Abstract
Background
Long COVID, also known as post-acute sequelae of COVID-19 (PASC), is characterized by persistent clinical symptoms following COVID-19.
Objective
To correlate biomarkers of endothelial dysfunction with persistent clinical symptoms and pulmonary function defects at distance from COVID-19.
Methods
Consecutive patients with long COVID-19 suspicion were enrolled. A panel of endothelial biomarkers was measured in each patient during clinical evaluation and pulmonary function test (PFT).
Results
The study included 137 PASC patients, mostly male (68%), with a median age of 55 years. A total of 194 PFTs were performed between months 3 and 24 after an episode of SARS-CoV-2 infection. We compared biomarkers evaluated in PASC patients with 20 healthy volunteers (HVs) and acute hospitalized COVID-19 patients (n = 88). The study found that angiogenesis-related biomarkers and von Willebrand factor (VWF) levels were increased in PASC patients compared to HVs without increased inflammatory or platelet activation markers. Moreover, VEGF-A and VWF were associated with persistent lung CT scan lesions and impaired diffusing capacity of the lungs for carbon monoxide (DLCO) measurement. By employing a Cox proportional hazards model adjusted for age, sex, and body mass index, we further confirmed the accuracy of VEGF-A and VWF. Following adjustment, VEGF-A emerged as the most significant predictive factor associated with persistent lung CT scan lesions and impaired DLCO measurement.
Conclusion
VEGF-A is a relevant predictive factor for DLCO impairment and radiological sequelae in PASC. Beyond being a biomarker, we hypothesize that the persistence of angiogenic disorders may contribute to long COVID symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long COVID, also referred to as post-acute sequelae of SARS-CoV-2 infection (PASC), is characterized by the enduring presence of clinical symptoms following the acute phase of SARS-CoV-2 infection [1,2,3]. This condition is defined as the persistence or emergence of new symptoms occurring at least three months after the initial SARS-CoV-2 infection, with these symptoms lasting for a minimum of two months without any other identifiable explanation [2]. Although the reported frequency of PASC symptoms is highly variable between studies, the literature suggests that main PASC symptoms are fatigue, dyspnea, chest pain, tightness, cough, and neurocognitive disorders [4,5,6,7,8]. However, a recent meta-analysis comprising 54 studies and two medical record databases from 22 countries revealed that 6.2% of individuals with symptomatic COVID-19 infection from March 2020 to January 2022 reported enduring clinical symptoms at the three-month mark. Patients experienced at least one of the following symptoms: persistent fatigue with bodily pain or mood swings (3.2%), cognitive disorder (2.2%), or ongoing respiratory defects (3.7%) [9]. Moreover, occurrence of PASC seems to depend on medical history, socio-economic factors, and the type of initial symptoms, while the recovery rate is low: only 7% of PASC cases have disappeared after 2 years [10].
Long COVID is believed to have multiple potential causes that often overlap, including the persistence of SARS-CoV-2 in tissues and immune dysregulation. This dysregulation can manifest as chronic activation of T cells, excessive production of cytokines, and even reactivation of underlying pathogens, including herpes viruses such as Epstein–Barr virus and human herpesvirus 6 [11,12,13]. While none of these mechanisms have been comprehensively studied or fully understood, recent studies have indicated that endothelial dysfunction may play a crucial role in triggering vasculopathy associated with long COVID, similar to what has been observed in acute COVID-19 cases [14, 15]. During the acute phase of COVID-19 illness, the occurrence of diffuse microthrombosis, especially in the lung vasculature, has been observed, leading to the development of acute respiratory distress syndrome (ARDS) [16, 17].
The process of microthrombosis has primarily been identified as a result of endothelial dysfunction associated with inflammation [17, 18]. Moreover, intussusceptive angiogenesis in the lung and heart has been demonstrated in autopsy studies in contrast to the ARDS associated with other viral infections [16, 19]. This angiogenic phenotype has also been described in biomarker studies of acute COVID-19 illness [20]. In long COVID patients, endothelial dysfunction may contribute to developing a broad spectrum of symptoms including fatigue, dyspnea, and cognitive impairment [3]. Endothelial dysfunction may also be involved in developing cardiovascular complications, such as thrombosis and myocarditis, possibly contributing to the mitochondrial dysfunction frequently observed in PASC. Impaired endothelium-dependent vasodilation has been demonstrated in long COVID [21], and endothelial dysfunction in biomarkers studies [22, 23] has been identified without strong evidence of a relationship to clinical symptoms.
Therefore, the objective of the present study was to investigate the potential correlation between biomarkers indicating endothelial dysfunction, activation of hemostasis, and angiogenesis. The study aimed to assess their relationship with persistent clinical symptoms and pulmonary function impairments following the acute phase of SARS-CoV-2 infection.
Methods
PASC cohort design and participants
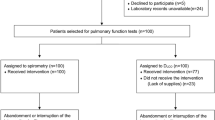
We conducted a prospective monocentric cohort study at the European Georges Pompidou Hospital in Paris, France. The study included consecutive adult patients (≥ 18 years old) who had an objectively confirmed acute COVID-19 diagnosis documented by a positive SARS-CoV-2 reverse transcriptase-polymerase chain reaction (RT-PCR) test dated at least 3 months prior to enrollment. PASC were defined as the presence of one or several symptoms persisting for at least 2 months. These symptoms included fatigue, dyspnea on exertion, ageusia, anosmia, fever, cough, chest pain, myalgia, arthralgia, headaches, diarrhea, neurocognitive disorders, and nausea. It was ensured that these symptoms were not present before the acute COVID-19 infection and were not explained by any alternative diagnosis. Patients were prospectively included in the study between November 2020 and June 2022, at least three and 24 months after their acute COVID-19 episode. Following acute COVID-19, patients were categorized based on the time of inclusion, specifically at month 3 (M3), month 6 (M6), month 9 (M9), month 12 (M12), and month 24 (M24). Patients were further categorized based on their clinical presentation at the time of the initial acute SARS-CoV-2 infection. Patients who did not require hospitalization were classified as outpatients. Those who were hospitalized but only needed supplemental oxygen within a medical ward were categorized as non-ARDS cases. Finally, patients who required mechanical ventilation in an intensive care unit (ICU) were classified as ARDS cases. At each inclusion visit and subsequent follow-up visits, all patients with PASC were evaluated by a pulmonologist. The pulmonologist collected patient characteristics, including age, sex, comorbidities, severity of the initial acute SARS-CoV-2 infection, and persistent PASC symptoms. Additionally, the pulmonologist oversaw the performance of pulmonary function tests (PFT), 6-min walk tests (6-MWT), chest high-resolution computed tomography (CT) scans, and blood sampling. If the pulmonologist deemed it necessary, patients were offered a follow-up visit three months after their initial visit (up to 24 months after inclusion) in cases of severe persistent respiratory symptoms such as dyspnea, chest pain, fatigue, or significant abnormalities in PFT or radiological findings. The follow-up visit procedure mirrored that of the initial visit, including data collection, blood sampling, PFT, and CT scan performance. There was no restriction on the number of follow-up visits that patients could undergo.
