Abstract
Agroforestry systems are widely practiced in tropical forests to recover degraded and deforested areas and also to balance the global carbon budget. However, our understanding of difference in soil respiration rates between agroforestry and natural forest systems is very limited. This study compared the seasonal variations in soil respiration rates in relation to fine root biomass, microbial biomass, and soil organic carbon between a secondary forest and two agroforestry systems dominated by Gmelina arborea and Dipterocarps in the Philippines during the dry and the wet seasons. The secondary forest had significantly higher (p < 0.05) soil respiration rate, fine root biomass and soil organic matter than the agroforestry systems in the dry season. However, in the wet season, soil respiration and soil organic matter in the G. arborea dominated agroforestry system were as high as in the secondary forest. Whereas soil respiration was generally higher in the wet than in the dry season, there were no differences in fine root biomass, microbial biomass and soil organic matter between the two seasons. Soil respiration rate correlated positively and significantly with fine root biomass, microbial biomass, and soil organic C in all three sites. The results of this study indicate, to some degree, that different land use management practices have different effects on fine root biomass, microbial biomass and soil organic C which may affect soil respiration as well. Therefore, when introducing agroforestry system, a proper choice of species and management techniques which are similar to natural forest is recommended.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tropical forests contain approximately 37 % of the global terrestrial carbon pool in their vegetation and soils (Dixon et al. 1994). Because of their dominant role in the global terrestrial carbon cycle, even a small change in tropical CO2 fluxes can modify the global carbon budget. Despite the importance of tropical forest systems, there are still high rates of forest conversion and deforestation practices which have results in the release of CO2 from terrestrial soils (Murphy et al. 2008). For example, the rapid shift of tropical forests to permanent croplands accounted for approximately 75 % of the total CO2 emission from tropical Asia in the 1980s (Houghton and Hackler Houghton and Hackler 1999). Soil respiration—the combined CO2 efflux of roots and microbial respiration from soils—is the largest component of the net terrestrial soil CO2 flux (Kosugi et al. 2007) and therefore serves as an important indicator to understanding how land use conversions affect the global carbon cycle. A number of studies on soil respiration have been undertaken in a variety of terrestrial ecosystems including forest and agricultural lands (Raich and Schlesinger 1992; Saviozzi et al. 2001).
Agroforestry is the system of growing trees or other woody perennials and crops or pastures on the same piece of land (Sanchez 1995). This system is widely recognized as an alternative land-management practice that can help to reduce the effects of forest land conversion, especially in tropical forests (Palm 1995). Agroforestry practices have received considerable attention recently as a strategy for increasing the carbon sink in soils (Lee and Jose 2003; Ross et al. 1999). Suitable agroforestry systems have a large potential for sequestering carbon into soils from the atmosphere because of the perennial vegetation and the high belowground production (Montagnini and Nair 2004). Despite wide adoption of agroforestry practices, little is known about the response of soil carbon flux resulting from conversion of forests to agroforestry systems (Tufekcioglu et al. 2001; Amatya et al. 2002; Lee and Jose 2003). Moreover, few comparisons of soil carbon flux between agroforestry and forest ecosystems have been conducted.
Soil respiration varies in time and space, and it is essential to understand the factors responsible for the variation of soil respiration to be able to predict changes resulting from conversions of forest lands into agroforestry lands (Zhu et al. 2009). Water content is a key physical factor responsible for the variation in soil respiration in tropical forests (Adachi et al. 2006; Xu and Qi 2001). In temperate and boreal forests however, soil temperature is considered the determining physical factor responsible for variations in soil respiration (Davidson 1998). Some studies have also reported the relationship between soil respiration and abiotic factors including fine root biomass (Hanson et al. Hanson et al. 1993; Adachi et al. 2006), microbial biomass (Rustad et al. 2000; Lee and Jose 2003) and soil properties (Xu and Qi 2001) in tropical forests and agroforestry systems, however no comparisons were made between these two systems in any of these studies.
