Abstract
The shea tree, Vitellaria paradoxa, is a socio-economically important tree for the rural population in parts of West Africa. Our study assessed the current status of this native tree species with regard to increasing human pressure in northern Benin. We compared distribution of adult shea trees, seedlings and saplings in farmed lands with protected areas in the Biosphere Reserve of Pendjari (BRP). At our study site near BRP, agricultural activities foster recruitment of shea trees by regularly cropping of vegetation cover. Furthermore, traditional farming practices preserve adult individuals thus permitting regular fruit harvests. Consequently, most of the tallest and largest individuals of shea trees are found in framed lands. In contrast, the highest density of juvenile trees including seedlings (dbh <5 cm) and saplings (dbh 5–10 cm) occurred within BRP. Saplings were negatively affected by farming activities. Furthermore, spatial point pattern analysis revealed differences in the spatial structure of juveniles. Juveniles showed significant aggregations at small scale (<20 m) in BRP as well as significant and positive small-scale associations with adult trees. This contrasts with farmed lands where we did not find such spatial patterns at similar small scale but only a weak aggregation between juveniles and absence of association (attraction) of adults to juveniles. Although our analyses indicate that shea trees are rather well preserved, we conclude that the observed severe reduction of saplings in farmed lands is likely to negatively impact the long-term viability of the tree population. Therefore agroforestry practices must consider the preservation of sapling populations in farming areas for long-term conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditional agroforestry parkland systems, where farmers grow annual crops in fields with scattered trees, are among the most widespread systems in sub-Saharan Africa (Teklehaimanot 2003). The agroforestry system in northern Benin as well as in Mali and Burkina Faso is built around the selection of desired tree species and individuals (Maranz and Wiesma 2003). The indigenous parkland farming system consists of alternating cycles of cultivation and fallows where natural regeneration of woody plants occurs around preserved trees (Boffa 1999; Lovett and Haq 2000) like shea trees (Vitellaria paradoxa). Therefore, phytodiversity in parklands and old fallows is mostly dominated by tree species that are useful for the local population. For example, density of the economically important shea tree averages 15 mature trees per hectare in Mali (Ruyssen 1957). It constitutes up to 70% of the woody vegetation in some areas of Benin (Agbahungba and Depommier 1989) and may reach over 80% in parts of northern Ghana (Lovett and Haq 2000) and Burkina Faso (Boffa 1999). Shea trees also dominate our study area in the Pendjari region situated in northwestern Benin.
Shea trees are a valuable source of a variety of non-timber forest products namely fruit and the nutrient rich seeds which serve as the basis for cooking oil as well as the production of shea butter. The latter is of substantial economic value as it is used for exportation because is ingredient of many cosmetics (Tran 1984). Women mainly exploit fruits and seeds. Shea tree products procure about 80% of their incomes in Mali (http://www.alterafrica.com) and in Benin. Agbahungba and Dopommier (1989) reported that women gained more income from shea trees than from work in cotton fields.
Although there is an apparent good preservation status of adult shea trees in farmed lands, the tree is on a constant decline on the population level. The Sudanian savanna zone, for instance, had the highest density of shea trees in the 1940s, with a population of 230 trees ha−1 (Chevalier 1946). This density has been seriously reduced nowadays to a minimum of 11 trees ha–1 (Nikiema et al. 2001) most likely because of profound changes in farmers’ practices. Recently, Kelly et al. (2004) studied the impact of farmers’ practices on size class distribution and spatial pattern of shea trees in Mali and reported that the spatial pattern of V. paradoxa is becoming progressively aggregated with a gradient ranging from cultivated fields to fallow and finally to forest.
Similar trends can be observed on the local level. With regard to the socio–economic importance of this plant in northern Benin in general and particularly in areas surrounding the Biosphere Reserve of Pendjari (BPR), shea trees are better preserved compared to other woody plants. However, increasing demographic pressure shortens the fallow cycle and leads to changes in farming patterns, in particular small- to large-scale conversion of land into cotton fields. Both factors seriously affect the regeneration of shea trees. As this important tree species is not cultivated by the local population, it depends heavily on natural regeneration, where seed dispersal by flying foxes (Chiroptera) and birds (Ruyssen 1957; Jackson 1968; Ayensu 1974) plays a crucial role (Killick 1959). Flying foxes are often the only remaining group of medium-sized animal dispersers in these areas as similar to many areas in Africa, large birds and mammals are often hunted out (Fa et al. 2000, 2002, 2005; Thibault and Blaney 2003).
In our study we assessed and compared demographic and morphological (dendrometic) characteristics of shea trees including density, tree height, crown area and general distribution patterns in farmed lands versus protected areas. We hypothesized that intense land use negatively affects the density of shea trees, leading to modifications in their distribution pattern because agricultural activities might require less vegetation coverage. Diminution in tree density and soil fertilization for agricultural needs favor adult trees’ height and/or crown areas as well as natural regeneration because seedlings demand light. We also assumed (hypothesized) that adult shea trees should show a clumped or random distribution pattern in protected areas and more regular distributions where they are heavily influenced by humans’ activities because of farming practices that go with a reduction in vegetation density. Criterions like need of space, growth speed, health, morphology, and productivity lead to a selective cutting of individuals of shea trees in farmed lands (Lovett and Haq 2000). Juvenile individuals might show similar distribution pattern like adult trees because they grow around adult trees and are treated similarly. As the cropping of vegetation leads to the preservation of only few selected juvenile plants this should also influence the spatial distribution pattern of juvenile plants around adults. Overall, we expect that adult and juvenile shea trees should show a positive association (attraction) in protected areas without cropping and a negative association (repulsion) where cropping of the vegetation occurs through agricultural activities.
