Abstract
The development of vascular system in vertebrates has been traditionally explained by early vasculogenic assembly of angioblasts followed by angiogenic outgrowth of pre-existing vessels. The discovery of adult endothelial progenitor cells (Asahara et al. in Science 275(5302):964–967, 1997) challenged this view, since postnatal vascular growth could be accomplished by recruitment of circulating cells with the ability to differentiate into endothelial cells. However, the existence of embryonic circulating endothelial progenitor cells and their actual contribution to vascular development is far less known. We review in this paper the literature concerning the features, origin and physiological functions of embryonic and foetal circulating endothelial progenitors. Our review includes the early (E7.5) progenitors isolated from yolk sac, the hematovascular progenitors identified in the foetal liver, the yolk sac-derived erythro-myeloid progenitors, circulating hematopoietic cells from the G2-GATA4 lineage and the endothelial colony-forming cells isolated from the placenta and umbilical cord blood. We highlight the need of further characterization of these populations and the relationships between them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development and growth of the vessels depend on two main processes involving the endothelial cells. Vasculogenesis is the de novo formation of endothelial tubes in the embryo by assembly of mesodermal cells called angioblasts. Angiogenesis is the process of vascular sprouting from pre-existing vessels [1]. This process involves activation, proliferation and migration of endothelial cells in response to angiogenic stimuli. During embryonic angiogenesis, tissue-resident angioblasts can eventually be recruited and incorporated to the growing vessels. Adult angiogenesis, however, can be accomplished only by mature endothelial cells.
It was thought for long time that these processes were necessary and sufficient to explain the full deployment of the vascular tree throughout the vertebrate body, including the postnatal vascular growth, either in physiological or pathological conditions (e.g. supporting tumour growth). This concept was challenged in the late 90s by Isner’s [2], who demonstrated that circulating endothelial progenitor cells (EPC) derived from bone marrow and expressing CD34 and VEGFR2, were able to differentiate into endothelial cells in culture. In vivo experiments suggested that they incorporated to the adult vessels in ischemic conditions, contributing to vascular growth. This process was known as “postnatal vasculogenesis”. This seminal paper gave rise to a large number of studies about the origin, characterization and clinical potential of the EPC (reviewed in [3]). Along the last two decades it has been realized that EPC are not a single, homogeneous progenitor population, as we will describe below. Within EPC, it is necessary to distinguish between myeloid angiogenic cells (MAC) and endothelial colony-forming cells (ECFC) [4, 5]. Nevertheless, it has been confirmed that both types of adult EPC do contribute to the vascular growth in the vascular response to ischemia, and they can be relevant for tumoral growth.
The knowledge about prenatal EPC is far less abundant. It is well known that ECFC can be isolated from umbilical vein blood and also from placenta (reviewed in [6]). Foetal ECFC show much higher proliferative, colony-forming and neovascularisation abilities than adult ECFC. However, it has been scarcely studied if circulating EPC actually contribute to vascular growth during embryonic development, besides the “classical” process of vasculogenesis and angiogenesis.
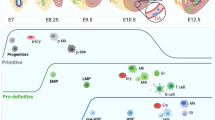
The aim of this paper is to review the relevant literature about the origin and features of the embryonic and foetal EPC, and the available evidence pointing to a significant participation of circulating EPC in the development of the embryonic vasculature. We are aware that the term EPC can be ambiguous when referred to adult organisms (see next section). However, we will keep EPC in our review as a comprehensive term to refer to those embryonic cells that are able to circulate throughout the blood stream and actually or potentially can contribute to the endothelium of different organs during development. As we will discuss below, these embryonic EPC are a set of heterogeneous populations that require a much better characterization (features summarized in Table 1, Fig. 1).
Graphic summary of all the types of embryonic and adult endothelial progenitor cells described in this paper. The main contributions of these cells to the foetal, normal adult and ischemic endothelium are shown by the arrows. Blue arrows represent endothelial differentiation of progenitor cells after transplant. The red surface represents expression of endothelial markers. EMP erythro-myeloid progenitors, eEPC embryonic endothelial progenitor cells, G2-GATA4 endothelial progenitors derived from a GATA4-expressing lineage, HVP hematovascular progenitors, MAC myeloid angiogenic cells, PL-ECFC and UC-ECFC placental and umbilical cord endothelial colony-forming cells, respectively
Adult EPC. A brief description
As stated above, the original term “endothelial progenitor cell” encompasses two main types of adult cells that can be recruited to sites of neovascularisation and efficiently contribute to postnatal growth of vessels [4] (Fig. 2). MAC, the type originally described in Asahara’s paper, have also been called “early endothelial progenitor cells”, since they appear soon (1 week) when mononuclear cells from human peripheral blood are cultured in endothelial medium [7]. They have a hematopoietic origin, derive from bone marrow, express CD45 and CD14 together with endothelial markers (CD31, VEGFR2) and support angiogenic growth through paracrine mechanisms. However, they do not differentiate into mature endothelial cells in vivo. ECFC or “late endothelial progenitor cells” appear in culture of mononuclear cells after 3 weeks, do not express CD45 or CD14, and they express the full set of canonical endothelial markers as well as the adhesion protein CD146 and the TGFβ receptor endoglin (CD105). They form tubes when cultured on matrigel, and they can differentiate into endothelial cells either in in vitro or in vivo assays [7].