The present study is part of the SARCODO cohort study (2020-A01048-31A, NCT04624997), which focuses on investigating coagulation and endothelium activity profiles in patients admitted to our hospital for SARS-CoV-2 infection. The study procedures and methods were approved by the French Ethics Committee (ID RCB: 2020-A00256-33).
Acute COVID-19 patients and healthy volunteers’ cohorts
To improve our understanding of the variations in biomarkers levels measured in PASC patients, we also measured this panel of biomarkers in a cohort of acute hospitalized COVID-19 patients within 48 h following hospitalization as a “positive control group” and a healthy volunteers (HV) cohort as a “negative control group.” The acute COVID-19 patient’s cohort comprised 88 adults (≥ 18 years old) with COVID-19 hospitalized in the European Georges Pompidou Hospital between March 13 and June 26, 2020. All included patients presented a confirmed diagnosis of SARS-CoV-2 infection by RT-PCR. Within this cohort, a total of 61 patients were classified as non-ARDS cases, indicating that they required supplemental oxygen in a medical ward during the first 48 h following hospitalization. Additionally, there were 27 patients classified as ARDS cases, indicating that they required mechanical ventilation in an ICU during the same time frame. Biomarkers were measured in all 88 patients included in the acute COVID-19 cohort in plasma sampled the first 48 h following hospitalization.
The HVs’ cohort was constituted of 10 unrelated healthy male volunteers aged 18 to 35 years (mean, 26.1; SD: 4 years) and 10 unrelated healthy women volunteers aged 18 to 38 years (mean, 24.9; SD: 5 years) recruited in the Clinical Investigation Center of European Georges Pompidou Hospital. All volunteers were non-smokers, had unremarkable personal and familial medical histories, and denied taking any medication for at least 10 days before blood collection.
Pulmonary function test
The PFTs included spirometry, functional residual capacity (FRC), total lung capacity (TLC), and diffusing capacity of the lungs for carbon monoxide (DLCO) by single-breath real-time CO/NH4 measurements. FRC was measured utilizing body plethysmography (Vyntus Body, DUOMED, Flaxlanden, France). Predicted values from the Global Lung Function Initiative were used for forced vital capacity and DLCO [24, 25]. We considered PFT findings abnormal below < 75% of the predicted values. A 6-MWD was performed according to ATS/ERS recommendations, with baseline SaO2 measured by pulse oximetry on index fingers [26]. The results were expressed as meters and percentages of predicted values calculated using a method described by Enright and colleagues in 1998 [27].
Chest CT scan visual lung damage score
All low-dose chest CT scans were performed on the same multi-row system (Somatom Definition Edge, Siemens) with a collimation of 128 × 0.6 mm (reconstruction at 1.5 mm in a mediastinal setting and 1 mm in a lung setting), and a 280-ms gantry rotation time. The chest CT scan was supine from the lung apices to the bases in a single breath-hold at maximum inspiration without electrocardiogram gating. Tube voltage (100–120 kVp) was selected by an automated tube voltage selection associated with 40–80 mAs, based on body size. In all PASC patients and as previously described regarding chest CT findings in COVID-19 [28, 29], lung injuries were assessed in each lung lobe for the presence of either ground-glass opacities (GGOs) defined as hazy areas of increased attenuation without obscuration of the underlying vasculature or consolidation defined as homogeneous opacification with obscuration of the underlying vasculature or both. Each of the five lung lobes was visually assessed to determine the presence of GGOs or consolidation and degree of extension of either GGO areas alone or GGO areas plus condensation opacity areas. Thus, each lung lobe extent was classified as having or not having sequelae.
Laboratory procedures
Blood was collected in 0.129 M trisodium citrate tubes (9NC BD Vacutainer, Plymouth, UK). Platelet-poor plasma (PPP) was obtained after centrifugation twice at 2500 × g for 15 min and stored at − 80 °C until analysis. The D-dimer level was measured using the Vidas D-dimers® assay (Biomérieux, Marcy-Etoile, France) according to the manufacturer’s instructions. The plasma concentrations of soluble vascular cell adhesion protein 1 (VCAM-1), soluble P-selectin (sP-sel), angiopoietin (Ang)-1, Ang-2, syndecan-1, vascular endothelial growth factor A (VEGF-A), fibroblast growth factor 2 (FGF-2), placental growth factor (PlGF) and endostatin were quantified in PPP using a Human Magnetic Luminex Assay (R&D Systems, Minneapolis, MN). Data were assessed with the Bio-Plex 200 using the Bio-Plex Manager 5.0 software (Bio-Rad, Marnes-la-Coquette, France). Measurement of von Willebrand factor antigen (VWF: Ag; (STA Liatest, Diagnostica Stago) was performed on a STA-R® Max coagulometer (Diagnostica Stago).
Statistical analysis
Continuous data were expressed as medians (interquartile ranges [IQRs]) with categorical data as proportions or in mean (SD). We performed QQ plot to assess normality and log-normality of all variables involved in statistical testing. For between-group comparison, we used the Welch’s t test and Fisher’s exact test for continuous and categorical variables, respectively. For comparison between more than two groups, we used the ANOVA and the chi-squared test for trends of continuous and categorical variables, respectively. For comparison between groups, the post hoc Tukey’s multiple comparisons tests followed the ANOVA test.
To assess the association between biomarkers levels and comorbidities and residual symptoms, we performed unadjusted logistic regression which was expressed as Odds ratio (OR) 95% confidence interval [CI]. To estimate the ability of biomarkers to predict in-hospital mortality, we used receiver operator characteristics (ROC) analysis. We estimated the area under the curve (AUC) and its 95% CI. In addition, we performed precision and recall metrics curves as sensitive analysis using the “prtab” command (Boston College Statistical Software Components (SSC) in STATA. Correlation coefficients and associated p-value were assessed using Pearson correlation test. We used the logistic regression model to investigate the relationships between the increase in VEGF-A and VWF levels (both treated as categorical variable using the median of the whole PASC cohort patient as cut-off) adjusted for age, sex and body mass index (BMI) with the following outcomes: residual lungs lesion on CT scan and residual DLCO < 75% of predicted value. The Bonferroni correction was used to control Type I error. In multiple comparisons, significance level of 0.05/κ (κ = number of comparisons) was set. All statistical analyses were performed using the Stata software package (Stata, version. 12.0; StataCorp) and GraphPad Prism version 9.0.0 for Windows (GraphPad Software, USA).