In this study we assess how farming activities affect structure and spatial distribution of shea tree populations. We focus on stand density, distribution of age classes, tree height and crown area and compare tree populations in areas of farmed lands surrounding the BRP with those of plants from protected areas inside the BRP. Ultimately, the main goal of this study was to deliver data useful for predicting the future of this important tree species in the region and to make recommendations for its long-term preservation.
Materials and methods
Study environment
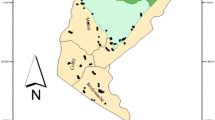
Our study took place in the vicinity and within the BRP (Fig. 1a, b). The region is located in the extreme northwest of Benin in the district of Atacora (10°40′–11°28′N and 0°57′–2°10′E). It covers an area of 4,661.4 km2 and is composed of the National Park of Pendjari (2,660.4 km2), the hunting zone of Pendjari (1,750 km2) and the hunting zone of Konkombri (251 km2). The Pendjari is the only important river that carries water all year round. It runs through the National Park of Pendjari and the Pendjari hunting zone (Delving et al. 1989). Other small streams dry out in the dry season including Magou, Bori, and Yapiti in the hunting zone of Pendjari and Podiega in the National Park.
The park is located in the Sudanian zone with one rainy season (April–May to October). The total rainfall averages 1,000 mm with 60% falling between July and September (Sinsin et al. 2002) and one dry season (November to March). During the rainy season large parts of the park are flooded. The dominant vegetation type is savanna intermingled with some patches of dry forests composed of deciduous trees (Sokpon et al. 2001). Temperature varies from around 21°C during the night up to around 40°C during the day. The annual mean varies from 25 to 28°C during the cooler period of the dry season and 30–33°C during the hot period of the dry season. Relative humidity varies between 17 and 99% (PAG2 2005). The border of Biosphere Reserve of Pendjari is lined with many small villages. Population density in this area is low compared with the whole country. It totals about 213,000 persons leading to 13 person per km2 (INSAE/RGPH 2002). In the area surrounding the BRP Berba, Waama, and Gourmantché are the dominant ethnic groups. The main activity of the local population is agriculture and herding with the Fulani as the ethnic group with the largest herds of cattle. Women mainly exploit non-timber forest resources like fruits of shea trees.
Studied species
Vitellaria paradoxa C.F. Gaertn. (Sapotaceae), the shea tree, is the source of vegetable oil obtained from the kernels which is second in importance only to palm oil in West Africa. Shea trees occur in a savanna belt stretching across Africa from Senegal to the border of Sudan and Ethiopia. A wide variety of economically useful products are made from this tree. The oil derived from the kernels of shea fruits (“shea butter”) is by far the most important product. Women through a laborious and time-consuming process traditionally extract it. From early on, this oil has been an important product in international trading activities in West Africa. After the First World War, shea butter contributed substantially to the flourishing trade between West Africa and Europe, where it was used in the production of vegetable margarine and in candle making (Dudgeon 1922). In 1998 Benin exported 1,000 tonnes of shea butter and gained 400,000 US$.
From central to northern part of Benin this tree species remains the most important source of non-timber resources for the rural population. Fruits from shea trees and the butter form a large portion of the diet and income of local households.
Despite its socio–economic importance, the cultivation of this tree species is not yet mastered by local populations who rely natural regeneration.
Inventory approach and data collection
Fourteen 1 ha-plots of ∼100 × 100 m were installed in homogeneous and comparable habitat types, comprising seven plots in farmed lands, i.e., fallows (1–3 years old) in village territories where farming activities had been previously conducted for a number of years and seven plots in protected areas inside the BRP (Fig. 1c). Plots were established randomly. In protected areas they were located in the Pendjari National Park and in the buffer zone (hunting zone) of Pendjari.
An individual plot consisted of a rectangle of 100 m for each side (1 ha). If some individual shea trees occurred next to the delineated plots, i.e., within 20–30 m, they were also included in the census to obtain comprehensive mapping of clusters. The positions of all individuals were recorded with GPS (GARMIN 76, Resolution 5 m). Plots in farmed lands were named T01 to T07 but C01 to C03 and P01 to P04 inside the BRP.
Data collection was conducted from September to October that coincided with the end of the rainy season allowing re-growth of all burned shea saplings and seedlings.
All V. paradoxa individuals were mapped and measured within each plot except seedlings with dbh ≤1 cm as many of them are typically burned in the following dry season. The diameter at breast height (dbh) of the trees was measured at 1.30 m above ground with ribbon “pi” (specific ribbon used by foresters to measure tree dbh) of 1-cm precision. Seedlings were measured near ground level. Trees with dbh > 10 cm were grouped in the adult category according to foresters’ classification (Rondeux 1999) and all trees with a dbh < 10 cm were grouped in the juvenile category, which comprises seedlings (dbh 1–5 cm) and saplings (5 cm ≥ dbh < 10 cm). Additionally, crown area radius was measured with ribbon of 5 m length scaled in centimeter (precision 1 cm). One operator put one end of the ribbon on the tree and second operator push the second end toward the vertical end of the crown area. Tree height was measured with “dendromètre” SUUNTO with formula h = L [tg (ß1) − tg (ß2)], were L means distance between operator and the tree, ß1 means angle of observation toward the top of the tree, and ß2 means angle of observation of the foot of the tree. Coordinates were determined with a handheld GPS receiver (GARMIN, GPS 76, Resolution 5 m). Coordinates were used to analyze the spatial distribution pattern of the shea tree species.