The precise origin of adult ECFC is controversial, but they do not derive from bone marrow. In fact, bone marrow cells do not originate endothelial cells in physiological or pathological conditions [8, 9]. Combining parabiosis with the hind limb ischemia model, Aicher et al. demonstrated that 75% of the cells incorporated to the ischemic area were not derived from bone marrow. Instead, liver and intestine were the main source of the recruited cells [10].
Probably, adult ECFC arise from the vessel walls [11,12,13], or from the endothelium itself (reviewed in [14]). Furthermore, they have been also localized in adipose tissue [15].
EPC in non-mammalian embryos
The existence of embryonic EPC in avian embryos has been well characterized, due to the ease of their experimental manipulation. A simple quail chick parabiosis, a model in which the bloodstream of both embryos are connected, showed that quail endothelial cells became integrated in the vessels of the chick embryos [16]. The presence of donor endothelial cells was detected by these authors in most tissues, but their number was low. This integration was much increased in wounded embryonic tissues or in tumour grafts. However, sprouting angiogenesis induced by VEGF in the chorioallantoic membrane did not enhance recruitment of EPC. Thus, EPC play a physiological, albeit minor role in the vascular growth of the avian embryos.
It was soon established that the origin of avian EPC was extraembryonic. Grafts of quail allantois into the coelomic cavity of chick embryos revealed the presence of endothelial cells in the host. These allantois-derived cells could only reach their destination through the circulation [17]. The existence of circulating EPC derived from the allantois was later confirmed [18] as well as their scarce contribution to the host endothelium (< 50 cells/mm3). Donor-derived cells were found mainly in heart, liver and wing buds. Besides the allantois, the yolk sac is also a source of embryonic EPC, as shown by yolk sac chimaeras [19]. The fusion of a chick embryo with a quail yolk sac allowed the study of the colonization of the host vascular system by yolk sac EPC. The process starts very soon but the density of donor endothelial cells decreases from 143 to 26 cells/mm3 between day 1 and day 4 of incubation. About 1% of the host endothelium was finally derived from the yolk sac. All the tissues incorporated EPC, mainly into the capillaries. However, this study did not detect EPC originating within the embryo.
In summary, extraembryonic, circulating EPC exist in the avian embryo although their contribution to vascular growth is scarce and their phenotype has been poorly characterized by the lack of specific markers.
Embryonic EPC have not hitherto been described in other non-mammalian models. However, it has been shown that human cord blood CD34+ cells injected in zebrafish embryos cosegregate with resident hemangiobasts and are recruited to developing vessels where they exert a paracrine proangiogenic activity. These human EPC also enhance vascular repair in the model of adult caudal fin amputation [20].
Early embryonic EPC in the mammalian embryo
An embryonic cell population obtained from dissociated egg cylinder and yolk sac from E7.5 mouse embryos and characterized by expression of thrombomodulin showed EPC features [21]. Their expression of thrombomodulin and GATA4 suggested that they derived from proximal lateral mesoderm. These cells were termed embryonic EPC (eEPC) by their discoverers, and their role as bona fide circulating endothelial progenitors with potential therapeutic applications was soon confirmed by a number of papers [22,23,24]. When injected into chick embryos, eEPC give rise to endothelial cells from brain, heart and liver. Induction with retinoic acid promotes their differentiation into endothelial cells in culture. Phenotypically, eEPC are characterized by the expression of the angiopoietin receptor Tie2, cKit and Sca1, but their expression of VEGFR2 is low or absent [21]. An advantage of eEPC is the lack of MHC-I expression as well as their resistance to killer cell-mediated cytolysis, allowing their use in non-syngeneic animals [25].
In physiological conditions, transplanted eEPC are not recruited by normal adult vessels. However, they are home to hypoxic areas and colonize tumor metastases in mice where they contribute to the tumoral endothelium. Their recruitment is mediated by both, E and P selectins, and P-selectin glycoprotein ligand-1 (PSGL1) [26]. In fact, incorporation of a “suicide gene” in eEPC allowed for targeting these cells to hypoxic lung metastases after intravenous injection, demonstrating antitumoral efficacy in a preclinic model [25]. When eEPC are pre-stimulated with tumor-conditioned medium they display macrophagic capacity in a murine model of melanoma metastasis, decreasing the number of metastases [27].
In the hind limb ischemia model, injected eEPC increase capillary density and limb perfusion [23]. This effect is produced also by eEPC encapsulated into hialuronic acid to protect them from systemic cytotoxicity [28]. The ability of eEPC to improve neovascularisation in the hind limb ischemia model is enhanced by pharmacological activation of the NFκB pathway before injection. This activation upregulates expression of E-selectin and PSGL1, increasing the adhesion of the eEPC to the endothelium of the ischemic areas [29].