Our objectives were to examine the influence of agroforestry practice on the seasonal patterns of soil respiration, fine root biomass, microbial biomass, and soil organic matter. We hypothesized that soil respiration would increase with increasing fine root biomass, microbial biomass, and soil organic matter, and that all these parameters would be higher in the secondary forests than the agroforestry sites during dry and wet season.
Materials and methods
Study area
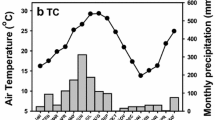
This study was conducted in the Agroforestry Field Laboratory of the Institute of Agroforestry, University of the Philippines in Los Baños (UPLB), Laguna, Philippines, which is situated in the midslopes of Mt. Makiling (14°09′N, 121°11′E, 80 m asl) (Fig. 1). The area has two main seasons; a wet season which occurs from June to October and a dry season from November to May. Mean annual temperature was 26.3 °C and mean annual precipitation was 308 mm in wet season and 35 mm in dry season in 2007 by a weather station in UPLB (Fig. 2).
After a forest fire occurred in 1990, parts of the area were planted with agroforestry trees and crops in 1991 while other parts were left untouched to revert to secondary forest. Two of the agroforestry sites, one dominated by Gmelina arborea and other dominated by Dipterocarps and one secondary forest site were chosen for the establishment of the research plots. In the G. arborea agroforestry site, species such as cacao (Theobroma cacao L.), coffee (Coffea sp.), and zinger (Zingiber officinale Roscoe) were planted as understory. In the Dipterocarps agroforestry site, ferns and cacao were planted as understory. The secondary forest was dominated by Dracontomelon dao, Dracontomelon edule, and Aleurites moluccana. Three plots (20 m × 20 m) were established in each of the three sites and diameter at breast height (DBH) of all trees above 10 cm in diameter were measured for basal area. Light intensity as an indicator of light availability was measured by a LICOR 250 (LICOR, Inc., Lincoln NE, U.S.A). Litterfall (leaves and twigs) was collected using litter traps (0.25 m2) (3 l traps per plot, total nine per stand). Litter was oven-dried at a temperature of 65 °C for at least 48 h or until weights of the samples became constant. The forest structure per stand is summarized in Table 1.
Measurement of soil respiration, soil temperature, and soil moisture
Soil respiration was measured five times each in the dry (December 2006 to February 2007) and wet (June to August 2007) seasons using an infrared gas analyzer system (LI-6400 survey system; LI-COR Biosciences, Lincoln NE, U.S.A.). Polyvinyl chloride (PVC) collars (5.5 cm in diameter, 5 cm length) were inserted into the top 2 cm of the litter layer at five locations in each plot (making a total of fifteen points per site). The collars remained in the same locations throughout the measurements in both the dry and the wet seasons. Soil CO2 efflux was measured over a period of 90–120 s at each sampling time. Simultaneous with the soil respiration measurements, soil temperature (°C) was measured using a temperature probe on the LI-COR 6400 (steel-embedded, copper-Constantan thermocouple, type T) at 5 cm depth for each soil respiration collar. Soil samples were collected from top soil (0–5 cm) with the use of stainless steel cylinders (8 cm in diameter, 5 cm length) after carefully removing the litter and organic layers. The samples were oven dried at 105 °C to constant weight to determine the soil water content and bulk density (Table 1).
Root and microbial biomass and soil characteristic analyses
Separate soil samples were collected from the 0–30 cm layer from each of the study plots in February (dry season) and August (wet season) to determine soil organic C (SOC), total nitrogen (TN) and soil pH. SOC was determined by the Walkley–Black method using correction factor of 1.33 (Sollins et al. 1999). TN was determined by the micro-Kjeldahl digestion procedure (McGill et al. 1993) and soil pH in water suspension at a ratio of 1: 2.5 (soil:distilled water) using a pH meter (Model 744, Metrohm Inc., Switzerland).