Spatial analysis
We used the pair-correlation function (Stoyan and Stoyan 1994) and G(y), the distribution of nearest neighbor distances y (Diggle 2003), to investigate (a) the type of distribution pattern shown by adult and juvenile plants of V. paradoxa (univariate point pattern) and (b) the type of association between adult and juvenile individuals (bivariate point pattern). The pair-correlation function g(r) is an intensity-normalized neighborhood density function indicating the density of neighboring trees as a function of distance r from an average tree (Wiegand and Moloney 2004) divided by the intensity λ of the pattern. The pair-correlation function is a non-accumulative version of Ripley’s K-function (Ripley 1981) satisfying g(r) = (1/2 πr) dK(r)/dr. Hence, it does not integrate the ‘memory’ (= trend at the beginning) of small-scale’s second-order effects to larger scales, as does Ripley’s K (Wiegand and Moloney 2004). Additionally it is more intuitive than an accumulative measure (Stoyan and Penttinen 2000) because it has a direct interpretation as normalized neighborhood density function.
The pair-correlation function and the distribution of nearest neighbor distances G can also be formulated for bivariate point patterns. The bivariate g 12(r) is the normalized density of neighboring type 2 trees (= pattern 2 represented here by juvenile trees) as a function of distance r from an average type 1 trees (= pattern 1 represented here by adult trees). The bivariate G describes the distribution of the distances from type 1 trees to its nearest type 2 neighbors. Note that g(r) and G(y) measure different features of the point pattern (Diggle 2003). The estimator of g and g 12 and the edge correction used are provided in the appendix. G(y) and G 12(y) were calculated without edge correction (Diggle 2003).
To determine statistical significance of the observed g(r), g 12(r), G(y) or G 12(y), 1% simulation envelopes of an appropriate null model were generated by 999 replicate Monte Carlo simulations of the null model. To this end, we used the 995th largest and the 995th smallest value, for example, g(r), taken from 999 simulations of a null model as simulation envelopes (Wiegand and Moloney 2004). In case that g(r) was for a given scale r outside the simulation envelopes, the null hypothesis was rejected at this scale; i.e., for an univariate point patterns with intensity λ, g(r) > λ indicates clumping, while values of g(r) < λ indicates regularity. For a bivariate point pattern with intensity λ 2 of pattern 2, g 12(r) > λ 2 indicates a positive association (attraction) whereas g 12(r) < λ 2 indicates a negative association (repulsion).
However, because of simultaneous inference (i.e., we tested r at several spatial scales simultaneously), type I error may occur if the value of the chosen statistic is close to a simulation envelope (i.e., the null model may be rejected even if it is true; Loosmore and Ford 2006). We therefore combined the common simulation envelop method with a goodness-of-fit test (Diggle 2003) recently advocated by Loosmore and Ford (2006). In short, the goodness-of-fit test collapses the scale dependent information contained for instance in g(r) into a single test statistic u i which represents the total squared deviation between the observed pattern and the theoretical result across the distances of interest (i = 1 for the data, and i = 2,...1,000 for the simulations). If the rank of u 1 is larger than 990, the summary statistic value, u 1, calculated for the observed pattern for the chosen statistic (G or g) over a specified range of distances is not different than those calculated for random instances of the hypothesized spatial process model on a 1% level.
For each treatment the data from the replicate plots were combined into one overall, mean weighted pair-correlation function (Diggle 2003; Riginos et al. 2005). Due to the low density of shea trees in the study area, we were not able to perform point pattern analysis for most of the individual plots. However, combining the information about the point-to-point distances from all replicate plots increased our sample size considerably. For more details see appendix.
Null models
As we choose our plots in uniform and homogeneous areas without apparent environmental heterogeneity, we applied complete spatial randomness (CSR; Diggle 2003; Wiegand and Moloney 2004) as null model to assess the spatial structure of the patterns of adult and juvenile trees.
In the bivariate pattern, when focusing on the relation between juvenile and adult trees, we assessed independence between both patterns (Goreaud and Pelissier 2003). To this end we applied a random toroidal shift of pattern 2 thus conserving the individual spatial structures of the two patterns, but breaking their possible dependence.
In all analyses we used a grid size of 1 m2, which is a fine enough resolution for our questions (Wiegand and Moloney 2004), and a ring width of 2 m. Because the point density was low, a smaller ring width would produce rugged plots of g(r) and wider rings would not allow separation of different distance classes. We calculated the statistics up to a scale of 50 m taking into account plots size (100 × 100 m).
Data analysis
To analyze the density of shea trees we averaged the total number of individuals per age class plus standard deviation (X̄ ± SD) in the seven 1 ha plots in the farmed lands and the seven others in protected areas of the BRP. We distinguished three age classes (seedlings, saplings, and adults) to compare densities between zones (farmed lands and protected area in BRP). We used Chi-square test to compare the proportion of individuals in each class between the two zones. In addition, we choose a One-Way ANOVA to depict possible differences in age, height, and crown area class distribution between farmed lands and protected areas.
For all point pattern analyses we used the software (Programita, Wiegand and Moloney 2004 improved version of 2007). Standard statistical analyses were conducted with SigmaStat 3.1 and Statistica 6.0. Spatial analysis was performed for first order intensity (Fig. 3) with ArcView GIS 3.2.
Results
Impact of land use on tree density
We mapped and measured (dbh, height and crown area) 178 adult and 346 juvenile trees in an area of 14 ha. Shea trees’ population structure is presented per hectare in the protected areas (BRP) and in the farmed lands (Table 1).
The proportions of juvenile trees (seedlings and saplings) differed significantly between farmed lands and protected areas (Table 1). There were more seedlings and saplings in BRP than in farmed lands but more adult shea trees in these areas than in BRP.
