Abstract
Amomum villosum grown in the traditional way for economic purpose in Xishuangbanna, southwest China, causes some damages to the local tropical seasonal rain forests. It is important to evaluate the effects of irradiance and soil moisture on A. villosum in order to find out ways to maintain the local forest ecosystems on the one hand and to promote its economic cultivation with agroforestry practices on the other hand. Soil moisture was the main determinant of plant growth. Seedling height, leaf number and area, biomass, relative growth rate (RGR) and net assimilation rate decreased significantly with the decrease of soil moisture. There were no interactions between soil moisture and irradiance on all the plant traits evaluated in this study. The effects of soil moisture and irradiance were orthogonal; drought reduced RGR at a similar degree at all irradiance levels. The plant can acclimate to high irradiance combined with low soil moisture. Under this condition, A. villosum modified its biomass allocation in favor to roots rather than to leaves so that whole-plant level water balance could be well maintained. Furthermore, many small-sized slender leaves were formed to facilitate leaf thermal loss, its carotenoid content increased in favor for photoprotection, and contents of chlorophyll and light-harvesting complex of photosystem II decreased so that its irradiance interception was better balanced for plant adaptation. Our results indicated that soil moisture is a more important factor to concern than irradiance when planting A. villosum in agroforestry practices and that A. villosum can be grown in high light habitats such as secondary forests and artificial forests when soil moisture is adequate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amomum villosum Lour. (Zingiberaceae) is a perennial evergreen herb and is considered as an obligate shade plant that occurs naturally in the understorey of humid subtropical forests. Its fruit is of importance for traditional medicine and spice in many Asian countries. For economic purpose, A. villosum was introduced into tropical seasonal rain forests of Xishuangbanna from Guangdong province, southeast China in 1963. Farmers often thin out some 30% of canopy trees and clear away all of the shrubs and herbs when they grow this plant in the forest, consequently leading to disappearance of 70%–90% of the species and simplification of the community structure (Zheng et al. 2004). Therefore, many ecologists and conservationists urge to change the current way to grow A. villosum in the seasonal rain forests in China even though the local minorities get used to this way and about half of their income comes from this plant. A. villosum can be grown in secondary forests (Feng et al.2005) and artificial forests (Zhou et al. 1997) although these agroforestry practices are uncommon in Xishuangbanna. To popularize its cultivation in these agroforestry systems and to increase economic returns from these practices it is necessary to study the effects of ecological factors on A. villosum. Irradiance and soil moisture are two main factors influencing growth and yield of A. villosum.
A. villosum can grow in the understorey with 5%–85% irradiance. It can grow and attain high yield even in the environment with several hours direct sunlight each day (He 1991; Feng et al.2002). In a survey of 12 populations grown under primary and secondary forests in Xishuangbanna, Feng et al. (2005) found that A. villosum performs best in a secondary forest located beside a brook. This is consistent with the previous findings that A. villosum has a high yield when grown at moisture sites (He 1991). A. villosum is susceptible to drought due to its shallow root system and low root mass fraction (Feng et al.2005). However, there are no experimental studies on the quantitative effects of soil moisture and the combined effects of irradiance and soil moisture on A. villosum.
The respective effects of irradiance and water on plants have been studied intensively, but few studies on their combined effects have been conducted (but see Sack and Grubb 2002; Aranda et al. 2005) and the results are inconsistent (Sack and Grubb 2002). The influential trade-off hypothesis (Smith and Huston 1989) predicts that drought has stronger impacts on individuals of a species grown in deep shade than on those under higher irradiance. Compared with plant grown at higher irradiance, shaded-plant generally has a higher specific leaf area (SLA) and invests more biomass to leaves but less to roots for efficient light capture (Poorter 1999; Feng et al. 2007; Franck et al. 2007), which might decrease its drought tolerant ability. According to above-ground facilitation hypothesis (Holmgren 2000) shade decreases leaf and air temperature, leaf-to-air vapour pressure deficit (Ishida et al. 1999), oxidative stress, and therefore can reduce the impacts of drought on growth and photosynthesis (Valladare and Pearcy 1997). The interplay hypothesis (Holmgren et al. 1997) predicts that the impacts of drought are strong at both high and low irradiances and weaker at intermediate irradiance due to facilitation. The independent effects model (Nobel 1999) predicts that the impacts of irradiance and soil moisture are orthogonal and drought reduce relative growth rate (RGR) by a given proportion at any irradiance.