Five soil cores (5.5 cm in diameter and 10 cm deep) were collected to next to the soil respiration collars after each seasonal measurement in the dry (February) and wet (August) seasons. Fine roots (<5 mm) were extracted from each soil core and oven-dried at 65 °C till weight loss ceased and weights were recorded as fine root biomass. The remaining soil samples were sent to Korea in sealed plastic bags and kept in ice-lined polystyrene containers for microbial biomass determinations. To determine the soil microbial biomass, colony forming units (CFU) were measured following serial dilution of samples in phosphate buffered saline solution. A range of media were tested including cold-extracted soil extract agar (Olsen and Bakken 1987) and water, agar (2 %, Difco) supplemented with 0.01, 0.1 or 1 % nutrient stock solution (Olsen and Bakken 1987), nutrient agar tested at full, l/10 and l/100 strength, R2A agar (Reasoner and Geldreich 1985), yeast extract agar, and half-strength tryptone soy agar. Counts were made in triplicate under ×10 magnification using light microscope, after incubation at 20 °C for 10 and 21 days.
Data analysis
Analysis of variance with repeated measures (ANOVAR) was used to compare the seasonal variations in soil respiration, fine root biomass and microbial biomass in the different sites. The effects of soil organic matter, soil N content, soil pH, soil temperature, and soil moisture on soil respiration was assessed with a Pearson correlation analysis. All statistical analysis were conducted using the statistical analysis software (SAS institute Inc., 2005).
Results
During the dry season, soil respiration rate in the secondary forest was significantly higher (p = 0.002) than in the two agroforestry systems (Fig. 3). During the wet season however, soil respiration in the secondary forest was only significantly higher than in the Dipterocarps dominated agroforestry site (p = 0.001) but not the G. arborea dominated agroforestry site (p = 0.25) (Fig. 3). The secondary forest had soil respiration rate that was 25 and 41 % higher than in the G. arborea and the Dipterocarps agroforestry systems in the dry season and 5 and 51 % higher than in the two agroforestry systems respectively in the wet season. There was significant seasonal difference in soil respiration at two of the sites. Soil respiration rate was significantly higher in the wet season than in dry season for the secondary forest site (p = 0.05) and the G. arborea agroforestry site (p = 0.0001). Soil respiration in the Dipterocarps agroforestry site did not vary significantly (p = 0.78) by season (Fig. 3).
Fine root biomass in the secondary forest was significantly greater in both the dry season (p = 0.04) and the wet season (p = 0.01) than in the two agroforestry systems. During the dry season, the secondary forest had 32 and 34 % more fine root biomass than the G. arborea and the Dipterocarps agroforestry systems. Fine root biomass in the secondary forest was also 42 and 74 % greater than in the two agroforestry systems respectively in the wet season (Fig. 4). In general, fine root biomass was not different between the two seasons (p = 0.31), and the seasonal pattern in fine root biomass among sites was similar to that of soil respiration. There was no significant difference in microbial biomass among the different land use systems (p = 0.44) and between the two seasons (p = 0.29) (Fig. 4). SOC on the other hand did not show any significant seasonal variability (p = 0.47) but it was significantly higher (p = 0.006) in the secondary forest than in the two agroforestry systems during the dry season (Fig. 4).
In general, fine root biomass and microbial biomass were positively correlated with soil respiration in all three sites. SOC also showed a positive relationship with soil respiration only in the Dipterocarp dominated agroforestry system but no relationship existed between SOC and soil respiration in the secondary forest and the G. arborea dominated agroforestry system (Fig. 5). The strength of these correlations differed among sites. In particular, soil respiration was strongly correlated with microbial biomass in the secondary forest and G. arborea dominated agroforestry system than in the Dipterocarp dominated agroforestry system. No significant correlations were found between soil respiration and the abiotic factors (Table 2). Soil N and pH showed some negative relationship with soil respiration in all sites, however, only the correlation between soil N and soil respiration in the secondary forest was strong (R = −0.58) in the dry season. Soil temperature and moisture did not show any correlation with soil respiration.