Impact of land use on stand structure of shea trees
Recruitment and number of young shea trees were high in farmed lands and in protected areas (Fig. 2a). However, the number of saplings was drastically reduced in the farmed lands. The distribution of tree diameters showed an opposite trend in farmed lands compared to the protected areas. The protected area was characterized by large numbers of medium height trees (2–4 m). The tallest and hence oldest trees (4–6 m and >6 m), however, were mainly found in the farmed lands (Fig. 2b). Those trees also attained larger crown areas (2–4 and 4–6 m) than those occurring in the protected areas (Fig. 2c).
The distribution of diameter classes (dbh) revealed a very low number of saplings (dbh 5–10 cm) in farmed lands leading to a statistically significant difference between both zones. A similar result was found for the crown area of the class of the largest individuals. There were no differences in tree height when data analysis was performed with a One-Way ANOVA (Table 2).
Impact of land use on spatial distribution patterns of shea trees
Typical examples of the spatial distribution of juvenile and adult trees in the two zones show differences in spatial distribution between zones with more juveniles near adult trees in plot from BRP (Fig. 3). Spatial analysis of distribution pattern of shea trees revealed differences between farmed lands (Fig. 4a, c, e) and protected areas (Fig. 4b, d, f).
Results of the point pattern analyses. The main figures show the results of the univariate analyses of adults (a, b), juveniles (c, d), and the bivariate analyses of juveniles around adults (e, f). The small insert figures show the corresponding results for the distribution function of nearest neighbor distances. Filled circles: statistic calculated from the data, gray solid lines: simulation envelopes being the 995 lowest and highest values of the 999 simulations of the null model, and the solid horizontal black lines show the average statistic taken from the 999 simulations of the null model (i.e., the theoretical expectation)
The pair-correlation function of adult trees in the farmed lands showed only a weakly significant aggregation (the GoF for scales r = 0–50 m yielded a rank of 997) with peaks around 6 and 15 m (Table 3, Fig. 4a). However, for the protected areas the CSR null model was overwhelmingly rejected (Fig. 4b), here the adult trees showed a strong aggregation up to 3 m and notable aggregation up to 10 m. Consequently, the GoF test yielded a rank of 1,000. The distribution of nearest neighbor distances were in both zones significantly different from the CSR null model (yielding rank 1,000), but comparing the distribution of the farmed lands (small insert in Fig. 4a) with the distribution of the protected areas (small insert in Fig. 4b) shows that adult trees in the farmed lands have much less nearest neighbors at small distances up to 4 m. Thus, adult shea trees in the farmed lands have at small scales a clearly more regular distribution than shea trees in the protected areas. This finding supports the hypothesis that in the farmed lands only selected large shea trees are preserved.
The spatial pattern of juveniles was similar in the farmed lands and protected zones (Fig. 4c, d); in both cases there was clear aggregation with the GoF test yielding ranks of 1,000 for both statistics g and G. The aggregation had a range of some 12 m.
The spatial relationship between juveniles and adults differed strongly between farmed lands and protected areas. Juveniles at the farmed lands were independent from adults (Fig. 4e; the GoF test for g yielded a rank of 988), whereas the null model of independence was overwhelmingly rejected for the protected areas (Fig. 4f; the GoF test for g yielded a rank of 1,000). Juveniles were significantly clustered around adults up to some 6 m (Fig. 4f). Similarly, the GoF test for the distribution of nearest neighbor distances yielded a rank of 999 for the farmed lands and 1,000 for the protected areas.
Discussion
Shea tree population under anthropic disturbances
Our study confirmed that land use impacts woody vegetation, particularly the distribution of shea trees in the Pendjari region. Small shea trees (dbh < 10 cm) were still frequent in the farmed lands, most likely because the local population intentionally preserves adult, reproductive shea trees. This allows natural regeneration around the adult trees (Boffa 1999; Lovett and Haq 2000). In agricultural areas still farmed in more traditional ways, overall vegetation coverage is low because of regular cropping. Laris and Wardell (2006) confirmed this finding with their study on fires in Mali when they stated that the expansion of the area under agriculture coupled with a reduction in the fallow cycle is a more probable cause of tree cover decline. This facilitates establishment of shea tree seedlings. However, we found that farming activities have a negative effect on saplings (Fig. 3a). The overall density of juveniles was lower in farmed lands than in protected areas. This result is in accordance with results from a study carried out in Mali, an other West African country, where individuals of small age classes occurred at lower numbers in village parklands and young fallows when compared to old fallows and forests where individuals of small age classes were found in larger numbers (Kelly et al. 2004). Seedlings need light for growth so that disturbance should foster regeneration; in addition, low density of woody plants in farmed lands reduces intra and interspecific competition and soil fertilization for agriculture also provide nutrients for seedlings. Despite these conditions we found an important reduction of the density of saplings (dbh 5–10 cm) in these areas.
At least two explanations can be given for those findings. First, fallow cycles are more and more shortened because of increasing demographic pressure where farmers constitute the largest part of the growing human population. Second, when farmers return to the fallows, they often cut the majority of shea trees that are <2 m in height preserving only the tallest ones (personal observations) because only these individuals could start fruit production in a near future.