The inconsistent results in literatures might be due at least partially to the facts that it is difficult to obtain the same soil moisture level at different irradiance levels. In general, subject to the same severe drought seedlings suffer more when grown in the understorey than in gaps due to their smaller root system. Therefore, such studies should be conducted under controlled conditions for more precise analyses and there are some of controlled experiments conducted for these purposes. But in most of these studies (Holmgren 2000; Sack and Grubb 2002), soil moisture is controlled simply through natural evapotranspiration loss of soil water, leading to generally severer drought for seedlings grown at high irradiance than at lower irradiance (Sack and Grubb 2002). Similar problem appears when soil moisture is controlled with different watering frequency. Soil moisture fluctuates greatly when the watering frequency is too low if a severe drought treatment is applied for more serious drought treatment. Furthermore, almost all of these studies focus only on forest tree seedlings (but see Baruch et al. 2000) under low levels of irradiance treatments. We hypothesized that soil moisture was the main factor affecting growth of A. villosum, and that there were significant interactions between soil moisture and irradiance, i.e. the optimal range of soil moisture contents for A. villosum was different at different irradiance levels and the same for irradiance. To test the hypotheses, the combined effects of four soil moisture levels (adjusted daily) and three irradiance levels in a wide range on herbaceous A. villosum were analyzed in this study. We also wanted to determine how the obligate shade plant with shallow root system acclimates to high irradiance and low soil moisture.
Materials and methods
Study site
This study was carried out at Xishuangbanna Tropical Botanical Garden (21°56′ N, 101°15′ E) of the Chinese Academy of Sciences in Mengla county, Yunnan province, southwest China. The mean annual temperature is 21.7°C in this area. The mean temperature in July, the hottest month is 25.3°C and that of January, the coolest month is 15.6°C. The average annual precipitation is 1557 mm with the dry period lasting from November to April (Feng et al. 2002). From November to February, there is a heavy fog cover almost daily from midnight to next midday. The fog contributes significantly to maintaining the moistures of both air and soil. Therefore, this duration of dry season is designated as foggy and cool season in this area, and the remaining months of dry season as dry and hot season. The fog also reduces integrated daily irradiance (24.8 versus 28.1 mol photons m−2 d−1 in sunny day).
Materials
From a 100-years-old seasonal rain forest, similar-sized seedlings (with two or three small-sized leaves) of A. villosum were collected in September 2003. The main tree species in the forest were Terminalia myriocarpa Van Heurck, Pometia tomentosa (Blume) Teysm. Et Binn., Barringtonia fusicarpa Hu, Baccaurea ramiflora Lour., Horsfieldia tetratepala Wu, Horsfieldia kingii (Hook. F.) Warb, Myristica yunnanensis Y. K. Li. The canopy height of the forest was 40–45 m. The transmission of daylight at the height of A. villosum was 30%–60%. The seedlings were transplanted singly into pots (35 cm in diameter and 25 cm in depth). The pots were filled with a mixture of river sand and top layer of forest soil (3:7). The river sand provided a texture with adequate drainage and facilitated harvest of the whole plant root system, including fine roots (Poorter 1999). The seedlings were divided into three groups randomly after being grown at 50% irradiance for three weeks, and each group was grown under one of the three irradiance levels applied in this study, respectively. The seedlings at each irradiance level were randomly divided into four sub-groups on 23 October 2003 after being grown for another three weeks, and each sub-group was grown under one of the four soil moisture levels applied in this study, respectively. Thirty seedlings per soil moisture per irradiance were grown. Four seedlings, one from each soil moisture treatment, were put together as a group in each shade house, and different groups were randomly positioned in the shade house and re-randomized within it fortnightly through the experiment. Seedlings were fertilized monthly with complex fertilizer (15% N, 7% P and 8% K) at 4 g per pot and kept away from diseases and pests.
