Biochem 32:1927–1933
Schinner F, Von Mersi W (1990) Xylanase-, CM-cellulase-and invertase activity in soil: an improved method. Soil Biol Biochem 22:511–515
Shamir I, Steinberger Y (2007) Vertical distribution and activity of soil microbial population in a sandy desert ecosystem. Microb Ecol 53:340–347
Tian L, Dell E, Shi W (2010) Chemical composition of dissolved organic matter in agroecosystems: correlations with soil enzyme activity and carbon and nitrogen mineralization. Appl Soil Ecol 46:426–435
Udawatta RP, Kremer RJ, Adamson BW, Anderson SH (2008) Variations in soil aggregate stability and enzyme activities in a temperate agroforestry practice. Appl Soil Ecol 39:153–160
Udawatta RP, Kremer RJ, Garrett HE, Anderson SH (2009) Soil enzyme activities and physical properties in a watershed managed under agroforestry and row-crop systems. Agr Ecosyst Environ 131:98–104
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Wang G, Cao F (2011) Integrated evaluation of soil fertility in Ginkgo (Ginkgo biloba L.) agroforestry systems in Jiangsu, China. Agrofor Syst 83:89–100
Wang H, Huang Y, Huang H, Wang KM, Zhou SY (2005) Soil properties under young Chinese fir-based agroforestry system in mid-subtropical China. Agrofor Syst 64:131–141
Wardle DA (1992) A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol Rev 67:321–358
Wick B, Tiessen H, Menezes R (2000) Land quality changes following the conversion of the natural vegetation into silvo-pastoral systems in semi-arid NE Brazil. Plant Soil 222:59–70
Xue D, Yao H, Huang C (2006) Microbial biomass, N mineralization and nitrification, enzyme activities, and microbial community diversity in tea orchard soils. Plant Soil 288:319–331
Zhou LK (1987) Soil enzyme. Science Press, Beijing, pp 45–48
Zhou XG, Yu GB, Wu FZ (2011) Effects of intercropping cucumber with onion or garlic on soil enzyme activities, microbial communities and cucumber yield. Eur J Soil Biol 47:279–287
Acknowledgments
The authors thank the staff of Sanfeng Farm in Changshu, Jiangsu Province, China, for providing sites for the experiments, and for help and support during the study. Also, thanks to all the postgraduate students in the laboratory of silviculture in Nanjing Forestry University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tian, Y., Cao, F. & Wang, G. Soil microbiological properties and enzyme activity in Ginkgo–tea agroforestry compared with monoculture. Agroforest Syst 87, 1201–1210 (2013). https://doi.org/10.1007/s10457-013-9630-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-013-9630-0