Although the juvenile trees are easily identified by the local population and although the socio-economic importance of this plant is known, the farmers cut some of the juveniles of shea trees together with other juveniles of undesired trees when cropping the vegetation cover. They preserve mainly the mature shea trees as they are of higher immediate value as they regularly produce large amounts of fruit. Furthermore, planning of the local population is mostly limited to their daily needs but very often not directed into the long-term future, knowing for example that it takes a long time until shea trees become productive. Indeed shea trees take 15–25 years to grow to maturity. They can live up to 200–300 years (Hall et al. 1996). Therefore, the current reduction of the density of sapling by agricultural practices does not immediately translate into a decline of the adult population but will certainly be noticeable within a few decades when the adult trees have reached old age and will subsequently die without adequate replacements.
Our data also revealed that most of the tall trees with large and extensive crowns occur in farmed lands. These findings are supported by Lovett and Haq (2000) who found in the Gonja districut of northern Ghana that anthropic selection of shea trees through elimination of other undesired trees as well as selected shea trees taking into account criteria such as spacing, growth, size, age, health, and productivity lead to an overrepresentation of large trees on farmed lands in comparison to smaller trees. This situation underlines the lack of appropriate management of this valuable natural resource where only exploitation is put into the foreground without consideration of long-term conservation of this resource for future generations. Explanation oriented toward the necessity of preserving enough saplings around adult trees in order to maintain similar shea tree population density have to be done with local human population in these areas to guaranty long-term conservation of this plant species.
Spatial distribution of shea tree with regard to land use
Regarding the spatial distribution pattern of shea trees under human impact, we documented a weakly aggregated distribution pattern of adult trees and no association between adults and juveniles in the farmed lands. This result shows that human influence on this plant species does not produce, at least now, regular distribution patterns or repulsion between adult and juvenile trees. Because as hypothesized, aggregation/attraction goes with absence or minimum disturbance whereas regular/repulsion means intense disturbance. Adult trees were much less aggregated than those in protected areas and juveniles were significantly clustered around adults in the protected areas. Thus, human impact made the patterns clearly more regular.
Since juveniles are the offspring of adult trees, we expected a clumped distribution pattern of juveniles and a positive association between juveniles and adult trees under protected conditions because juveniles are supposed to establish around adult trees. Fruit bats in these areas actively disperse shea seeds, as shea fruit constitute a key resource for them (B. A. Djossa, submitted data). Inside the protected areas of the BRP where plots were established, humans do not harvest fruit. Fruits are removed only by animals (primates, elephants, rodents, birds, and bats) that consume part of the fruit crop which is produced during a season. As the animals do not fully deplete the tree, enough fruits and hence seeds remain available as source for natural regeneration. Frugivores that then take the fruit and hence the seeds away and eat it in close proximity of the tree leading to the positive association of parent tree and juvenile. Here the parent tree acts so to speak as a source. The clumped distribution of the juveniles might then be the results of feeding roosts where animals drop then several seeds on the forest floor. These seeded fruit cannot be carried too far from parent trees and cannot pass alimentary canal so that seeds are simply dropped in the vicinity. Some seeds germinate even next to the parent tree, thus creating a clumped distribution. Taking humans into account with their use of shea fruits, they usually harvest almost all fruit and thus reduce natural regeneration because there are few fruits left. In addition, when natural regeneration occurs with the remained seed bank, agricultural activities also reduce saplings. Kelly et al. (2004) carried out a similar study in Mali and concluded that the spatial pattern of V. paradoxa became progressively more aggregated from cultivated fields to fallows and then to forest. Kelly et al. (2004) concluded that differences in aggregation between sites were mostly explained by the intensity of fruit production and subsequent recruitment whereas increasing regularity of tree distribution found in farmed lands and fallows was due to human intervention. In our study we only considered fallows and protected areas (equivalent to forest in their study) because we were concerned with natural regeneration of the target tree species whereas Kelly et al. (2004) did not analyze seedlings (diameter girth at breast height from 20 cm equivalent to dbh > 5 cm). Our study had better resolution on population structure.
We found the largest individuals mainly in farmed lands whereas the smallest individuals occurred more frequently in protected areas. Our spatial pattern analysis provides evidence that disturbance may cause more regular distributions. In accordance with our findings, a regular distribution of trees was found in Mali at the most cultivated site whereas trees were more aggregated at the site with fewer disturbances.
The lack of a spatial association between juvenile and adult trees in farmed lands may also be caused by the fact that trees in fields are generally much larger than in protected areas with extensive crown areas that affect seedlings negatively through out-shading since V. paradoxa is considered a light demanding species. Consequently, seedlings could be negatively affected when they are too close to adult trees because of competition for light and nutrients (Hall et al. 1996). The Janzen–Connell model that states that seeds fall under parent trees and subsequent seedlings are under the pressure of predators and pathogens of parents that reduce there density (Janzen 1970 and Connell 1971; Condit et al. 1992, 1996; Hyatt et al. 2003) is not applicable here because most seeds are collected by women so that seed predation by domestic animals is unlikely and pathogens like vegetal parasites (Tapinanthus sp.) were found exclusively on adult trees but not on seedlings. Inside the protected areas (BRP) the absence of a clear aggregation in the distribution pattern of individuals and the association of juvenile and adult trees at large scales can be explained by the Janzen–Connell model because seeds are removed by seed predators such as rodents and primates (i.e., baboons) which are often seen eating fruits of shea trees. This supports colonization hypothesis of Janzen–Connell that states the negative correlation between the amount of seed transported from parent trees and distance leading to a reduction of density of offspring.
Conservation status
In terms of the conservation status of shea trees, the negative effect of intense local agricultural practices by humans might push the distribution pattern of the trees from aggregation to regularity (univariate) or from attraction to repulsion (bivariate), finally leading toward the distribution of only few isolated individuals. As we still found random (univarite) and independent (bivariate) distribution patterns of shea trees in farmed lands, we conclude that shea trees are still rather well preserved. However, additional studies are crucial to appreciate the population dynamics of shea trees linking for example age class with seed production, seedling establishment and finally viability of juveniles in time (in French “matrice de transition”). This information is necessary to appreciate in more detail the conservation status of this plant species in this region.