Irradiance and water treatments
The potted seedlings were grown in three shade houses with 25%, 50% and 80% irradiance, respectively, which were created by covering the shade houses with different layers of nylon shade netting and one layer of colorless transparent plastic film. The plastic film was used to prevent rain from influencing soil moisture control. The light intensity applied in this study represented the light continuum that A. villosum encountered in forests in Xishuangbanna. The relative irradiance in each shade house was estimated by comparing the integrated photosynthetic photon flux density (PPFD) in the house during a clear day in the summer with that in a fully open site. The PPFD was measured with Li-1400 (Li-Cor, Lincoln, Nebraska, USA). The lower 30 cm of each shade house was open to facilitate air flow and to reduce the potential effects of other environmental factors except irradiance. Four soil moisture levels, i.e. 40%, 60%, 80% and 100% (percent of field capacity soil water content), were used in this study. Before the seedlings were transplanted, the weight of each pot (W p) and the weight of each pot plus soil with field capacity water content (W p+s+w), and field capacity soil water content (0.21 g H2O g−1 dry soil in this study) were determined. For controlling soil moisture, the total weight of each pot (W t, including the weight of pot, soil, saturated water and seedling) was measured after being fully watered. The weight of each pot under designated soil moisture can be calculated as W t−0.21 * (1-soil moisture) * (W p+s+w−W p)/(1 + 0.21), where soil moisture were 0.4, 0.6, 0.8 and 1, respectively. Soil moisture was controlled by watering daily at 17:00 according to the mean daily evapotranspiration under each treatment, which was calculated by weighing five pots per light per soil moisture. The water was added to each pot through a 5 cm depth (1.5 cm diameter) hole in the center of each pot.
Measurements
Height and leaf number of 20 seedlings per treatment were recorded every 23–25 d. For each treatment, the initial harvest was carried out on 5 November 2003 after the seedlings grew under their new soil moisture for two weeks. The final harvest was carried out on 5 May 2004. Eight seedlings per irradiance per soil moisture were used at each harvest. Seedling height, total leaf area, and biomass of leaves, stem and roots were determined at the harvest. The length, width and area of the third - seventh leaves (from tip) of each seedling were measured for determining mean leaf size (MLS) and the ratio of leaf length to width (L:W). Leaf area was determined with Li-3000A leaf area meter (Li-Cor, Lincoln, Nebraska, USA). Plant parts were oven-dried for 48 h at 80°C.
The following variables were derived from the primary data: (1) root mass fraction (RMF, root mass/total seedling mass, in g g−1); (2) stem mass fraction (SMF, stem mass/total seedling mass, in g g−1); (3) leaf mass fraction (LMF, leaf mass/total seedling mass, in g g−1); (4) leaf area root mass ratio (LA:RM, leaf area/root mass, in dm2 g−1 ); (5) leaf area ratio (LAR, leaf area / total seedling mass, in cm2 g−1 ); (6) SLA (leaf area/leaf mass, in dm2 g−1); (7) MLS (total leaf area/leaf number, in cm2); (8) L:W (leaf length/width, in cm cm−1); (9) RGR (biomass growth per unit plant biomass, in mg g−1 d−1); (10) net assimilation rate (NAR, biomass growth per unit leaf area, in mg cm−2 d−1); (11) mean leaf area ratio (LARm, mean leaf area per plant mass, in cm2 g−1). The growth analysis was conducted according to Hunt et al. (2002).