Results
PASC patients displayed PFT and CT scan impairments
We enrolled 137 PASC patients (93 males and 44 females) with a median age of 55 years (IQR 46.5–66.5). As expected, comorbidities were frequent, including hypertension (28.5%), dyslipidemia (19%), diabetes (12.4%), and asthma (11%). Among these 137 patients, 62 (45.2%) were included at M3, 10 (7.3%) at M6, 21 (15.3%) at M9, 11 (8%) at M12 and 33 (24.1%) at M24 following the acute phase of SARS-CoV-2 infection. The median delay between acute COVID-19 illness and inclusion was 202 days (IQR 105–611). Upon inclusion, the most common residual symptoms were dyspnea on exertion (66.4%), fatigue (65%), chest pain (16.8%), and cough (16.8%). At the time of acute SARS-CoV-2 infection, 43 PASC patients (31.4%) were hospitalized in an ICU with ARDS, 41 PACS patients (29.9%) were hospitalized in the medical wards (non-ARDS patients) and 53 PASC patients (38.7%) had an acute infection as outpatients. The associations between residual symptoms frequency and the initial clinical severity are summarized in supplemental Table 1. Regarding PFT parameters, median basal Sa02 was 96% (IQR 95.0–98.0), median FVC was 95% (IQR 80.0–107.5), median DLCO was 73% (IQR 61.0–83.0) and median TLC was 91% (IQR 78.8–105.0). Furthermore, 95 (69.3%) displayed residual CT scan lungs lesion at inclusion. Patients’ characteristics, comorbidities, biological characteristics, and outcomes are summarized in Table 1. Among these 137 PASC patients, 93 (67.9%) had attended one visit, while 44 (32.1%) had one or more iterative follow-up visits as long as deemed necessary by the pulmonologist. Pooling the initial inclusion and follow-up visits, we conducted 194 visits (each visit including PFT and CT scan performance, residual symptoms recording and blood sampling). Overall, 62 (45.3%) PASC patients were explored at M3, 34 (24.8%) at M6, 34 (24.8%) at M9, 27 (19.7%) at M12, and 37 (27%) at M24. Interestingly, among patients who had one or more iterative visit, we observed an overall increase between M3 and M24 in PFT parameters: FVC (p = 0.0024) and DLCO (p = 0.006), Sa02 (p < 0.001), as well as an overall improvement in residual scan lesion prevalence (p = 0.0019) (Table 2). In contrast, we did not observe a significant variation of COVID-19 residual symptoms frequency over time.
PASC was associated with increased angiogenesis-related biomarkers and VWF levels
To identify the pathophysiological mechanisms associated with PASC, we measured circulating biomarkers related to inflammation, coagulation activation, endothelial and platelet dysfunction and angiogenesis at M3, M6, M9, M12, and M24 after acute COVID-19 symptoms appearance. This panel of biomarkers was also measured in a cohort of 88 acute hospitalized COVID-19 patients, including 27 ARDS and 61 non-ARDS patients within the first 48 h of hospitalization and a cohort of 20 HVs.
As expected, circulating levels of inflammatory markers, coagulation activation, endothelial lesion, and coagulation activation markers were increased in acute SARS-CoV-2 infection (especially in ARDS patients) compared to HVs (Fig. 1A–L). However, only half remained elevated in PASC patients. D-dimer levels were significantly higher in PASC patients (302.5 ng/mL, IQR [205.8–465.3]) than in HVs (187.0 ng/mL, IQR [142.5–252.5], p < 0.0001, Fig. 1B). However, there was no significant variation in D-dimer levels between M3 and M24. VWF:Ag levels were also higher in PASC patients (125%, IQR [100.0–167.5]) than in HVs (89.81%, IQR [72.3––106.2], p < 0.0001, Fig. 1C) but progressively decreased over time between M3 and M24 (p = 0.0004). Similarly, median Ang-1 levels were significantly higher in PASC patients (4554.0 pg/mL, IQR [3463.0–6689.0]) compared to HVs (1558.0 pg/mL, [1102.0–2282.0], p < 0.0001, Fig. 1H) and decreased progressively after acute COVID-19 from M3 to M24 (p < 0.0001). Median VEGF-A levels were significantly higher in PASC patients (31.22 pg/mL, IQR [21.4–41.00]) than in HVs (13.6 pg/mL, IQR [10.3––16.5], p < 0.0001, Fig. 1I) and normalized progressively at a distance from acute COVID-19 infection from M3 to M24 (p < 0.0001). Median FGF-2 levels were significantly higher in PASC patients (14.3 pg/mL, IQR [12.4–17.8]) than in HVs (11.0 pg/mL, [8.4–13.6], p < 0.0001, Fig. 1J) and decreased progressively at a distance from acute COVID-19 from M3 to M24 (p = 0.0008). Median PlGF levels were significantly higher in PASC patients (50.5 pg/mL, IQR 40.7–61.5) than in HVs (40.9 pg/mL, [35.4––47.9], p < 0.0001, Fig. 1K) and decreased progressively at a distance from acute COVID-19 from M3 to M24 (p = 0.0008). In contrast, circulating levels of CRP (Fig. 1A), soluble P-selectin (sP-sel; Fig. 1D), VCAM-1 (Fig. 1E), syndecan-1 (Fig. 1F), Ang-2 (Fig. 1G), and endostatin (Fig. 1L) in PASC patients were similar to HVs. Furthermore, we investigated the relationship between biomarkers measured in PASC patients and comorbidities. We found that high VWF levels were associated with older age (OR 4.27 95% CI 2.77–8.52, p < 0.0001), male sex (OR 2.95 95% CI 1.47–5.94, p = 0.003), higher BMI (OR 2.59 95% CI 1.19–6.49], p = 0.008), and hypertension (OR 2.95 95%CI 1.34–6.54, p = 0.007). In contrast, high VEGF-A levels were associated with older age (OR 3.59 95%CI 1.90–9.53, p = 0.003) and male sex (OR 2.37 95%CI 1.10–5.11, p = 0.028). All associations between biomarkers levels measured in PASC patients and comorbidities are summarized in supplemental Table 2. The study did not identify any significant association between the measured levels of biomarkers and the residual symptoms related to PASC. More information are detailed in supplemental Table 3.