Many authors reported that trees in tropical ecosystems show in general random or clumped distribution pattern (Condit et al. 1992, 2000; Wills et al. 1997; Hubbell et al. 1999). Our findings are in accordance with these findings. Aggregation was mostly limited to neighborhood scales of about 12 m. This may be related to seed dispersal where seeds are dispersed for short distances away from the parent tree and thus rapidly decrease in density with increasing distance to the source (Harper 1977; Howe and Smallwood 1982; Willson 1993 in Nathan and Casagrandi 2004). In the BRP, the annual burning of the vegetation can also play an important role with regard to the distribution pattern and survival rates of seedlings, in particular in years with a lot of dry biomass. Bruner et al. (2001) pointed out the effectiveness of park in biodiversity conservation and negative effects of fire with regard to biodiversity conservation. Fire is annually used inside the BRP as a management tool mainly for renewal of resources (i.e., pastures) for native herbivores such as antelopes (see Sinsin and Saïdou 1998). Prescribed burning is used at the end of the rainy season when the biomass still not very dried in order to avoid intense fires that could be detrimental to plants, soil microorganisms as well as a large number of invertebrates and vertebrates. Nikiema (2005) reported from Burkina Faso that early fire (end of rainy season) is less violent. However, fire is a problem for the fragile seedlings of shea trees (Grime 2001; Hölzel 2005) because if the seedling is not completely burned it has to renew its stem growth and leaves each season, generally starting from near ground level. This slows down firm seedling establishment and growth. Even for mature trees, the annual vegetation burning causes the smaller tree crowns as noticed in BRP compared with farmed lands where vegetation fire also exist but with less biomass to burn and thus fewer damage. Moreover, similar to what Laris and Wardell (2006) reported from Mali, early fire is used by local population living around BRP to reduce risks of damaging trees preserved or planted by them. Our findings are supported by Hjerpe et al. (2001) who reported that 5 years after a cyclone and fire had impacted a rain forest reserve on the Tafua peninsula Savai’I in Samoa, canopy cover increased less in burned area than in cyclone destroyed area. The fire favored fast growing pioneer species more than the cyclone leading to higher mean numbers of species per plot in the unburned area compared to the burned area. We derive from those results that the small size of the shea trees (dbh and height of productive trees) found in BRP zones compared to farmed lands could also be attributed in part to the effects of the annual fire that stresses trees and slows down their growth even if fire stress has also been recognized to produce positive and fast phenological activities on shea trees (Hall et al. 1996) so that leaves renewing and flowering start short after vegetation burning.
Conclusion
This study assessed the distribution of shea trees (V. paradoxa) in protected areas (BRP) and in farmed lands. Overall, shea trees are well preserved in parklands. Furthermore, traditional farming activities foster the growth of young shea trees (seedlings) by reducing potential competition with other tree species. This leads to parklands that are dominated by shea trees. As a consequence of the traditional agricultural practices, most of the large, mature individuals are in farmed lands. Point pattern analysis of the mapped tree locations in plots in farmed lands revealed random distribution patterns for adult and juvenile trees and no association of juveniles to adults. In plots located in the BRP, we found significant small-scale aggregation of juveniles and significant and positive small-scale associations of juveniles to adults. The absence of small-scale aggregations in plots of farmed lands demonstrates to our opinion the influence of this type of land-use. The low number of saplings (dbh 5–10 cm) in farmed lands compared to protected lands is likely to have long-term consequences on the viability of shea tree populations and may in the long run even lead to a collapse of the V. paradoxa population. As a suggestion to managers we recommend the establishment of meaningful management plans for shea trees in the region, particularly the village territories surrounding the BRP for maintenance of the current shea tree density and a better preservation for saplings. This is the only way to guarantee the presence of this economical important plant species also for future generations.