Cardiac output is also improved by eEPC infusion after myocardial ischemia–reperfusion injury in pigs. Part of these effects are probably due to paracrine action, since eEPC express a number of angiogenic factors [23] as well as thymosin β4 [30]. In addition to this effect, eEPC express the neuregulin 1 receptors erbB2 and erbB3. Treatment of eEPC with neuregulin 1β before injection in the model of myocardial ischemia increased phosphorilation of Akt, ERK1-2 and GSK3β, promoting anti-apoptotic activity on cardiomyocytes [31]. On the other hand, stimulation of the adenosine receptor 1 in eEPC increases their expression of P-selectin, their adhesion to cardiac microvascular endothelial cells and their retention in isolated mouse hearts [32]. It was well known that extracellular adenosine concentration increases in sites of ischemia promoting neovascularisation. In a rat model of chronic cerebral ischemia, eEPC also increase collateralization and promotes hemodynamic rescue [24].
The interaction of eEPC with platelets seems to be very important for their functions. Platelets increase recruitment of eEPC to sites of damage and induce their differentiation into endothelium. The adhesion is enhanced in both, static and dynamic conditions and it is mediated by P-selectin and β1 integrin [33].
Thus, eEPC can be detected in the mouse embryo as soon as E7.5 and they share properties with adult ECFC, particularly the ability to contribute to sites of neovascularization. However, a detailed comparison of their phenotypic profiles as well as an estimation of their actual contribution to the normal development of the embryonic vascularization have not been performed yet.
Foetal liver hematovascular progenitors
The presence of putative EPC in the foetal liver had been suggested by culture of CD31+ /Sca1+ cells obtained from dissociated livers on a layer of liver feeder fibroblasts. Colonies of EPC emerge between 2 and 3 weeks of culture (a time corresponding to late adult EPC, i.e. ECFC). These foetal liver EPC express VEGFR2, CD34, CD133, VCAM and VE-Cadherin [34]. On the other hand, it was well known that foetal liver contains a large number of hematopoietic stem cells that can be transplanted to myeloablated mice where they show long-term reconstitution potential of all the hematopoietic system [35]. The features of the hematovascular foetal liver cells was further investigated using a SCL-3′ enhancer lineage tracing system, and it was observed that a population of foetal hematopoietic liver cells was also able to give rise to endothelial cells after transplantation [36]. Injection of these hematovascular progenitors was performed in two models, newborns treated with the myeloablative drug busulfan and irradiation of adult mice. In both cases, donor-derived endothelial cells were found in liver, heart and kidneys. However, when the mice were injected in the same conditions with adult bone marrow cells, only a few donor-derived endothelial patches were found. This demonstrated the EPC ability of a subset of foetal liver cells, probably corresponding to the cultured CD31+/Sca1+ cells described by Cherqui et al. [34]. More recently, the foetal liver EPC were characterized as a VE-Cadherin+/CD45− population derived from the SCL-3′ enhancer lineage. This population showed a clearly endothelial profile, expressing Lyve1, CD31, Tie2, VEGFR2 and CD34, but being negative for Mac1 or cKit [37]. The endotheliogenic potential of foetal liver hematovascular progenitors is maintained along the embryonic development and it can be detected even postnatally. Although this has not been experimentally demonstrated it is conceivable that this population contributes to the endothelium of different organs along the embryonic development. However, Bockamp et al. did not find SCL-tagged endothelial cells between the stages E9 and E12.5 using a different reporter system [38].
G2-GATA4 lineage endothelial progenitors
GATA4 is a transcription factor widely expressed in mesoderm and endoderm. Its expression is regulated by a number of tissue-specific enhancers. The G2 enhancer drives GATA4 expression in the lateral mesoderm and allantois [39]. Recently, it was shown that about 20% of the adult murine hematopoietic stem cells derive from progenitors where GATA4 expression is driven by the G2 enhancer. Probably, most of these progenitors arise from the placenta [40]. A number of endothelial cells derived from this lineage were found in different organs. Furthermore, transplantation of cells obtained from the placenta or the aorta-gonad-mesonephros (AGM) area into busulfan-treated newborns gives rise to patches of donor-derived endothelial cells in the liver and heart. This was interpreted as the differentiation of endothelial cells from circulating hematopoietic progenitors [40]. More recently, it was shown that about 20% of all the cardiac endothelial cells of the mouse embryo belong to the G2-GATA4 lineage [41] (Fig. 3). A minor part of these endothelial cells derive from the epicardium, a tissue that expresses the G2-GATA4 lineage reporter, but at least 15% of all the embryonic cardiac/coronary endothelium arises from circulating progenitors, according to this report. In fact, most G2-GATA4 endothelial cells appear associated to the ventricular endocardium, while the endothelial cells expressing epicardial lineage reporters such as WT1 or GATA5 are mainly located into the compact ventricular layer. It is important to remark that the embryonic ventricular endocardium is a main contributor of the coronary endothelium [42]. Consequently, the recruitment of circulating cells by the endocardium would contribute to the rapid expansion of the coronary vessels throughout the compact myocardial layer. G2-GATA4 endothelial cells were also observed in the brain vessels, where they can only be derived from blood-borne cells. Thus, circulating endothelial progenitors from a G2-GATA4 lineage, probably originated into the placental mesoderm, contribute to the vascularisation of the heart and, probably, of other organs.