Levels of inflammation, coagulation activation, endothelial dysfunction, and angiogenesis biomarkers in acute COVID-19 and PASC patients. Datapoints indicate individual measurements, whereas horizontal bars represent the median with the interquartile range. Green shaded areas indicate the normal range of value for von Willebrand factor antigen (VWF:Ag). For each biomarker circulating plasma level (A-L), the results were divided into three sections separated by dotted lines. First, in the left section, the plasma levels of each biomarker were compared in acute hospitalized COVID-19 patients, between 20 healthy volunteers (HVs), 61 non-acute respiratory distress syndrome (non-ARDS) COVID-19 patients and 27 ARDS COVID-19 patients (p-value from the ANOVA followed eventually by Tukey’s multiple comparisons tests). Then, in the middle section, the circulating plasma levels of each biomarker were compared between HVs and all PASC patients’ 194 samples/timepoints concomitant with pulmonary function test (PFT), whether at inclusion or follow-up (p-value from the Welch’s t test). Finally, in the right section, the circulating plasma levels of each biomarker were compared in PASC patients according to the time from acute COVID-19 (p-value from the ANOVA test). This methodology for analysis and presentation of results was applied to the levels of C-reactive protein (CRP ; A), D-dimer (B), (VWF:Ag ; C), soluble P-selectin (sP-sel ; D), soluble vascular cell adhesion molecule (VCAM-1 ; E), syndecan-1 (F), angiopoietin-2 (Ang-2 ; G), angiopoietin-1 (Ang-1 ; H), vascular endothelial growth factor A (VEGF-A ; I), basic fibroblast growth factor (FGF-2 ; J), placental growth factor (PlGF ; K) and endostatin (L)
Angiogenesis-related biomarkers and VWF levels are associated with persistent CT scan lesions in PASC patients
We evaluated the association between the persistence of GGOs and crazy paving pattern assessed on CT scans and plasma biomarkers tested. PASC patients with persistent lung involvement displayed higher median levels of VWF:Ag (154.5%, IQR 109.8–189.3), VEGF-A (37.1 pg/mL IQR 26.8–48.8), FGF-2 (15.5 pg/mL IQR 13.0–18.9), PlGF (53.4 pg/mL IQR 45.3–63.5), and endostatin (53,000.0 pg/mL IQR 46075.0–60208.0) compared with PASC patients with normal lung CT scan (VWFAg: 107% IQR 93.0–149.8, p = 0.0006, Fig. 2C; VEGF-A: 37.1 pg/mL IQR 26.8–48.8, p = 0.0008, Fig. 2I; PlGF: 49.2 pg/mL IQR 39.9–54.8, p = 0.0017, Fig. 2K; endostatin: 42,568.0 pg/mL IQR 36412.0–58283.0, p = 0.0034, Fig. 2L). Consistently, both ROC curve and precision–recall curve analyses showed that VWF:Ag (AUC = 0.70 95% CI 0.58–0.80, Fig. 3A; AUC = 0.85, Fig. 3B), VEGF-A (AUC = 0.68, 95% CI 0.57–0.80, Fig. 3C; AUC = 0.86, Fig. 3D), endostatin (AUC = 0.67, 95% CI 0.55–0.80, Fig. 3E; AUC = 0.82, Fig. 3F), and PlGF (AUC = 0.66, p = 0.016, 95% CI 0.54–0.78, Fig. 3G; AUC = 0.84, Fig. 3H) could divide PASC patients with or without CT scan residual GGOs and crazy paving pattern.
Association between inflammation, coagulation activation, endothelial dysfunction, and angiogenesis biomarkers levels and persistent CT scan lesion in PASC patients. Datapoints indicate individual measurements, whereas horizontal bars represent the median with the interquartile range. Comparison of plasma levels of C-reactive protein (CRP ; A), D-dimer (B), von Willebrand factor antigen (VWF:Ag ; C), soluble P-selectin (sP-sel ; D), soluble vascular cell adhesion molecule (VCAM-1 ; E), syndecan-1 (F), angiopoietin-2 (Ang-2 ; G), angiopoietin-1 (Ang-1 ; H), vascular endothelial growth factor A (VEGF-A ; I), basic fibroblast growth factor (FGF-2 ; J), placental growth factor (PlGF ; K), and endostatin (L) in all PASC patients’ 194 samples concomitant with pulmonary function test (PFT) (whether at inclusion or follow-up) between PASCS patients displaying no persistent CT scan lesion and PASCS with persistent CT scan lesion. (p-value from the Welch’s t test).
Predictive value of von Willebrand factor and angiogenesis-related biomarkers levels regarding persistent CT scan lesion in PASC patients. Receiver operating curves evaluating unadjusted von Willebrand factor antigen (VWF: Ag; A), vascular endothelial growth factor A (VEGF-A ; B), endostatin (C), placental growth factor (PlGF ; D), and basic fibroblast growth factor (FGF-2 ; E) ability to predict the presence of residual CT scan lesion in PASC patients. The diagonal red dotted segment is the reference line. AUC: area under the curve.
VEGF-A, Ang-1, and VWF levels are associated with impairment of PFT in PASC patients
First, we studied the association between biomarkers levels and DLCO impairment. PASC patients with persistent DLCO reduction (< 75%) displayed higher levels of VWF:Ag (144%, IQR 110.8–183.3) than in patients with normal DLCO (114%, IQR 90.8–154.0, p < 0.0001, Fig. 4C). PASC patients with persistent DLCO reduction displayed higher levels of VEGF-A (34.2 pg/mL IQR 25.1–43.1) than in patients with normal DLCO (23.6 pg/mL IQR 20.3–35.2, p = 0.00024, Fig. 4I). Consistently, both ROC curve and precision–recall curve analysis showed that VWF:Ag (AUC = 0.65, 95% CI 0.58–0.73, Fig. 5-A; AUC = 0.72, Fig. 5B) and VEGF-A (AUC: 0.65, 95% CI 0.56–0.74, Fig. 5C; AUC = 0.69, Fig. 5D) could classify PASC patients with or without DLCO impairment. Finally, we studied the association between PFT parameters, including blood oxygen level (SaO2) at rest, FVC, DLCO, and TLC and biomarkers of inflammation, coagulation activation, endotheliopathy, and angiogenesis (Fig. 6). Among measured biomarkers, VEGF-A and VWF:Ag levels were the most correlated with SaO2 (r = − 0.19 and r = − 0.20, respectively), FVC (r = − 0.28 and r = − 0.24, respectively), DLCO (r = − 0.26 & r = − 0.35, respectively) and TLC (r = − 0.30 and r = − 0.27, respectively, Fig. 6). Other angiogenesis biomarkers were also inconstantly and less frequently associated with PFT parameters. Indeed, FGF-2 levels were associated solely with FVC (r = − 0.19) and TLC (r = − 0.21). In addition, PlGF levels were associated alone with SaO2 (r = − 0.18) and TLC (r = − 0.20). Finally, Ang-1 levels were associated only with SaO2 (r = − 0.21) and DLCO (r = − 0.19).
Association between inflammation, coagulation activation, endothelial dysfunction, and angiogenesis biomarkers levels and persistent DLCO reduction in PASC patients. Datapoints indicate individual measurements, whereas horizontal bars represent the median with the interquartile range. Comparison of plasma levels of C-reactive protein (CRP ; A), D-dimer (B), von Willebrand factor antigen (VWF:Ag ; C), soluble P-selectin (sP-sel ; D), soluble vascular cell adhesion molecule (VCAM-1 ; E), syndecan-1 (F), angiopoietin-2 (Ang-2 ; G), angiopoietin-1 (Ang-1 ; H), vascular endothelial growth factor A (VEGF-A ; I), basic fibroblast growth factor (FGF-2 ; J), placental growth factor (PlGF ; K), endostatin (L) in all PASC patients’ 194 samples concomitant with a PFT (whether at inclusion or follow-up) between PASCS patients displaying a normal diffusing capacity of the lung for carbon monoxide (DLCO ≥75%) and PASCS patients with an abnormal DLCO (< 75%) (p-value from the Welch’s t test).
Predictive value of von Willebrand factor and angiogenesis-related biomarkers levels regarding persistent DLCO reduction in PASC patients. Receiver operating curves evaluating unadjusted von Willebrand factor antigen (WF: Ag; A), vascular endothelial growth factor A (VEGF-A; B), angiopoietin-1 (Ang-1; C), ability to predict the persistent diffusing capacity of the lung for carbon monoxide (DLCO) reduction, defined as < 75% of predicted values. The diagonal red dotted segment is the reference line. AUC: area under the curve.