References
Agbahungba G, Depommier D (1989) Aspects du parc a karites1-neres (Vitellaria paradoxa Gaertn. f. Parkia biglobosa Jacq. Benth) dans le sud du Borgou (Benin). Bois et Forets des Tropiques 222:41–54
Ayensu EA (1974) Plant and bat interaction in West Africa. Ann Mo Bot Gard 61:702–727
Boffa JM (1999) Agroforestry parklands in sub-Saharan Africa. FAO conservation guide 34. FAO, Rome
Bruner AG, Gullison RE, Rice RE, da Fonseca GAB (2001) Effectiveness of parks in protecting tropical biodiversity. Science 291:125–128
Chevalier A (1946) L’arbre à beurre d’Afrique et l’avenir de sa culture. Oléagineux 1:7–11
Condit R, Ashton PS, Baker P, Bunyavejchewin S, Gunatilleke S, Gunatilleke N, Hubbell SP, Foster R, Itoh A, Lafrankie JV, Lee HS, Losos E, Manokaran N, Sukumar R, Yamakura T (2000) Spatial patterns in the distribution of tropical tree species. Science 288:1414–1418
Condit R, Hubbell S, Foster R (1992) Recruitment near conspecific adults and the maintenance of tree and shrub diversity in a neotropical forest. Am Nat 140:261–286
Connell JH (1971) On the role of natural enemies in preventing competitive exclusion in some marine animals and rain forest trees. In: den Boer PJ, Gradwell PR (eds) Dynamics of populations. Centre for agricultural publishing and documentation, Wageningen, pp 289–312
Delvingt W, Heymans JC, Sinsin B (1989) Guide du parc national de la Pendjari. Communauté Economique Européenne (C.E.E.), Bruxelles
Dudgeon WR (1922) The agricultural and forest products of West Africa. Murray, London
Fa JE, Garcia Yuste JE, Castelo R (2000) Bushmeat markets on Bioko Island as a measure of hunter presence. Conserv Biol 14:1602–1613
Fa JE, Peres CA, Meeuwig J (2002) Bushmeat exploitation in tropical forests: an intercontinental comparison. Conserv Biol 16:232–241
Fa JE, Ryan SF, Bell DJ (2005) Hunting vulnerability, ecological characteristics and harvest rates of bushmeat species in afrotropical forests. Biol Conserv 121:167–176
Goreaud F, Pelissier R (2003) Avoiding misinterpretation of biotic interactions with the intertype K-12-function: population independence vs. random labeling hypotheses. J Vegetation Sci 14:681–692
Grime JP (2001) Plant strategies, vegetation processes and ecosystem properties, 2nd edn. Wiley, Chichester, UK
Hall JB, Aebischer DP, Tomlinson HF, Amaning EO, Hindle JR (1996) Vitellaria paradoxa: a monograph, school of agricultural and forestry sciences. Publication Number: 8, University of Wales, Bangor, UK, p 105
Hjerpe J, Hedena H, Elmqvist T (2001) Tropical rain forest recovery from cyclone damage and fire in Samoa. BIOTROPICA 33(2):249–259
Hölzel N (2005) Seedling recruitment in flood-meadow species: the effects of gaps, litter and vegetation matrix. Appl Vegetation Sci 8:115–224
Hubbell S, Foster R, O’Brien S, Wechsler B, Condit R, Harms K, Wright S, De Loo Lau S (1999) Light gaps, recruitment limitation and tree diversity in a neotropical forest. Science 283:554–557
Hyatt LA, Rosenberg MS, Howard TG, Bole G, Fang W, Anastasia J, Brown K, Grella R, Hinman K, Kurdziel JP, Gurevitch J (2003) The distance dependence prediction of the Janzen-Connell hypothesis: a meta-analysis. Oikos 103:590–602
INSAE/MPPD (2002) Rapport du troisième recensement général de la population et de l’habitat. RGPH-3, Bénin
Jackson G (1968) Notes on West African Vegetation. 3. The seedling morphology of Butyrospermum paradoxum (Gaertn. f.) Hepper. J West Afr Sci Assoc 13:215–222
Janzen DH (1970) Herbivores and the number of tree species in tropical forests. Am Nat 104:501–528
Kelly BA, Hardy JO, Bouvet J-M (2004) Temporal and spatial genetic structure in Vitellaria paradoxa (shea tree) in an agroforestry system in southern Mali. Mol Ecol 13:1231–1240
Killick HJ (1959) The ecological relationship of certain plants in the forest and savanna of central Nigeria. J Ecol 47:115–127
Laris P, Wardell AD (2006) Good, bad or ‘necessary evil’? Reinterpreting the colonial burning experiments in the savanna landscapes of West Africa. Geogr J 172(4):271–290
Loosmore NB, Ford ED (2006) Statistical inference using the G or K point pattern spatial statistics. Ecology 87:1925–1931
Lovett PN, Haq N (2000) Diversity of the Sheanut tree (Vitellaria paradoxa C.F. Gaertn.) in Ghana. Genet Resour Crop Evol 47:293–304
Maranz S, Wiesman Z (2003) Evidence for indigenous selection and distribution of the shea tree, Vitellaria paradoxa, and its potential significance to prevailing parkland savanna tree patterns in sub-Saharan Africa north of the equator. J Biogeogr 30:1505–1516
Nathan R, Casagrandi R (2004) A simple mechanistic model of seed dispersal, predation and plant establishment: Janzen-Connell and beyond. J Ecol 92:733–746
Nikiema A, van der Maesen LJG, Hall JB (2001) The impact of parkland management practices on plant resources diversity. In: Teklehaimanot Z (ed) Improved management of agroforestry parkland systems in Sub-Saharan Africa. EU/INCO Project Contract IC18-–CT98-0261, 3rd Annual Progress Report, University of Wales Bangor, UK, pp 57–66
Nikiema A (2005) Agroforestry parkland species diversity: uses and management in semi-arid West Africa (Burkina Faso). Dissertation, Wageningen Universty, The Netherlands, p 102
PAG2 (2005) Plan d’Aménagement Participatif et de Gestion 2004–2013. Parc National de la Pendjari, Bénin, pp 83 + Annexes
Ripley BD (1981) Spatial statistics. Wiley, New York
Rondeux J (1999) Mesure des arbres et peuplements forestiers. Presses Agronomiques de Gembloux, Belgium, p 521
Ruyssen B (1957) Le karité au Soudan, Premiere Partie, Aire géographique du karité en Afrique et au Soudan. L’Agronomie Trop 11:143–171
Sinsin B, Tehou AC, Daouda I, Saidou A (2002) Abundance and species richness of larger mammals in Pendjari National Park in Benin. Mammalia 66(3):369–380
Sinsin B, Saïdou A (1998) Impact des feux contrôlés sur la productivité des pâturages naturels des savanes soudano-guinéennes du ranch de l’Okpara au Bénin. Annales des Sci Agronomiques du Bénin 1:11–30
Sokpon N, Biaou H, Hounhyet O, Ouinsavi C, Barbier N (2001) Inventaire et caractérisation des formations végétales du complexe national de la Pendjari, zone cynégétique de la Pendjari et de l’Atacora: région de Konkombri. Rapport, UNB/Bénin III, pp 56 + Annexes
Stoyan D, Stoyan H (1994) Fractals, Random shapes and point fields. Methods of geometrical statistics. Wiley, Chichester
Stoyan D, Penttinen A (2000) Recent application of point process methods in forest statistics. Stat Sci 15:61–78
Teklehaimanot Z (2003) Improved management of agroforestry parkland systems in Sub-Saharan Africa. EU/INCO Project Contract IC181-CT981-0261, 3rd Annual Progress Report, University of Wales Bangor, UK, pp 57–66
Thibault M, Blaney S (2003) The oil industry as an underlying factor in the bushmeat crisis in central Africa. Conserv Biol 17(6):1807–1813
Tran T (1984) Shea butter—more than just a cosmetic ingredient. Drug Cosmet Ind 54:54–61 and 114–116
Wiegand T, Moloney K (2004) Rings, circles and null1-models for point pattern analysis in ecology. Oikos 104:209–229
Wills C, Condit R, Foster R, Hubbell S (1997) Strong density—and diversity—related effects help to maintain tree species diversity in a neotropical forest. Proc Natl Acad Sci 94:1252–1257
Acknowledgments
We are very grateful to BIOTA West Africa for financial support during this work and to members of stochastic department of University of Ulm for contributing on statistical aspect. We also want to thank the manager team of the BRP for creating a good working environment. Finally we want to express our gratitude to all farmers in villages surrounding the BRP that collaborated and accepted that we used their fields for data collection.