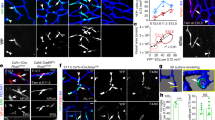
Circulating endothelial progenitors derived from a GATA4-expressing cell lineage under control of the G2 enhancer [40]. a, b Ventricle of G2-GATA4Cre;YFP mouse embryos, E11.5. Some circulating cells expressing the reporter (arrows) can be seen in the ventricular lumen (a) and close to the endocardial lining (b), where endothelial cells of the G2-GATA4 lineage appear (arrowheads). In the insert, two large cells from the G2-GATA4 lineage can be seen attached to the endocardium of a myocardial trabecula (M), one of them expressing the endothelial marker CD31. c When foetal liver cells from E11.5 G2-GATA4Cre; YFP embryos are injected into the bloodstream of busulfan-treated newborns, donor endothelial cells can be found in the lungs of the recipient mice after 1 month (arrowheads). Scale bar: 25 µm for all the figures
Erythro-myeloid endothelial progenitors
Tissue-resident macrophages originate in mice from the yolk sac endothelium in two waves, first between E7.0 and E8.25 and then from E8.25 onwards [43, 44]. The early wave colonizes the embryo and gives rise to the microglia and Langerhans cells of the epidermis, while the late wave colonizes the liver. When the promoter of the colony-stimulating factor receptor 1 (Csfr1) was used as a driver to track the lineage of the tissue-resident macrophages, a number of endothelial cells appeared as derived from this lineage [45]. The erythro-myeloid progenitors of these endothelial cells were localized by the stage E12.5 in liver and peripheral blood and they showed a CD31−/CD45low/cKit+ profile. They contribute mainly to the liver endothelium (about 60% of all the endothelial cells) and to the heart, lung and brain in a lesser extent. Interestingly, the endothelial cells derived from the Csfr1 lineage keep a particular transcriptional signature in adults. This study concluded that the vascularisation of fast-growing organs is supported by recruitment of circulating endothelial progenitors and thus, it does not depend only of angiogenic proliferation of the local endothelium or integration of tissue-resident angioblasts.
ECFC have been localized in the human placenta. They are characterized by the expression of CD31, CD105, CD144 and VEGFR2, but they are negative for CD45 and CD34 [46]. According to that study, placental ECFC are phenotypically similar to cord blood ECFC, but they exhibit a higher vasculogenic potential in vivo. Placental ECFC were also studied by [47], who found a CD45−/CD34+/CD31low profile, somewhat different to that reported by [46] as well as expression of CD105, CD144 and CD146. All these markers are similar to those from the ECFC isolated from human cord blood. However, the number of ECFC found in one placenta is 27 times higher than those found in cord blood. According to this report, both, placental and cord blood ECFC show similar vessel forming ability in vivo and in vitro, and their performance in the hind limb ischemia model is similar. Further comparisons between placental and cord blood ECFC were reviewed by [48].
A comparison between ECFC derived from microvascular (i.e. maternal) and macrovascular (i.e. foetal) circulation was performed by Solomon et al. [49]. In both cases ECFC were characterized by a similar profile (positive for CD31, CD105, CD146, CD144, negative for CD45 and CD14). Foetal ECFC showed higher clonogenic, proliferative and in vitro tube formation potential, while maternal ECFC showed a higher potential in in vivo vasculogenic assays.
The mammalian placenta is an evolutionary derivative of the allantois, and it is important to remark that circulating endothelial progenitors were localized into the avian allantois [17, 18] as commented above. Thus, the presence of ECFC in the placenta can be considered as the evolutionary conservation of a property of the allantois.
Umbilical cord blood ECFC
The best characterized foetal endothelial progenitors are those derived from the umbilical cord blood. They were first identified by Ingram et al. [50]. The methods of their isolation, cloning and expansion were described by Prasain et al. and Zhang et al. who phenotypically characterized cord blood ECFC as CD31+/CD144+/CD34+/CD146+/CD45−/CD14−/CD133− cells [51, 52]. A detailed comparison between endothelial cells differentiated in vitro from cord blood ECFC, HUVEC (human umbilical vein endothelial cells) and adult endothelial cells has been performed by Bompais et al. [43]. Despite a similar expression of endothelial markers, fully differentiated endothelial cells derived from cord blood ECFC showed higher proliferative potential, were more sensitive to angiogenic stimuli and exhibited more hematopoietic supportive activity than HUVEC or adult endothelial cells.