Relationship between soil respiration, microbial population, fine root biomass, and soil organic matter in secondary forest (circles), G. arborea agroforestry site (squares), and Dipterocarps agroforestry site (triangles). Solid shapes and lines are during wet season and open shapes and lines are during dry season
Discussion
We observed that soil respiration increased by 22 % in the secondary forest, 19 % in the G. arborea dominated agroforestry system, and 14 % in the Dipterocarps dominated agroforestry system from dry to wet season. Using soil respiration as an indicator of ecosystems productivity (Raich and Schlesinger 1992; Hanson et al. 1993), our results suggest that both the secondary forest and the agroforestry systems have higher productivity in the wet season than in the dry season. However, neither fine root biomass nor microbial biomass differed between wet and dry season while soil respiration was significantly higher in wet season than in dry season indicating that high precipitation in wet season could enhance direct carbon supply by photosynthesis from aboveground to roots (Hogberg et al. 2001).
Despite the distinct differences between the two seasons, we did not observe any relationship between soil respiration and soil moisture. In other studies, soil moisture has been identified as one of the main factors that influence soil respiration in tropical forests (Davidson et al. 2000). The lack of relationship between these two soil variables in our study area is probably because the soil moisture in this study area is not under soil water stress by drought or by too much rain leading to anaerobic condition so as to induce roots and microbial activity (Bowden et al. 1993; Liu et al. 2002).
As we expected, conversion of secondary forest to two agroforestry systems reduced soil respiration. Similarly, Hertel et al. (2009) reported significantly higher soil respiration rate in undisturbed and slightly disturbed forest stands than in a cacao agroforestry system. He also observed that soil respiration rate in the intensively managed agroforestry system with planted shading trees was comparable to that of the slightly disturbed forest site. Soil respiration rate in natural forests of the tropics have been observed to be higher than or the same as disturbed forests; primary or secondary forest and oil palm plantation (Adachi et al. 2006), pastures and adjacent forest (Fernandes et al. 2002), and secondary forest and plantation (Li et al. 2005). In our study, the lower soil respiration in the agroforestry system than the secondary forestry indicates that agroforestry practices can reduce soil respiration as a result of reductions in fine root biomass, microbial biomass, and soil organic C accompanying the conversion. The no clear difference in soil respiration, fine root biomass, microbial biomass, and soil organic C between the two agroforestry systems suggests that tree species in our agroforestry systems do not affect soil carbon flux.
Fine root biomass was greater in the secondary forest than in the agroforestry systems and followed the same pattern as soil respiration. Many studies have shown that root respiration, accounts for about half of soil respiration in forest ecosystems (Ewel et al. 1987; Nakane et al. 1996; Epron et al. 2006), and it is positively correlated with fine root biomass (Zhu et al. 2009; Maier and Kress 2000) because decaying dead roots and roots exudates provide carbon substrates to the soil microflora (Epron et al. 2006; Lee and Jose 2003) and increase soil respiration. In other studies, up to 300 % more root biomass has been observed in secondary forests than in multi-strata agroforestry systems and tree crop monocultures due to higher tree density in the forest stands. However, we cannot attribute the higher root biomass and soil respiration in the secondary forest than the agroforestry systems in this study to tree density because our initial measurements showed that the secondary forest had the lowest basal area although not significant.
The two agroforestry systems in this study also had lower SOC compared to the secondary forest however, there was no detectable trend in microbial biomass between the secondary forest and the agroforestry systems. SOC is one of the sources of microbial respiration because it can be broken down to CO2 by microbes. Microbial biomass is also an important source of soil organic matter which is needed for microbial respiration. High SOC and microbial biomass have been observed in an agroforestry system with high litter production and decomposition rate and consequently high microbial respiration (Insam and Domsch 1988). Further study of litter production and decomposition rate between secondary forest and agroforestry systems could support the effects of microbial biomass on soil respiration.
The positive contribution of fine root biomass, microbial biomass, and SOC to total soil respiration is well documented (Raich and Tufekciogul 2000; Lee and Jose 2003). This study also showed that soil respiration increased with increase in fine root biomass and microbial biomass in all sites. However soil respiration increased with an increase in SOC only in the Dipterocarps dominated agroforestry system. The slopes of the correlation between soil respiration and fine root biomass and between soil respiration and microbial biomass were higher in the wet season than in the dry season (Fig. 5). This is probably due to higher root and microbial activities in the wet season resulting from higher root and microbial biomass, higher soil moisture and decomposition rate than during dry season (Davidson et al. 2000). The different slopes of correlation in the three different sites also suggest that the relative sensitivity of soil respiration to fine root biomass, microbial biomass, and SOC vary between forest systems and tree species.