Correlation matrix between inflammation, coagulation activation, endothelial dysfunction, and angiogenesis biomarkers levels and pulmonary function test parameters in PASC patients. The heat map represents the color-coded correlation factors between circulating plasma levels of von Willebrand factor antigen (VWF:Ag), C-reactive protein (CRP), angiopoietin-2 (Ang-2), vascular endothelial growth factor A (VEGF-A), basic fibroblast growth factor (FGF-2), placental growth factor (PlGF) angiopoietin-2 (Ang-1), endostatin and at rest blood oxygen saturation (SaO2), forced vital capacity (FVC), diffusing capacity of the lung for carbon monoxide (DLCO), and total pulmonary capacity (TPC) in all PASCS patients’ 194 samples concomitant with pulmonary function test (PFT) (whether at inclusion or follow-up)
VEGF-A was the best predictive marker of residual CT scan lesion and persistent DLCO impairment
First, since VEGF-A and VWF:Ag were the two best biomarkers regarding association with residual CT scan lesion and association with impairment in PFT parameters, we hypothesized a relationship between VEGF-A and VWF:Ag. As a result, we found that VWF:Ag and VEGF-A circulating levels were significantly associated (r = 0.42, p < 0.0001, Fig. 7A). In contrast, there was no evidence for an inflammation-driven synthesis of VWF or VEGF-A given the absence of correlation between VWF:Ag and CRP (r = 0.08, p = 0.269, Fig. 7B) and between VEGF-A and CRP (r = 0.04, p = 0.617, Fig. 7C). Moreover, we performed multivariate analyses by logistic regression to evaluate whether high VWF:Ag levels and/or high VEGF-A levels were independent predictors of residual CT scan lesion and persistent DLCO impairment with an adjustment on VWF:Ag levels, VEGF-A levels, age, sex and BMI. Second, we found that after adjustment, the only significant predictor regarding residual CT scan lesion were high VEGF-A levels (OR 4.26, 95% CI 1.26–14.38) and above median age (OR 9.61, 95% CI 2.41–38.32, Fig. 7D). For DLCO impairment, the only significant predictor was high VEGF-A levels (OR = 2.71 95% CI 1.31–5.63; Fig. 7E).
VEGF-A level is an independent predictor of persistent DLCO reduction and CT,scan lung lesion after adjustment for von Willebrand factor levels and demographic data Association between circulating plasma levels of vascular endothelial growth factor A (VEGF-A) and von Willebrand factor antigen (VWF: Ag) (A), between circulating plasma levels of VWF: Ag and C-reactive protein (CRP) (B), and between circulating plasma levels of VEGF-A and CRP (C) in all PASCS patients’ 194 samples concomitant with pulmonary function test (PFT) (whether at inclusion or follow-up). Datapoints represent individual measurements. The bold black line indicates the line of best fit at a 95% confidence interval. Forest plots showing the multiple logistic regression model for VEGF-A levels adjusted for VWF: Ag levels, age, sex and body mass index (BMI) with persistent DLCO reduction as the outcome (D) or persistent CT scan lung lesion as the outcome (E). The red dot represents the adjusted Odds ratio. The horizontal lines show the 95% confidence intervals (CIs). Cut-offs are defined as the median value of the population.
Discussion
In this study, we were able to establish a correlation between circulating angiogenesis biomarkers and the severity of lung function parameters in patients with long COVID. To the best of our knowledge, this is the first study to comprehensively examine a wide range of endothelial circulating biomarkers and evaluate pulmonary function in the context of PASC. Among the angiogenic biomarkers studied, VEGF-A emerged as the most predictive marker for decreased DLCO and CT scan abnormalities. This finding highlights the relevance of evaluating VEGF-A and other angiogenic biomarkers in PASC patients and provides valuable insights into the underlying pathophysiological mechanisms contributing to persistent respiratory symptoms.
Studies concerning angiogenesis or vessel regeneration demonstrated increased circulating angiogenic growth factors or vasculogenic progenitor cells which may play a role in developing of COVID-19-related vascular complications [20, 30]. Moreover, intussusceptive angiogenesis, a process of blood vessel formation in which existing blood vessels split into two or more smaller vessels [31], has been described as a specific mechanism of damaged vessels in COVID-19 ARDS patients [16]. Nevertheless, knowledge about long-lasting sequelae, angiogenic disorders, and PASC symptoms is still limited. However, impaired microcirculation and altered angiogenesis seem at the origin of fibrotic remodeling in PASC [32, 33]. Our study found that VEGF-A was the best biomarker associated with decreased lung functions, including DLCO. VEGF-A is a key player in angiogenesis and the growth formation of new blood vessels and has been proposed as a key angiogenic factor in several pulmonary disorders. Indeed, in ARDS, VEGF-A has been found to increase and could lead to increased vascular permeability, pulmonary edema, and impaired gas exchange [34]. In addition, in chronic pulmonary disease like pulmonary hypertension (PH), VEGF-A has been involved in the pathogenesis of the disease through various mechanisms, including pulmonary vascular remodeling, endothelial dysfunction, and the promotion of maturation and stability of new blood vessels [35,36,37]. Moreover, in PH associated with congenital cardiopathies, we have previously described that remodeling vessels in irreversible PH expresses higher VEGF-A levels than in reversible PH [38]. VEGF-A is also involved in developing pulmonary arterial hypertension (PAH) in animal models and its inhibition has been shown to attenuate PAH development in these models [39]. Nonetheless, VEGF-A implication in idiopathic pulmonary fibrosis (IPF) seems controversial [40]. Some studies have suggested that VEGF-A may play a protective role in IPF by promoting angiogenesis and inhibiting fibrosis [40]. However, other studies have demonstrated increased circulating levels of VEGF-A in IPF patients [41], suggesting that VEGF-A may contribute to IPF pathogenesis. Moreover, the standard of care in IPF patients is nintedanib, a multikinase inhibitor that blocks VEGF-A. [42]. In the context of COVID-19, VEGF-A levels have been found to increase in acute COVID-19 patients, particularly in those with severe disease, even if other VEGF family members, such as placental growth factor seems more specific of severity at acute phase [20]. In PASC, several studies found that levels of VEGF-A were increased [43,44,45], without specific relationship with clinical presentation. One study suggested that VEGF-A may play a role in developing fatigue, a common symptom of long COVID [46].
VEGF-A is a protein that can be overproduced by various cells inside or outside the lung including alveolar epithelial cells, fibroblasts, immune cells and pulmonary microvascular endothelial cells. Angiogenic biomarkers have been recently found significantly increased in acute COVID-19 patients developing virus mutations [47]. This upregulation of angiogenic growth factors has been proposed as a consequence of SARS-CoV-2–induced lung damage. Thus, in the context of PASC, this phenomenon could be related to the persistence of the virus in the body or an overactive immune response to the acute infection [48].