Before the final harvest, the diurnal change of net photosynthetic rate (P n) was measured at the intervals of 1.5 h from 09:00 to 17:00 on three clear days (12–14 March 2004) using Li-6400 (LI-COR, Lincoln, Nebraska, USA). Lately fully expanded leaves (the third leaf from tip) of four randomly chosen seedlings were measured for each treatment. The leaves for determining P n were also used to detect chloroplast pigments content. Pigments were extracted with 80% acetone (v/v) and determined using spectrophotometry (Lichtenthale and Wellburn 1983).
Statistical analysis
Effects of soil moisture, irradiance and their interaction on variables measured in this study were evaluated with a two-way ANOVA. The relative importance of each independent factor on each variable was estimated according to the value of Eta squared. The differences in each variable among different levels of the same factor (irradiance or soil moisture) were analyzed using a one-way ANOVA (LSD test). SPPS 10.0 (SPSS Inc. Chicago, Illinois, USA) was used for all the statistical analysis.
Results
Growth
Leaf number increased almost linearly with the time of treatments (Fig. 1d–f). Seedling height increased slowly at the beginning of the experiment but faster 100 days after treatments (Fig. 1a–c), and at this time the effects of soil moisture on both height and leaf number became evident. During the six months of treatments, A. villosum grew by about 40 cm in height and developed into 16 new leaves at 50% irradiance and 100% soil moisture (Fig. 1b, e).
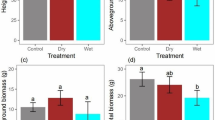
Soil moisture was the main determinant of the final seedling height, leaf number, total leaf area, biomass, RGR and NAR (Table 1). The six variables increased significantly with the increase of soil moisture (Fig. 2). The effects of irradiance were also significant on seedling height, leaf number, total leaf area and biomass, but not on RGR and NAR (Table 1). Across the irradiance levels, seedling height, total leaf area and biomass were lowest at 80% irradiance, while leaf number was lowest at 25% irradiance (Fig. 2). The differences in LARm among treatments were not significant (Table 1), indicating that the differences in RGR among treatments were determined mainly by NAR.
(a) Seedling height; (b) leaf number; (c) total leaf area; (d) biomass; (e) relative growth rate (RGR); and (f) net assimilation rate (NAR) for Amomum villosum grown at 25% (circle), 50% (triangle up), 80% (triangle down) irradiances and 40%, 60%, 80% and 100% relative soil moistures for six months. Mean ± SE (n = 8). Different small and capital letters indicate significant differences among soil moistures and among irradiances, respectively (LSD-test of a one-way ANOVA, P < 0.05)
Biomass allocation and morphology
Irradiance was the main determinant of RMF, SMF, LA:RM and L:W, while soil moisture was that of LMF, SLA and MLS (Table 1). At 80% and 50% irradiance levels and with decreasing soil moisture, RMF increased while LMF and LA:RM decreased (Fig. 3a–c). The effects of irradiance on LMF were not significant (Table 1). Root mass fraction was significantly higher while LA:RM lower at 80% and 50% irradiances than at 25% irradiance. With increasing soil moisture, SLA and L:W decreased while MLS increased (Fig. 3d–f). The mean leaf size was the highest at 50% irradiance. When soil moisture was in the range of 40%–80%, A. villosum showed the lowest MLS and SLA and the highest L:W at 80% irradiance, leading to small-sized, thin and slender leaves.