Conclusions
This study has demonstrated that tropical soil respiration, fine root biomass, microbial biomass and SOC differ significantly between secondary forest and agroforestry systems and by season. The results indicate that, in the dry season, the secondary forest had significantly higher values of soil respiration, fine root biomass and SOC than the agroforestry systems. Soil respiration rate was higher in the wet season than in the dry season however fine root biomass, microbial biomass, and SOC did not show any seasonal variability. Soil respiration increased with increase in fine root biomass, microbial biomass, and SOC irrespective of the land use management system. The results of this study also indicate, to some degree, that the different land use management practices affect the fine root biomass, microbial biomass and SOC which in turn could influence soil respiration. The selection of proper tree species and management techniques that mimics a natural forest system is therefore recommended when introducing agroforestry system. Further studies into the influences of litter production and carbon supply by photosynthesis to belowground on soil respiration in secondary forest and agroforestry systems would be necessary to give a clearer insight into the difference in soil respiration rates between these ecosystems.
References
Adachi M, Bekku Y, Rashidah W, Okuda T, Koizumi H (2006) Differences in soil respiration between different tropical ecosystems. Appl Soil Ecol 34(2–3):258–265
Amatya G, Chang S, Beare M, Mead D (2002) Soil properties under a Pinus radiata-ryegrass silvopastoral system in New Zealand. Part II. C and N of soil microbial biomass, and soil N dynamics. Agrofor Syst 54(2):149–160
Bowden RD, Nadelhoffer KJ, Boone RD, Melillo JM, Garrison JB (1993) Contributions of aboveground litter, belowground litter, and root respiration to total soil respiration in a temperate mixed hardwood forest. Can J For Res 23(7):1402–1407
Davidson EA, Verchot LV, Cattanio JH, Ackerman IL, Carvalho J (2000) Effects of soil water content on soil respiration in forests and cattle pastures of eastern Amazonia. Biogeochemistry 48(1):53–69
Davidson EC (1998) Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob Chang Biol 4(2):217–227
Dixon R, Brown S, Houghton R, Solomon A, Trexler M, Wisniewski J (1994) Carbon pools and flux of global forest ecosystems. Science (Washington) 263(5144):185–189
Epron D, Bosc A, Bonal D, Freycon V (2006) Spatial variation of soil respiration across a topographic gradient in a tropical rain forest in French Guiana. J Trop Ecol 22(05):565–574
Ewel K, Cropper W Jr, Gholz H (1987) Soil CO2 evolution in Florida slash pine plantations. II. Importance of root respiration. Can J For Res 17(4):330–333
Fernandes S, Bernoux M, Cerri C, Feigl B, Piccolo M (2002) Seasonal variation of soil chemical properties and CO2 and CH4 fluxes in unfertilized and P-fertilized pastures in an Ultisol of the Brazilian Amazon. Geoderma 107(3–4):227–241
Hanson P, Wullschleger S, Bohlman S, Todd D (1993) Seasonal and topographic patterns of forest floor CO2 efflux from an upland oak forest. Tree Physiol 13(1):1
Hertel D, Harteveld AM, Leuschner C (2009) Conversion of a tropical forest into agroforest alters the fine root-related carbon flux to the soil. Soil Biol Biochem 41:481–490
Hogberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Hogberg MN, Nyberg G, Ottosson-Lofvenius M, Read DJ (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411(6839):789–792
Houghton R, Hackler J (1999) Emissions of carbon from forestry and land use change in tropical Asia. Glob Chang Biol 5(4):481–492
Insam H, Domsch K (1988) Relationship between soil organic carbon and microbial biomass on chronosequences of reclamation sites. Microb Ecol 15(2):177–188