Capillary rarefaction has been emphasized in the context of long COVID [49] and may also contribute to symptoms of PASC. An increased angiogenic signal is not really in line with observed rarefaction. However, this vascular density has been quantified with a camera evaluating sublingual microvasculature. PASC patients have parenchymal sequelae on CT scan, some of which are similar to those observed during IPF, another pathology characterized by pulmonary parenchymal alterations. In IPF, pulmonary vasculature can differ significantly according to the area. Indeed, IPF is associated with major pulmonary vascular remodeling and aberrant angiogenesis, with a capillary rarefaction in the fibrotic area and redistribution of blood vessels in areas of interstitial thickening. Thus, in IPF-adjacent areas of fibrotic tissue, we can find non-fibrotic area highly vascularized [50]. The same phenomenon could exist in microcirculation induced by COVID-19 and long COVID. Since angiogenic disorders explored by circulating growth factors are associated with CT scan sequelae, we can speculate that angiogenic disorders are close to the fibrotic area. Ackerman’s group proposed that vascular disease is a preliminary step to fibrosis, confirming our hypothesis of a relationship between respiratory sequelae and angiogenic disorder [32]. In the TUN-EndCOV Study, Charfeddine et al. reported endothelial dysfunction in PASC and proposed a glycocalyx dysfunction at the origin of the higher endothelial quality index and the slower response to the reperfusion phase [51]. Impaired glycocalyx was reversed after treatment with sulodexide, a highly purified mixture of glycosaminoglycans [52] associated with decreased PASC symptoms (i.e., chest pain and palpitations). Similarly, Osiaevi et al. previously described a variation of endothelial glycocalyx dimensions who underwent sublingual video microscopy in 27 patients with persistent symptoms > 12 weeks after recovery [49]. However, our data do not align with a glycocalyx involvement in PASC since circulating syndecan-1 levels (and all other endothelial activation markers) are not increased in PASC patients are not associated with abnormalities on CT lung scan. Nevertheless, these levels of syndecan-1 and previous “clinical” glycocalyx evaluation should be investigated in the same study thoroughly understand its involvement in PASC.
We found an association between VEGF-A and VWF levels independently of inflammation, contrasting with what we observed in acute COVID-19 phase [18]. We and others have previously described increased of VWF at acute phase of COVID-19 and its ability to predict severity and mortality [18]. Moreover, increased VWF has been proposed as a main trigger in microthrombosis associated to COVID-19 [17]. Several studies reported increased of VWF in convalescent patients after COVID-19 [53,54,55] without any correlation to symptoms. However, it seems that this increased levels of VWF are associated not only with an imbalance of ADAMTS13 [23] as already described in acute COVID-19 [56] but also with an increase in platelet binding on both collagen and anti-vWFA3 [57]. Two groups were able to link these increased VWF:Ag levels to impaired exercise capacity in PASC [22, 58], but no association to symptoms like fatigue or PFT has been described to date.
VEGF-A may be involved in the pathogenesis of COVID-19 by promoting angiogenesis while also interfering with inflammation, the development of thrombosis, and endothelial dysfunction. Some evidence in the literature suggests that VEGF-A can modify VWF synthesis or secretion release by endothelial cells, as shown in vitro [59,60,61]. It is important to note that these studies were conducted in vitro, so the in vivo effects of VEGF-A on VWF may differ. However, the absence of endothelial activation in our cohort with VWF increase aligns with a specific increase in VWF. VEGF-A may be at the origin of these increased levels of VWF in PASC patients previously described in the Irish COVID-19 Vasculopathy Study [22, 23]. Importantly, VWF and VEGF-A may also be released from activated platelets. However, in our study, no change in platelet number or activation was observed, as evidenced by normal soluble P-selectin levels, rendering a potential platelet origin of VEGF-A unlikely. In solid malignancy therapy, bevacizumab, a monoclonal antibody targeting VEGF-A, decreases the tumoral angiogenic signal and normalizes vessel phenotype while also acting as a key immune response modulator [62]. In acute COVID-19, VEGF inhibitors have been proposed as a therapeutic option for COVID-19-related vascular complications. Indeed, Bevacizumab has been tested in severe acute COVID-19 patients [63] and has shown clinical efficacy by improving oxygenation and shortening the duration of oxygen support. Therefore, in severe PASC patients with incapacitating clinical manifestations, VEGF-A may represent a future therapeutic target that should be validated in future hypothesis-testing studies and, in the longer term, in randomized controlled trials testing medications such as Bevacizumab.
Within the study’s limitations, two points are noteworthy. (i) First, this study mainly focused on analyzing and understanding of respiratory symptoms in patients with COVID-19. Moreover, patients were addressed for assessment of pulmonary symptoms. Thus, the results of our study cannot be applied to non-pulmonary symptoms associated with PASC. (ii) A second limitation of this study is the lack of inclusion of patients who fully recovered from acute COVID-19 infection. The absence of such a group makes comparing PASC patients, acute patients, and those who completely recovered is more challenging, potentially impacting the study’s conclusions and general implications. Another limitation of this work is the lack of systemic documentation of the underlying virus variant responsible for SARS-CoV-2 infection.
However, our present study suggests that VEGF-A may play a role in the pathogenesis of PASC.
All in all, our study provides new insights demonstrating that long COVID is probably a related angiogenic disorder. Our results highlight the relevance of plasma VEGF-A quantification as a quick, easy, and non-invasive way to discriminate long COVID-19 patients with respiratory impairment and may help to identify and target patients for new therapeutic approaches. Further studies are needed to identify which cell overproduces VEGF-A in PASC. However, beyond its involvement as a biomarker, VEGF-A emphasizes the link between angiogenesis and respiratory functions during long COVID and may open a new area for long COVID prevention or treatment with antiangiogenic therapy.
Abbreviations
- Ang:
-
Angiopoietin
- AP-HP:
-
Assistance Publique - Hôpitaux de Paris
- ARDS:
-
Acute respiratory distress syndrome
- ATS:
-
American Thoracic Society
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CRP:
-
C-reactive protein
- CT:
-
Computer Tomography
- DLCO:
-
Diffusing capacity of the lung for carbon monoxide
- FGF-2:
-
Fibroblast growth factor 2
- FRC:
-
Functional residual capacity
- GLI:
-
Global Lung Function Initiative
- GGO:
-
Ground glass opacities
- HR-CT:
-
High-resolution computed tomography
- Hs-cTnI:
-
High-sensitivity cardiac troponin I
- IPF:
-
Idiopathic pulmonary fibrosis
- IQR:
-
Interquartile range
- 6-MWD:
-
6-minute walk distance
- PAH:
-
Pulmonary arterial hypertension
- PASC:
-
Post-acute sequelae of COVID-19
- PH:
-
Pulmonary hypertension
- PIGF:
-
Placental growth factor
- PFT:
-
Pulmonary function test
- PPP:
-
Platelet-poor plasma
- ROC:
-
Receiver operator characteristics
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus-2
- SD:
-
Standard deviation
- sP-sel:
-
Soluble P-selectin
- VCAM-1:
-
Vascular cell adhesion protein 1
- VEGF-A:
-
Vascular endothelial growth factor A
- VWF:
-
von Willebrand factor
- TLC:
-
Total lung capacity
References
Centers for disease control and prevention (CDC). Post-COVID Conditions: Information for Healthcare Providers. :https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html.
Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV (2022) WHO clinical case definition working group on post-COVID-19 condition. a clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 22(4):e102-7
Montani D, Savale L, Noel N, Meyrignac O, Colle R, Gasnier M et al (2022) Post-acute COVID-19 syndrome. Eur Respir Rev Off J Eur Respir Soc 31(163):210185
Carfì A, Bernabei R, Landi F (2020) Gemelli against COVID-19 Post-acute care study group. persistent symptoms in patients after acute COVID-19. JAMA 324(6):603–5
Xiong Q, Xu M, Li J, Liu Y, Zhang J, Xu Y et al (2021) Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 27(1):89–95
Ballering AV, van Zon SKR, Olde Hartman TC, Rosmalen JGM (2022) Lifelines corona research initiative persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet Lond Engl 400(10350):452–61
Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y et al (2021) 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet Lond Engl 398(10302):747–758
Huang L, Li X, Gu X, Zhang H, Ren L, Guo L et al (2022) Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med 10(9):863–876
Global Burden of Disease Long COVID Collaborators, Wulf Hanson S, Abbafati C, Aerts JG, Al-Aly Z, Ashbaugh C et al (2022) Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 328(16):1604–1615
Mateu L, Tebe C, Loste C, Santos JR, Lladós G, López C, et al. Determinants of the Onset and Prognosis of the Post-COVID-19 Condition: A 2-Year Prospective Cohort Study [Internet]. SSRN; 2023. Retrieved July 13, 2023, from https://www.ssrn.com/abstract=4505315
Yang C, Zhao H, Espín E, Tebbutt SJ (2023) Association of SARS-CoV-2 infection and persistence with long COVID. Lancet Respir Med. https://doi.org/10.1016/S2213-2600(23)00142-X
Swank Z, Senussi Y, Manickas-Hill Z, Yu XG, Li JZ, Alter G et al (2023) Persistent Circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin Infect Dis Off Publ Infect Dis Soc Am 76(3):e487–e490
Patel MA, Knauer MJ, Nicholson M, Daley M, Van Nynatten LR, Cepinskas G et al (2023) Organ and cell-specific biomarkers of Long-COVID identified with targeted proteomics and machine learning. Mol Med 29(1):26
Gyöngyösi M, Alcaide P, Asselbergs FW, Brundel BJJM, Camici GG, da Costa Martins P et al (2023) Long COVID and the cardiovascular system-elucidating causes and cellular mechanisms in order to develop targeted diagnostic and therapeutic strategies: a joint Scientific Statement of the ESC Working Groups on cellular biology of the heart and myocardial and pericardial diseases. Cardiovasc Res 119(2):336–56
Chioh FW, Fong SW, Young BE, Wu KX, Siau A, Krishnan S et al (2021) Convalescent COVID-19 patients are susceptible to endothelial dysfunction due to persistent immune activation. eLife 10:e64909
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F et al (2020) Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 383(2):120–128
Smadja DM, Mentzer SJ, Fontenay M, Laffan MA, Ackermann M, Helms J et al (2021) COVID-19 is a systemic vascular hemopathy: insight for mechanistic and clinical aspects. Angiogenesis 24(4):755–788
Philippe A, Chocron R, Gendron N, Bory O, Beauvais A, Peron N et al (2021) Circulating Von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality. Angiogenesis 24(3):505–517
Werlein C, Ackermann M, Stark H, Shah HR, Tzankov A, Haslbauer JD et al (2022) Inflammation and vascular remodeling in COVID-19 hearts. Angiogenesis 12:1–16
Smadja DM, Philippe A, Bory O, Gendron N, Beauvais A, Gruest M et al (2021) Placental growth factor level in plasma predicts COVID-19 severity and in-hospital mortality. J Thromb Haemost JTH 19(7):1823–1830
Faconti L, Farukh B, McNally RJ, Brett S, Chowienczyk PJ (2023) Impaired β2-adrenergic endothelium-dependent vasodilation in patients previously hospitalized with coronavirus disease 2019. J Hypertens. https://doi.org/10.1097/HJH.0000000000003420
Fogarty H, Townsend L, Morrin H, Ahmad A, Comerford C, Karampini E et al (2021) Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost JTH 19(10):2546–2553
Fogarty H, Ward SE, Townsend L, Karampini E, Elliott S, Conlon N et al (2022) Sustained VWF-ADAMTS-13 axis imbalance and endotheliopathy in long COVID syndrome is related to immune dysfunction. J Thromb Haemost 20(10):2429–2438
Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH et al (2012) Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 40(6):1324–1343
Stanojevic S, Graham BL, Cooper BG, Thompson BR, Carter KW, Francis RW et al (2017) Official ERS technical standards: global lung function initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J 50(3):1700010
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002 Jul 1; 166(1):111–7
Enright PL, Sherrill DL (1998) Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 158(5 Pt 1):1384–1387
Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N et al (2020) Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology 295(3):200463
Wang Y, Dong C, Hu Y, Li C, Ren Q, Zhang X et al (2020) Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology 296(2):E55-64
Guerin CL, Guyonnet L, Goudot G, Revets D, Konstantinou M, Chipont A et al (2021) Multidimensional proteomic approach of endothelial progenitors demonstrate expression of KDR restricted to CD19 Cells. Stem Cell Rev Rep 17(2):639–651
Dudley AC, Griffioen AW (2023) Pathological angiogenesis: mechanisms and therapeutic strategies. Angiogenesis 15:1–35
Ackermann M, Kamp JC, Werlein C, Walsh CL, Stark H, Prade V et al (2022) The fatal trajectory of pulmonary COVID-19 is driven by lobular ischemia and fibrotic remodelling. EBioMedicine 4(85):104296
Kamp JC, Werlein C, Plucinski EKJ, Neubert L, Welte T, Lee PD et al (2023) Novel insight into pulmonary fibrosis and long COVID. Am J Respir Crit Care Med 207(8):1105–1107
Barratt S, Medford AR, Millar AB (2014) Vascular endothelial growth factor in acute lung injury and acute respiratory distress syndrome. Respir Int Rev Thorac Dis 87(4):329–342
Miao H, Qiu F, Zhu L, Jiang B, Yuan Y, Huang B et al (2021) Novel angiogenesis strategy to ameliorate pulmonary hypertension. J Thorac Cardiovasc Surg 161(6):e417–e434
Eddahibi S, Humbert M, Sediame S, Chouaid C, Partovian C, Maître B et al (2000) Imbalance between platelet vascular endothelial growth factor and platelet-derived growth factor in pulmonary hypertension. effect of prostacyclin therapy. Am J Respir Crit Care Med 162:1493–9