Cord blood ECFC abundance and properties change along gestation. ECFC isolated between weeks 33 and 36 showed the same clonogenic potential than those obtained from full-term pregnancies. However, ECFC obtained between weeks 24 and 28 give rise to less colonies in culture. Anyway, the foetal ECFC can give rise to 100 times more progeny than the ECFC isolated from adult blood [54]. Other studies have reported more ECFC in cord blood of preterm infants [55]. Safranow et al. found higher early ECFC (CD133+) and late ECFC (CD133−) in cord blood of preterm infants [56]. The number of ECFC was inversely correlated with APGAR score of preterm infants. This observation is consistent with the decrease in the number of ECFC along the gestational age [57]. These authors found that an abnormal low number of ECFC in preterm infants was associated with higher risk of bronchopulmonary dysplasia.
An increase of ECFC along the third trimester of gestation was reported by Muñoz-Hernández et al. [58]. In this report, a low number of ECFC in the cord blood was related with preeclampsia risk. A lower number of ECFC, showing lower proliferation, migration and vasculogenic potential in vitro, has also been reported in cases of preeclampsia. This defect was attributed to Vitamin D3 deficiency and, in fact, treatment of ECFC with vitamin D3 promoted the vasculogenic ability of ECFC and the upregulation of VEGF expression [59, 60]. The close relationships between ECFC and preeclampsia and the controversial findings obtained in some cases have been recently reviewed by Attar et al. [61].
Low body weight at birth has also been associated with lower number of cord blood ECFC-derived colonies and tube formation ability in vitro. ECFC from low birth weight preterm infants showed increased expression of thrombospondin-1, an antiangiogenic glycoprotein [62]. It is important to remark in this context that murine foetal ECFC are able to migrate to the maternal circulation and contribute to uterine vasculature potentiating placental perfusion and consequently the foetal growth [63]. This finding can be related with the lower number, lower proliferation, impaired migration and reduced in vitro vasculogenic potential of ECFC isolated from foetus suffering intrauterine growth restriction [64]. When implanted into an in vivo vasculogenic assay, these ECFC showed a sixfold decrease in de novo capillary formation.
Conflicting data on cord blood ECFC have also been obtained in gestational diabetes mellitus. Some studies report no changes in ECFC number, while other have found a decrease in the number of colonies generated by ECFC in culture, as well as lower migration and tube formation potential. In general, gestational diabetes mellitus seems to provoke functional abnormalities in ECFC function (reviewed in [6]). As described above for the impaired function of ECFC in preeclampsia, Vitamin D can rescue the quantity and the impaired angiogenesis-related function of ECFC obtained from gestational diabetes mellitus pregnancies [65].
As described above for eEPC, platelet function seems to be relevant for cord blood ECFC function. A platelet lysate increased cord blood ECFC survival via activation of the Akt pathway, as well as tube formation in vitro [66].
Recently, Reid et al. have used human cord blood ECFC for cellular therapy of ischemic retinopathies. They isolated these cells by plating CD31+/CD105+/CD45-/CD14− mononuclear cells and performed systemic and intravitreal injections in a murine model of ischemic retinopathy, obtaining good therapeutic efficacy. However, the injected cells did not persist in the retina for more than 24 h [67].
About the origin of the cord blood ECFC, an interesting proposal was made by Ingram et al. comparing HUVEC with HAEC (human aortic endothelial cells). They found that HUVEC were able of 40 doubling population in vitro. These cells expressed the typical markers of umbilical cord ECFC (CD31, C105, CD146, CD144, VEGFR2). The authors concluded that HUVEC could be a main source of the cord blood endothelial progenitors [68]. It may be significant in this context that adult ECFC can be expanded in vitro using blood plasma instead of FBS [69]. However, as stated above, functional differences between cord blood ECFC and HUVEC have been reported [53].
Conclusions and future directions
The classical view about the development of the vascular system in vertebrates (early vasculogenic assembly of angioblasts followed by angiogenic outgrowth of pre-existing vessels) has been challenged by the increasing evidence of a prenatal incorporation of circulating progenitor cells of the endothelial lining of the vessels. However, many uncertainties remain, since embryonic circulating EPC have been described in many different contexts, and frequently they have only been characterized by in vitro assays or for their potential of neovascularisation in pathological conditions. We still do not know if the different sets of embryonic EPC herein described reflect an actual physiological complexity concerning their origin, phenotype and contribution to the prenatal vascular growth. We do not know if the late ECFC populations found in umbilical cord blood or placenta are related in some extent with the early EPC populations (hematovascular foetal liver, G2-GATA4 and erythro-myeloid progenitors as well as the earliest eEPC). We do not know what the total amount of endothelial cells that are derived from EPC recruitment during development is. We know that circulating EPC in avian embryos have a real, albeit limited, contribution to the embryonic blood vessels, and some of the reports quoted in this review suggest that this contribution could be even higher in mammals, at least in fast-growing organs like the liver or heart. We do not know what the meaning of the differences in EPC number reported among organs is, or if the EPC-derived endothelial cells keep some differential features in adults. Could these differential features have translational interest? For example, the origin of adult EPC could be related with the persistence of embryonic EPC progenitors in the vessel wall.