(a) Root mass fraction (RMF); (b) leaf area root mass ratio (LA:RM); (c) leaf mass fraction (LMF); (d) specific leaf area (SLA); (e) leaf length to width ratio (L:W); and (f) mean leaf size (MLS) for Amomum villosum grown at 25% (circle), 50% (triangle up), 80% (triangle down) irradiances and 40%, 60%, 80% and 100% relative soil moistures for 6 months. Mean ± SE (n = 8). Different small and capital letters indicate significant differences among soil moistures and among irradiances, respectively (LSD-test of a one-way ANOVA, P < 0.05)
Photosynthesis and chloroplast pigments
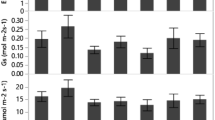
In the morning P n increased and peaked at noon, then decreased in the afternoon and the magnitude of decrease was more significant at 80% irradiance than at 50% and 25% irradiances (Fig. 4). At all irradiances, P n increased with the increase of soil moisture.
Chlorophyll content per unit leaf area (Chl) was lower, while the ratio of carotenoid to chlorophyll (Car/Chl) and the ratio of chlorophyll a to b (Chl a/b) were higher at 80% irradiance than at 25% and 50% irradiances (Fig. 5). At 80% irradiance, Chl decreased whereas Car/Chl increased with the decrease of soil moisture (below 80%). However, the effects of soil moisture on Chl and Car/Chl were not significant at 25% and 50% irradiance levels. With the decrease of soil moisture, Chl a/b increased although the increase was not significant.
(a) Leaf chlorophyll content per unit area (Chl); (b) the ratio of carotenoid to chlorophyll (Car/Chl); and (c) the ratio of chlorophyll a to b for Amomum villosum grown at 25% (circle), 50% (triangle up), 80% (triangle down) irradiances and 40%, 60%, 80% and 100% relative soil moisture for 6 months. Mean ± SE (n = 4). Different small and capital letters indicate significant differences among soil moistures and among irradiances, respectively (LSD-test of a one-way ANOVA, P < 0.05)
Discussion
Orthogonal effects of soil moisture and irradiance
As expected, soil moisture was the main determinant of growth in A. villosum although the effects of irradiance were also significant on some growth-related traits (Table 1). This is different with the results of Baruch et al. (2000). They found that the effects of water stress on four Melastomataceae species are weaker than those of light stress. In contrast to our expectation, there were no interactions between soil moisture and irradiance on all traits measured in this study. The effects of soil moisture and irradiance were orthogonal; drought reduced RGR at a similar degree at all the three irradiance levels (Fig. 2). The results are consistent with the predictions of independent effects model (Nobel 1999). The model has no strong theoretical foundation, and is considered as a null hypothesis (Sack and Grubb 2002). However, several recent studies support the hypothesis within irradiance below the excessive range (Sack and Grubb 2002; Sack 2004; Aranda et al. 2005). In this study, it was confirmed at irradiance above excessive range with herbaceous plant. Implications of this model for potential niche differentiation and modeling plant performance are discussed by Sack and Grubb (2002) and Sack (2004).
Acclimation to high irradiance combined with low soil moisture
At high irradiance and low soil moisture, A. villosum invested more biomass to roots but less to leaves, resulting in low LA:RM (Fig. 3a–c), which is beneficial to maintaining water balance at whole-plant level. Leaf morphological changes under different irradiances were also beneficial for A. villosum to acclimate to the environments (Fig. 3d–f). These results are consistent with those in literatures (Poorter 1999; Baruch et al. 2000; Sack and Grubb 2002; Sack 2004; Feng et al. 2004, 2007). The changes of root hydraulic conductivity and osmotic adjustment might also contribute to maintaining whole-plant level water balance at high irradiance and low soil moisture condition (Aranda et al. 2005; Shimizu et al. 2005).