Christou H, Yoshida A, Arthur V, Morita T, Kourembanas S (1998) Increased vascular endothelial growth factor production in the lungs of rats with hypoxia-induced pulmonary hypertension. Am J Respir Cell Mol Biol 18(6):768–776
Smadja DM, Gaussem P, Mauge L, Israël-Biet D, Dignat-George F, Peyrard S et al (2009) Circulating endothelial cells: a new candidate biomarker of irreversible pulmonary hypertension secondary to congenital heart disease. Circulation 119(3):374–381
Godinas L, Guignabert C, Seferian A, Perros F, Bergot E, Sibille Y et al (2013) Tyrosine kinase inhibitors in pulmonary arterial hypertension: a double-edge sword? Semin Respir Crit Care Med 34(5):714–724
Papaioannou AI, Kostikas K, Kollia P, Gourgoulianis KI (2006) Clinical implications for vascular endothelial growth factor in the lung: friend or foe? Respir Res 7(1):128
Smadja DM, Nunes H, Juvin K, Bertil S, Valeyre D, Gaussem P et al (2014) Increase in both angiogenic and angiostatic mediators in patients with idiopathic pulmonary fibrosis. Pathol Biol (Paris) 62(6):391–394
Fukihara J, Kondoh Y (2016) Nintedanib (OFEV) in the treatment of idiopathic pulmonary fibrosis. Expert Rev Respir Med 10(12):1247–1254
Alfadda AA, Rafiullah M, Alkhowaiter M, Alotaibi N, Alzahrani M, Binkhamis K et al (2022) Clinical and biochemical characteristics of people experiencing post-coronavirus disease 2019-related symptoms: a prospective follow-up investigation. Front Med 9:1067082
Patel MA, Knauer MJ, Nicholson M, Daley M, Van Nynatten LR, Martin C et al (2022) Elevated vascular transformation blood biomarkers in Long-COVID indicate angiogenesis as a key pathophysiological mechanism. Mol Med 28(1):122
Patterson BK, Guevara-Coto J, Yogendra R, Francisco EB, Long E, Pise A et al (2021) Immune-based prediction of COVID-19 severity and Chronicity decoded using machine learning. Front Immunol 28(12):700782
Patterson BK, Yogendra R, Guevara-Coto J, Mora-Rodriguez RA, Osgood E, Bream J et al (2023) Case series: maraviroc and pravastatin as a therapeutic option to treat long COVID/Post-acute sequelae of COVID (PASC). Front Med 10:1122529
Gupta A, Konnova A, Smet M, Berkell M, Savoldi A, Morra M et al (2023) Host immunological responses facilitate development of SARS-CoV-2 mutations in patients receiving monoclonal antibody treatments. J Clin Invest 133(6):e166032
Vojdani A, Vojdani E, Saidara E, Maes M (2023) Persistent SARS-CoV-2 infection, EBV, HHV-6 and other factors may contribute to inflammation and autoimmunity in long COVID. Viruses 15(2):400
Osiaevi I, Schulze A, Evers G, Harmening K, Vink H, Kümpers P et al (2023) Persistent capillary rarefication in long COVID syndrome. Angiogenesis 26(1):53–61
Ebina M, Shimizukawa M, Shibata N, Kimura Y, Suzuki T, Endo M et al (2004) Heterogeneous increase in CD34-positive alveolar capillaries in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 169(11):1203–1208
Charfeddine S, Ibn Hadj Amor H, Jdidi J, Torjmen S, Kraiem S, Hammami R et al (2021) Long COVID 19 syndrome: is it related to microcirculation and endothelial dysfunction? insights from TUN-EndCOV study. Front Cardiovasc Med 8:745758
Charfeddine S, Ibnhadjamor H, Jdidi J, Torjmen S, Kraiem S, Bahloul A et al (2022) Sulodexide significantly improves endothelial dysfunction and alleviates chest pain and palpitations in patients with long-COVID-19: insights from TUN-EndCOV study. Front Cardiovasc Med 9:866113
Willems LH, Nagy M, Ten Cate H, Spronk HMH, Groh LA, Leentjens J et al (2022) Sustained inflammation, coagulation activation and elevated endothelin-1 levels without macrovascular dysfunction at 3 months after COVID-19. Thromb Res 209:106–114
Willems LH, Jacobs LMC, Groh LA, Ten Cate H, Spronk HMH, Wilson-Storey B et al (2023) Vascular function, systemic inflammation, and coagulation activation 18 months after COVID-19 infection: an observational cohort study. J Clin Med 12(4):1413
Fan BE, Wong SW, Sum CLL, Lim GH, Leung BP, Tan CW et al (2022) Hypercoagulability, endotheliopathy, and inflammation approximating 1 year after recovery: assessing the long-term outcomes in COVID-19 patients. Am J Hematol 97(7):915–923
Philippe A, Gendron N, Bory O, Beauvais A, Mirault T, Planquette B et al (2021) Von Willebrand factor collagen-binding capacity predicts in-hospital mortality in COVID-19 patients: insight from VWF/ADAMTS13 ratio imbalance. Angiogenesis 24(3):407–411
Constantinescu-Bercu A, Kessler A, de Groot R, Dragunaite B, Heightman M, Hillman T et al (2023) Analysis of thrombogenicity under flow reveals new insights into the prothrombotic state of patients with post-COVID syndrome. J Thromb Haemost 21(1):94–100
Prasannan N, Heightman M, Hillman T, Wall E, Bell R, Kessler A et al (2022) Impaired exercise capacity in post-COVID-19 syndrome: the role of VWF-ADAMTS13 axis. Blood Adv 6(13):4041–4048
Lorenzi O, Frieden M, Villemin P, Fournier M, Foti M, Vischer UM (2008) Protein kinase C-delta mediates von Willebrand factor secretion from endothelial cells in response to vascular endothelial growth factor (VEGF) but not histamine. J Thromb Haemost 6(11):1962–1969
Yang X, Jian Sun H, Rong Li Z, Zhang H, Jun Yang W, Ni B et al (2015) Gastric cancer-associated enhancement of von Willebrand factor is regulated by vascular endothelial growth factor and related to disease severity. BMC Cancer 15:80
Matsushita K, Yamakuchi M, Morrell CN, Ozaki M, O’Rourke B, Irani K et al (2005) Vascular endothelial growth factor regulation of Weibel-Palade-body exocytosis. Blood 105(1):207–214
Geindreau M, Ghiringhelli F, Bruchard M (2021) Vascular endothelial growth factor, a key modulator of the anti-tumor immune response. Int J Mol Sci 22(9):4871
Pang J, Xu F, Aondio G, Li Y, Fumagalli A, Lu M et al (2021) Efficacy and tolerability of bevacizumab in patients with severe Covid-19. Nat Commun 12(1):814
Funding
This work was funded with grants from French national agency for research ANR SARCODO (Fondation de France). Aurélien Philippe was funded with grants from Mécénat Crédit Agricole Ile de France programme jeune talent.
Author information
Authors and Affiliations
Contributions
DMS, SG, and JLD designed the study. AP and DMS analyzed the data and wrote the manuscript. AP and PC performed statistical analysis. All authors reviewed and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Philippe, A., Günther, S., Rancic, J. et al. VEGF-A plasma levels are associated with impaired DLCO and radiological sequelae in long COVID patients. Angiogenesis 27, 51–66 (2024). https://doi.org/10.1007/s10456-023-09890-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-023-09890-9