Finally, we think that the issue of the existence of embryonic EPC must be regarded in a wider evolutionary context. The ontogeny of endothelial and blood cells has been closely related by concepts such as the hemangioblast, the hemogenic endothelium (reviewed in [70]) or the origin of endothelial cells from hematopoietic progenitors herein reviewed. We think that the still existing controversies about these concepts should consider that the endothelial cells can be regarded as a specialized type of blood cells whose evolutionary origin is related to the origin of vertebrates [71]. In this way, the transitions between hematopoietic and endothelial phenotypes during ontogeny can be much better understood, since we are actually dealing with a single lineage of cells related by a common evolutionary origin.
References
Ribatti D, Nico B, Crivellato E (2015) The development of the vascular system: a historical overview. Methods Mol Biol 1214:1–14. https://doi.org/10.1007/978-1-4939-1462-3_1
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275(5302):964–967. https://doi.org/10.1126/science.275.5302.964
Chong MS, Ng WK, Chan JK (2016) Concise review: endothelial progenitor cells in regenerative medicine: applications and challenges. Stem Cells Transl Med 5(4):530–538. https://doi.org/10.5966/sctm.2015-0227
Medina RJ, Barber CL, Sabatier F, Dignat-George F, Melero-Martin JM, Khosrotehrani K, Ohneda O, Randi AM, Chan JKY, Yamaguchi T, Van Hinsbergh VWM, Yoder MC, Stitt AW (2017) Endothelial progenitors: a consensus statement on nomenclature. Stem Cells Transl Med 6(5):1316–1320. https://doi.org/10.1002/sctm.16-0360
Chopra H, Hung MK, Kwong DL, Zhang CF, Pow EHN (2018) Insights into endothelial progenitor cells: origin, classification, potentials, and prospects. Stem Cells Int 2018:9847015. https://doi.org/10.1155/2018/9847015
Gumina DL, Su EJ (2017) Endothelial progenitor cells of the human placenta and fetoplacental circulation: a potential link to fetal, neonatal, and long-term health. Front Pediatr 5:41. https://doi.org/10.3389/fped.2017.00041
Minami Y, Nakajima T, Ikutomi M, Morita T, Komuro I, Sata M, Sahara M (2015) Angiogenic potential of early and late outgrowth endothelial progenitor cells is dependent on the time of emergence. Int J Cardiol 186:305–314. https://doi.org/10.1016/j.ijcard.2015.03.166
Göthert JR, Gustin SE, van Eekelen JA, Schmidt U, Hall MA, Jane SM, Green AR, Göttgens B, Izon DJ, Begley CG (2004) Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood 104(6):1769–1777
Purhonen S, Palm J, Rossi D, Kaskenpää N, Rajantie I, Ylä-Herttuala S, Alitalo K, Weissman IL, Salven P (2008) Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci USA 105(18):6620–6625. https://doi.org/10.1073/pnas.0710516105
Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fändrich F, Siebert R, Cooke JP, Dimmeler S, Heeschen C (2007) Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res 100(4):581–589
Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergün S (2006) Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development 133(8):1543–1551
Ergün S, Tilki D, Hohn HP, Gehling U, Kilic N (2007) Potential implications of vascular wall resident endothelial progenitor cells. Thromb Haemost 98(5):930–939
Klein D, Hohn HP, Kleff V, Tilki D, Ergün S (2010) Vascular wall-resident stem cells. Histol Histopathol 25(5):681–689
Yoder MC (2010) Is endothelium the origin of endothelial progenitor cells? Arterioscler Thromb Vasc Biol 30(6):1094–1103. https://doi.org/10.1161/ATVBAHA.109.191635
Lin RZ, Moreno-Luna R, Muñoz-Hernandez R, Li D, Jaminet SC, Greene AK, Melero-Martin JM (2013) Human white adipose tissue vasculature contains endothelial colony-forming cells with robust in vivo vasculogenic potential. Angiogenesis 16(4):735–744. https://doi.org/10.1007/s10456-013-9350-0
Pardanaud L, Eichmann A (2006) Identification, emergence and mobilization of circulating endothelial cells or progenitors in the embryo. Development 133(13):2527–2537
Pardanaud L, Altmann C, Kitos P, Dieterlen-Lièvre F, Buck C (1987) Vasculogenesis in the early quail blastodisc as studied with a monoclonal antibody recognizing endothelial cells. Development 100:339–349