Author information
Authors and Affiliations
Corresponding author
Appendix: combining the data from individual mapped replicate plots into mean, weighted O(r), and G(y) functions
Appendix: combining the data from individual mapped replicate plots into mean, weighted O(r), and G(y) functions
For statistical analysis it is common to map several replicate plots of a larger point pattern under identical conditions. In this case the resulting second-order statistics of the individual replicate plots can be combined into average second-order statistics (Diggle 2003). This is of particular interest if the number of points in each replicate plot is relatively low. In this case the simulation envelopes of individual analyses would become wide, but combining the data of several replicate plots into average second-order statistics increases the sample size and thus narrows the confidence limits. Average second-order statistics are also an effective way of summarizing the results of several replicate plots.
When the patterns are strict replicates of an underlying process, the corresponding estimates \( \hat{K}_{i} (r) \) of the K-functions from plots i are identically distributed and a reasonable overall estimate can be obtained by simply averaging the individual K-functions (Diggle 2003: Eq. 4.20 at page 52). Using the grid-based estimator of Programita, the resulting estimator of the O-ring statistic is given in the appendix of Riginos et al. (2005).
However, because Ripley’s K-function K(r) is defined as a ratio of expected number of points in circles [= λK(r)] divided by the intensity λ, a better strategy may be to pool separately estimates of λ and λK(r). The resulting average K-function is a weighted average of the individual estimates \( \hat{K}_{i} (r) \), where the weight is the number of points in plot i divided by the total number of points in all replicate plots (Diggle 2003: Eq. 8.11, page 123). Note that the resulting average K-function is also an appropriate estimator if the replicates would be differentially thinned versions of a common underlying process (Diggle 2003: p 123).
Using the grid-based estimators of Programita and following the notation in Wiegand and Moloney (2004) (their Eq. 11), the numerical estimator of the bivariate pair-correlation function g 12(r) is calculated as:
where n 1 is the number of points of pattern 1, R w1,i (r) is the ring with radius r and width w centered in the ith point of pattern 1, Points 2 [X] counts the points of pattern 2 in a region X, and the operator Area[X] determines the area of the region X.
To integrate the data of N different replicates into a single weighted pair-correlation function, the formula for one replicate (Eq. A1) is extended by calculating, for each spatial scale r, the average weighted number of points of pattern 2 taken over all N replicates and the average weighted area taken over all N replicates:
where i j is the ith point of pattern 1 and replicate j, n j1 is the number of points of pattern 1 and replicate j, and N = ∑j n j1 is the total number of points of pattern 1 in all replicates. Equation A2 simplifies to:
Following the strategy of Diggle (2003) to pool separately estimates of λ 2 and λ 2 K 12(r) [and analogously estimates of λ 2 and λ 2 g 12(r) for estimating the pair-correlation function] the overall intensity λ 2 is estimated as λ 2 = N 2/A where
is the total number of points of pattern 2 in all N replicates j, and A the total area
of all replicates j with area A j.
The univariate estimator of g(r) is calculated in a manner analogous to the bivariate functions by setting pattern 1 equal to pattern 2.
Because we did not use edge correction for the distribution function G of nearest neighbor distances we simply summed up the frequency distributions of single replicated and normalized the frequency distribution after combining all replicated to yield the normalized G 12(y) and G(y).
Literature cited in appendix
Diggle PJ (2003) Statistical analysis of point patterns, IInd edn. Arnold, London
Riginos C, Milton SJ, Wiegand T (2005) Context-dependent negative and positive interactions between adult shrubs and seedlings in a semi-arid shrubland. J Vegetation Sci 16:331–340
Wiegand T, Moloney KA (2004) Rings, circles and null-models for point pattern analysis in ecology. Oikos 104:209–229
Rights and permissions
About this article
Cite this article
Djossa, B.A., Fahr, J., Wiegand, T. et al. Land use impact on Vitellaria paradoxa C.F. Gaerten. stand structure and distribution patterns: a comparison of Biosphere Reserve of Pendjari in Atacora district in Benin. Agroforest Syst 72, 205–220 (2008). https://doi.org/10.1007/s10457-007-9097-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-007-9097-y