In contrast to some previous reports, our results showed that soil moisture was the main determinant of SLA (Table 1). SLA increased significantly with the decrease of soil moisture (Fig. 3d). It has been reported that irradiance is the main factor influencing SLA (Muraoka et al. 1997; Sack 2004; Aranda et al. 2005) and that the effects of soil moisture on it are not significant (Sack 2004; Aranda et al. 2005). The increased SLA at low soil moisture can decrease leaf-level water utilization efficiency (McDowell 2002) as thin leaf reduces the distance that water diffuses within it, and therefore decreasing the retaining time of water in it (Van den Boogaard and Villar 1998). The increased SLA might also adversely affect water balance at whole-plant level as it will contribute to the increase of total plant leaf area, which is the product of plant mass, LMF and SLA. However, LAR (LMF * SLA) changed only slightly across all levels of soil moisture and irradiance (from 37.27 cm2 g-1 at 40% moisture and 50% irradiance to 42.56 cm2 g−1 at 60% moisture and 25% irradiance) as LMF decreased with decreasing soil moisture (Fig. 3c), which counteracted the effects of the increased SLA on LRA.
Forming small-sized leaves at low soil moisture (Fig. 3f) would result in more leaf number when compared to the similar-sized plant grown at higher soil moisture because LAR changed little across treatments. Indeed, with the decrease of soil moisture leaf number per gram mass increased from 0.56 at the treatment of 100% moisture and 25% irradiance to 0.75 at the treatment of 40% moisture and 80% irradiance. Therefore, forming many small-sized slender leaves at low moisture (Fig. 3e, f) can not help A. villosum to reduce whole-plant level water loss, which is also inconsistent with the general viewpoint about leaf morphology (Gibson 1998). We hypothesized that formation of such leaves in A. villosum could facilitate thermal loss at low soil moisture and maintain leaf area for carbon assimilation as well. High leaf temperature could induce photoinhibition of photosynthesis (Ishida et al. 1999) and damage photosynthetic machinery. The decrease of soil moisture will reduce leaf transpiration rate, leading to a decreased thermal loss. Therefore, other ways for leaf to dissipate heat are necessary. A small-sized leaf has a thin boundary layer which allows for a better convective heat loss to environment and can decrease its temperature (Gibson 1998). Some desert plants also form compact canopy with many small-sized slender leaves (Gibson 1998; Klich 2000). Forming many small-sized slender leaves at dry condition might be a common characteristic of different plant species occurred at different habitats.
At stressful conditions, A. villosum maintained relatively high photosynthesis (Fig. 4), and no long-term photoinhibition (Long et al. 1994) occurred, as indicated by the high value (about 0.85) of maximum photochemical efficiency of photosystem (PS) II at dawn (data not shown). At high irradiance, thermal dissipation and leaf rolling degree increased with the decrease of soil moisture (data not shown), which can help A. villosum to protect photosystem from photodamage (Feng et al. 2002). The increased thermal dissipation was consistent with the increase of Car/Chl (Fig. 5b). Carotenoid is not only associated with thermal dissipation but also can scavenge reactive oxygen species (Bungard et al. 1999). Plants can protect photosystem from photodamage through increasing Car/Chl (Munné-Bosch and Alegre 2000). The increase of Chl a/b is an indicator of the decrease of light-harvesting complex of PS II (Anderson and Aro 1994). Therefore, a low level of Chl and high value of Chl a/b at high irradiance under low soil moisture (Fig. 5a, c) could help A. villosum to reduce light capture, especially that of PS II, alleviating the oxidation pressure of PS II (Park et al. 1997), which is more vulnerable to photooxidation than PS I.
Implications for A. villosum cultivation
In this study, we proved for the first time that A. villosum can acclimate to high irradiance combined with low soil moisture with a controlled experiment. Soil moisture is a more important ecological factor to concern than irradiance when growing A. villosum in agroforestry pratices; it can be grown in high light habitats such as secondary forests and artificial forests when water availability is adequate. In forests, A. villosum often forms dense populations (Feng et al. 2005), leading to serious shading among different individuals, which can further increase its high light tolerant ability at whole-plant level. It has been reported that surface soil moisture content is generally higher in gaps than in the adjacent understorey (Denslow et al. 1998). Thus, we hypothesize that A. villosum will grow better in gaps than in understorey. This is consistent with the result that fruit yield of A. villosum in a tropical forest increased with the increase of irradiance (Zheng et al. 2004).