Kosugi Y, Mitani T, Itoh M, Noguchi S, Tani M, Matsuo N, Takanashi S, Ohkubo S, Rahim Nik A (2007) Spatial and temporal variation in soil respiration in a Southeast Asian tropical rainforest. Agric For Meteorol 147(1–2):35–47
Lee K, Jose S (2003) Soil respiration and microbial biomass in a pecan–cotton alley cropping system in Southern USA. Agrofor Syst 58(1):45–54
Li Y, Xu M, Zou X, Shi P, Zhang Y (2005) Comparing soil organic carbon dynamics in plantation and secondary forest in wet tropics in Puerto Rico. Glob Chang Biol 11(2):239–248
Liu X, Wan S, Su B, Hui D, Luo Y (2002) Response of soil CO2 efflux to water manipulation in a tallgrass prairie ecosystem. Plant soil 240(2):213–223
Maier CA, Kress L (2000) Soil CO2 evolution and root respiration in 11 year-old loblolly pine (Pinus taeda) plantations as affected by moisture and nutrient availability. Can J For Res 30(3):347–359
McGill W, Figueiredo C, Carter M (1993) Total nitrogen. In: Carter M (ed) Soil sampling and methods of analysis. CRC Press, Boca Raton, pp 201–211
Montagnini F, Nair P (2004) Carbon sequestration: an underexploited environmental benefit of agroforestry systems. Agrofor Syst 61(1):281–295
Murphy M, Balser T, Buchmann N, Hahn V, Potvin C (2008) Linking tree biodiversity to belowground process in a young tropical plantation: impacts on soil CO2 flux. For Ecol Manag 255(7):2577–2588
Nakane K, Kohno T, Horikoshi T (1996) Root respiration rate before and just after clear-felling in a mature, deciduous, broad-leaved forest. Ecol Res 11(2):111–119
Olsen R, Bakken L (1987) Viability of soil bacteria: optimization of plate-counting technique and comparison between total counts and plate counts within different size groups. Microb Ecol 13(1):59–74
Palm C (1995) Contribution of agroforestry trees to nutrient requirements of intercropped plants. Agrofor Syst 30(1):105–124
Raich J, Schlesinger W (1992) The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 44(2):81–99
Raich J, Tufekciogul A (2000) Vegetation and soil respiration: correlations and controls. Biogeochemistry 48(1):71–90
Reasoner D, Geldreich E (1985) A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol 49(1):1
Ross D, Tate K, Scott N, Feltham C (1999) Land-use change: effects on soil carbon, nitrogen and phosphorus pools and fluxes in three adjacent ecosystems. Soil Biol Biochem 31(6):803–813
Rustad L, Huntington T, Boone R (2000) Controls on soil respiration: implications for climate change. Biogeochemistry 48(1):1–6
Sanchez P (1995) Science in agroforestry. Agrofor Syst 30(1):5–55
Saviozzi A, Levi-Minzi R, Cardelli R, Riffaldi R (2001) A comparison of soil quality in adjacent cultivated, forest and native grassland soils. Plant Soil 233(2):251–259
Sollins P, Glassman C, Paul EA, Swanston C, Lajtha K, Heil JW, Elliott ET, Robertson G (1999) Soil carbon and nitrogen: pools and fractions. Standard soil methods for long-term ecological research, pp 89–105
Tufekcioglu A, Raich J, Isenhart T, Schultz R (2001) Soil respiration within riparian buffers and adjacent crop fields. Plant Soil 229(1):117–124
Xu M, Qi Y (2001) Soil-surface CO2 efflux and its spatial and temporal variations in a young ponderosa pine plantation in northern California. Glob Chang Biol 7(6):667–677
Zhu J, Yan Q, Fan A, Yang K, Hu Z (2009) The role of environmental, root, and microbial biomass characteristics in soil respiration in temperate secondary forests of Northeast China. Trees Struct Funct 23(1):189–196
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bae, K., Lee, D.K., Fahey, T.J. et al. Seasonal variation of soil respiration rates in a secondary forest and agroforestry systems. Agroforest Syst 87, 131–139 (2013). https://doi.org/10.1007/s10457-012-9530-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-012-9530-8