Caprioli A, Jaffredo T, Gautier R, Dubourg C, Dieterlen-Lièvre F (1998) Blood-borne seeding by hematopoietic and endothelial precursors from the allantois. Proc Natl Acad Sci USA 95(4):1641–1646
Pardanaud L, Eichmann A (2011) Extraembryonic origin of circulating endothelial cells. PLoS ONE 6(10):e25889. https://doi.org/10.1371/journal.pone.0025889
Pozzoli O, Vella P, Iaffaldano G, Parente V, Devanna P, Lacovich M, Lamia CL, Fascio U, Longoni D, Cotelli F, Capogrossi MC, Pesce M (2011) Endothelial fate and angiogenic properties of human CD34+ progenitor cells in zebrafish. Arterioscler Thromb Vasc Biol 31(7):1589–1597. https://doi.org/10.1161/ATVBAHA.111.226969
Hatzopoulos AK, Folkman J, Vasile E, Eiselen GK, Rosenberg RD (1998) Isolation and characterization of endothelial progenitor cells from mouse embryos. Development 125(8):1457–1468
Kupatt C, Horstkotte J, Vlastos GA, Pfosser A, Lebherz C, Semisch M, Thalgott M, Büttner K, Browarzyk C, Mages J, Hoffmann R, Deten A, Lamparter M, Müller F, Beck H, Büning H, Boekstegers P, Hatzopoulos AK (2005) Embryonic endothelial progenitor cells expressing a broad range of proangiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J 19(11):1576–1578
Kupatt C, Hinkel R, Lamparter M, von Brühl ML, Pohl T, Horstkotte J, Beck H, Müller S, Delker S, Gildehaus FJ, Büning H, Hatzopoulos AK, Boekstegers P (2005) Retroinfusion of embryonic endothelial progenitor cells attenuates ischemia-reperfusion injury in pigs: role of phosphatidylinositol 3-kinase/AKT kinase. Circulation 112(9 Suppl):117–122
Hecht N, Schneider UC, Czabanka M, Vinci M, Hatzopoulos AK, Vajkoczy P, Woitzik J (2014) Endothelial progenitor cells augment collateralization and hemodynamic rescue in a model of chronic cerebral ischemia. J Cereb Blood Flow Metab 34(8):1297–1305. https://doi.org/10.1038/jcbfm.2014.78
Wei J, Blum S, Unger M, Jarmy G, Lamparter M, Geishauser A, Vlastos GA, Chan G, Fischer KD, Rattat D, Debatin KM, Hatzopoulos AK, Beltinger C (2004) Embryonic endothelial progenitor cells armed with a suicide gene target hypoxic lung metastases after intravenous delivery. Cancer Cell 5(5):477–488
Vajkoczy P, Blum S, Lamparter M, Mailhammer R, Erber R, Engelhardt B, Vestweber D, Hatzopoulos AK (2003) Multistep nature of microvascular recruitment of ex vivo-expanded embryonic endothelial progenitor cells during tumor angiogenesis. J Exp Med 197(12):1755–1765
Defresne F, Bouzin C, Grandjean M, Dieu M, Raes M, Hatzopoulos AK, Kupatt C, Feron O (2011) Preconditioned endothelial progenitor cells reduce formation of melanoma metastases through SPARC-driven cell-cell interactions and endocytosis. Cancer Res 71(14):4748–4757. https://doi.org/10.1158/0008-5472.CAN-10-2449
Ratliff BB, Ghaly T, Brudnicki P, Yasuda K, Rajdev M, Bank M, Mares J, Hatzopoulos AK, Goligorsky MS (2010) Endothelial progenitors encapsulated in bioartificial niches are insulated from systemic cytotoxicity and are angiogenesis competent. Am J Physiol Renal Physiol 299(1):F178–186. https://doi.org/10.1152/ajprenal.00102.2010
Pfosser A, El-Aouni C, Pfisterer I, Dietz M, Globisch F, Stachel G, Trenkwalder T, Pinkenburg O, Horstkotte J, Hinkel R, Sperandio M, Hatzopoulos AK, Boekstegers P, Bals R, Kupatt C (2010) NF kappaB activation in embryonic endothelial progenitor cells enhances neovascularization via PSGL-1 mediated recruitment: novel role for LL37. Stem Cells 28(2):376–385. https://doi.org/10.1002/stem.280
Hinkel R, El-Aouni C, Olson T, Horstkotte J, Mayer S, Müller S, Willhauck M, Spitzweg C, Gildehaus FJ, Münzing W, Hannappel E, Bock-Marquette I, DiMaio JM, Hatzopoulos AK, Boekstegers P, Kupatt C (2008) Thymosin beta4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Circulation 117(17):2232–2240. https://doi.org/10.1161/CIRCULATIONAHA.107.758904
Safa RN, Peng XY, Pentassuglia L, Lim CC, Lamparter M, Silverstein C, Walker J, Chen B, Geisberg C, Hatzopoulos AK, Sawyer DB (2011) Neuregulin-1β regulation of embryonic endothelial progenitor cell survival. Am J Physiol Heart Circ Physiol 300(4):H1311–1319. https://doi.org/10.1152/ajpheart.01104.2009
Ryzhov S, Solenkova NV, Goldstein AE, Lamparter M, Fleenor T, Young PP, Greelish JP, Byrne JG, Vaughan DE, Biaggioni I, Hatzopoulos AK, Feoktistov I (2008) Adenosine receptor-mediated adhesion of endothelial progenitors to cardiac microvascular endothelial cells. Circ Res 102(3):356–363