During the first three months of treatments, A. villosum grew slowly and responded sluggishly to different levels of soil moisture (Fig. 1a–f). This may be associated with A. villosum’s inherent growth rhythm and/or the low temperature in this duration. For example, the mean monthly temperature in Nov, Dec, Jan, Feb, Mar and April was 19.3, 17.3, 16.3, 18.0, 21.0 and 23.9°C, respectively. The heavy fog occurred daily in the duration might also reduce the effects of soil moisture. Plants can absorb fog water directly through leaf and recover water balance in 5 h (Zheng and Feng 2006). These results indicated that the low water availability in the first half of dry season might not be a serious problem for A. villosum in Xishuangbanna. However, our results were only based on a one-year experiment with potted seedlings. Long-term field studies are necessary to evaluate the combined effects of soil moisture and irradiance on A. villosum.
References
Anderson JM, Aro EM (1994) Grana stacking and protection of photosystem II in thylakoid membranes of higher plant leaves under sustained high irradiance: a hypothesis. Photosynth Res 41:315–326
Aranda I, Castro L, Pardos M, Gil L, Pardos JA (2005) Effects of the interaction between drought and shade on water relations, gas exchange and morphological traits in cork oak (Quercus suber L.) seedlings. Forest Ecol Manag 210:117–129
Baruch Z, Pattison RR, Goldstein G (2000) Responses to light and water availability of four invasive Melastonmataceae in the Hawaiian islands. Int J Plant Sci 161:107–118
Bungard RA, Ruban AV, Hibberd JM, Press MC, Horton P, Scholes JD (1999) Unusual carotenoid composition and a new type of xanthophylls cycle in plants. P Natl Acad Sci USA 96:1135–1139
Denslow J, Ellison AM, Sanford RE (1998) Treefall gap size effects on above- and below-ground processes in a tropical wet forest. J Ecol 86:597–609
Feng YL, Cao KF, Feng ZL (2002). Thermal dissipation, leaf rolling and inactivation of PS II reaction centres in Amomum villosum. J Trop Ecol 18:865–876
Feng YL, Cao KF, Zhang JL (2004) Photosynthetic characteristics, dark respiration, and leaf mass per unit area in seedlings of four tropical tree species grown under three irradiances. Photosynthetica 42:431–437
Feng YL, Wang JF, Sang WG (2007) Biomass allocation, morphology and photosynthesis of invasive and noninvasive exotic species grown at four irradiance levels. Acta Oecol 31:40–47
Feng ZL, Gan JM, Zheng Z, Feng YL (2005) Comparative study of two kinds of Amomum villosum cultivation model in Xishuangbanna. Acta Phytoecol Sin 29:137–143
Franck N, Winkler S, Pastenes C, Infante R (2007) Acclimation to sun and shade of three accessions of the Chilean native berry-crop murta. Agr Syst 69:215–229
Gibson AC (1998) Photosynthetic organs of desert plants. Bioscience 48:911–920
He ZX (1991) Investigation on Amomum villosum high yield habitat. China J Chinese Material Medica 16:405–406
Holmgren M (2000) Combined effects of shade and drought on tulip poplar seedlings: trade-off in tolerance or facilitation? Oikos 90:67–78
Holmgren M, Scheffer M, Huston MA (1997) The interplay of facilitation and competition in plant communities. Ecology 78:1966–1975
Hunt R, Causton DR, Shipley B, Askew AP (2002) A modern tool for classical plant growth analysis. Ann Bot London 90:485–488
Ishid AA, Toma T, Marjenah (1999) Leaf gas exchange and chlorophyll fluorescence in relation to leaf angle, azimuth, and canopy position in the tropical pioneer tree, Macaranga conifera. Tree Physiol 19:117–124
Klich MG (2000) Leaf variations in Elaeagnus angustifolia related to environmental heterogeneity. Environ Exp Bot 44:171–183
Lichtenthaler HK, Wellburn AR (1983) Determination of total carotenoids and chlorophyll a and b of leaf extracts in different solvents. Bioch Soc T 603:591–592
Long SP, Humphries S, Folkowski PG (1994) Photoinhibition of photosynthesis in nature. Ann Rev Plant Physiol Plant Mol Biol 45:633–662
McDowell SCL (2002) Photosynthetic characteristics of invasive and noninvasive species of Rubus (Rosaceae ). Am J Bot 89:1431–1438
Munné-Bosch S, Alegre L (2000) The xanthophylls cycle is induced by light irrespective of water status in field-grown lavender (Lavandula stoechas) plants. Physiol Plant 108:147–151
Muraoka H, Tang Y, Koizumi H, Washitani I (1997) Combined effects of light and water availability on photosynthesis and growth of Arisaema heterophyllum in the forest understorey and an open site. Oecologia 112:26–34
Nobel PS (1999) Physiochemical and environmental plant physiology. 2nd edn. Academic Press, San Diego
Park YI, Chow WS, Anderson JM (1997) Antenna size dependency of photoinactivation of photosystem II in light acclimated pea leaves. Plant Physiol 115:151–157
Poorter L (1999) Growth responses of 15 rain forest tree species to a light gradient: the relative importance of morphological and physiological traits. Funct Ecol 13:396–410
Sack L (2004) Responses of temperate woody seedlings to shade and drought: do trade-offs limit potential niche differentiation? Oikos 107:110–117
Sack L, Grubb PJ (2002) The combined impacts of deep shade and drought on the growth and biomass allocation of shade-tolerant woody seedlings. Oecologia 131:175–185
Shimizu M, Ishida A, Hogetsu T (2005) Root hydraulic conductivity and whole-plant water balance in tropical saplings following a shade-to-sun transfer. Oecologia 143:189–197
Smith T, Huston M (1989) A theory of the spatial and temporal dynamics of plant-communities. Vegetation 83:49–69
Valladares F, Pearcy RW (1997) Interactions between water stress, sun-shade acclimation, heat tolerance and photoinhibition in the sclerophyll Heteromeles arbutifolia. Plant Cell Environ 20:25–36
Van den Boogaard R, Villar R (1998) Variation in growth and water-use efficiency: a comparison of Aegilops L. species and Triticum aestivum L. cultivars. In: Lambers H, Poorter H, van Vuuren MMI (eds) Inherent variation in plant growth: physiological mechanisms and ecological consequences. Bachuys, Leiden, The Netherlands, pp 289–308
Zheng Y, Feng YL (2006) Leaf water absorption of epiphytes and non-epiphytes in Xishuangbanna. Chinese J Appl Ecol 27:977–981
Zheng Z, Gan JM, Feng ZL, Meng Y (2004) Dynamics of Amomum villosum growth and its fruit yield cultivated under tropical forests. Chinese J Appl Ecol 15:5–8
Zhou ZZ, Zheng HS, Yang ZJ, Yin GT, Chen KT (1997) Research on biological cycle of nutrient elements in plantation of rubber intercropped with Amomum Longiligulare. Forest Res 10:464–471
Acknowledgements
The authors are grateful to the two anonymous referees for their advice and comments on an earlier version of the manuscript. This study was funded by the Key Project of Knowledge Innovation Engineering of Chinese Academy of Sciences (KSCX1-SW-13-0X-0X) and the Project of Distinguished Scientists in West China from the Chinese Academy of Sciences granted to Y. L. F..
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, YL., Li, X. The combined effects of soil moisture and irradiance on growth, biomass allocation, morphology and photosynthesis in Amomum villosum . Agroforest Syst 71, 89–98 (2007). https://doi.org/10.1007/s10457-007-9076-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-007-9076-3