Langer H, May AE, Daub K, Heinzmann U, Lang P, Schumm M, Vestweber D, Massberg S, Schönberger T, Pfisterer I, Hatzopoulos AK, Gawaz M (2006) Adherent platelets recruit and induce differentiation of murine embryonic endothelial progenitor cells to mature endothelial cells in vitro. Circ Res 98(2):e2–10
Cherqui S, Kurian SM, Schussler O, Hewel JA, Yates JR 3rd, Salomon DR (2006) Isolation and angiogenesis by endothelial progenitors in the fetal liver. Stem Cells 24(1):44–54
Göthert JR, Gustin SE, Hall MA, Green AR, Göttgens B, Izon DJ, Begley CG (2005) In vivo fate-tracing studies using the Scl stem cell enhancer: embryonic hematopoietic stem cells significantly contribute to adult hematopoiesis. Blood 105(7):2724–2732
García-Ortega AM, Cañete A, Quinter C, Silberstein L, Piquer-Gil M, Álvarez-Dolado M, Dekel B, Gottgens B, Sánchez MJ (2010) Enhanced hematovascular contribution of SCL 3' enhancer expressing fetal liver cells uncovers their potential to integrate in extramedullary adult niches. Stem Cells 28(1):100–112. https://doi.org/10.1002/stem.228
Cañete A, Comaills V, Prados I, Castro AM, Hammad S, Ybot-Gonzalez P, Bockamp E, Hengstler JG, Gottgens B, Sánchez MJ (2017) Characterization of a fetal liver cell population endowed with long-term multiorgan endothelial reconstitution potential. Stem Cells 35(2):507–521. https://doi.org/10.1002/stem.2494
Bockamp E, Antunes C, Liebner S, Schmitt S, Cabezas-Wallscheid N, Heck R, Ohnngemach S, Oesch-Bartlomowicz B, Rickert C, Sanchez MJ, Hengstler J, Kaina B, Wilson A, Trumpp A, Eshkind L (2009) In vivo fate mapping with SCL regulatory elements identifies progenitors for primitive and definitive hematopoiesis in mice. Mech Dev 126(10):863–872. https://doi.org/10.1016/j.mod.2009.07.005
Rojas A, De Val S, Heidt AB, Xu SM, Bristow J, Black BL (2005) Gata4 expression in lateral mesoderm is downstream of BMP4 and is activated directly by Forkhead and GATA transcription factors through a distal enhancer element. Development 132:3405–3417
Cañete A, Carmona R, Ariza L, Sánchez MJ, Rojas A, Muñoz-Chápuli R (2017) A population of hematopoietic stem cells derives from GATA4-expressing progenitors located in the placenta and lateral mesoderm of mice. Haematologica 102(4):647–655. https://doi.org/10.3324/haematol.2016.155812
Carmona R, Barrena S, López Gambero AJ, Rojas A, Muñoz-Chápuli R (2020) Epicardial cell lineages and the origin of the coronary endothelium. FASEB J. https://doi.org/10.1096/fj.201902249RR
Wu B, Zhang Z, Lui W, Chen X, Wang Y, Chamberlain AA, Moreno-Rodriguez RA, Markwald RR, O'Rourke BP, Sharp DJ, Zheng D, Lenz J, Baldwin HS, Chang CP, Zhou B (2012) Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell 151(5):1083–1096. https://doi.org/10.1016/j.cell.2012.10.023
Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC, Poidinger M, Zolezzi F, Larbi A, Ng LG, Chan JK, Greter M, Becher B, Samokhvalov IM, Merad M, Ginhoux F (2015) C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42(4):665–678. https://doi.org/10.1016/j.immuni.2015.03.01
McGrath KE, Frame JM, Fegan KH, Bowen JR, Conway SJ, Catherman SC, Kingsley PD, Koniski AD, Palis J (2015) Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep 11(12):1892–1904. https://doi.org/10.1016/j.celrep.2015.05.036
Plein A, Fantin A, Denti L, Pollard JW, Ruhrberg C (2018) Erythro-myeloid progenitors contribute endothelial cells to blood vessels. Nature 562(7726):223–228. https://doi.org/10.1038/s41586-018-0552-x
Rapp BM, Saadatzedeh MR, Ofstein RH, Bhavsar JR, Tempel ZS, Moreno O, Morone P, Booth DA, Traktuev DO, Dalsing MC, Ingram DA, Yoder MC, March KL, Murphy MP (2011) Resident endothelial progenitor cells from human placenta have greater vasculogenic potential than circulating endothelial progenitor cells from umbilical cord blood. Cell Med 2(3):85–96. https://doi.org/10.3727/215517911X617888
Patel J, Seppanen E, Chong MS, Yeo JS, Teo EY, Chan JK, Fisk NM, Khosrotehrani K (2013) Prospective surface marker-based isolation and expansion of fetal endothelial colony-forming cells from human term placenta. Stem Cells Transl Med 2(11):839–847. https://doi.org/10.5966/sctm.2013-0092
Gumina DL, Black CP, Balasubramaniam V, Winn VD, Baker CD (2017) Umbilical cord blood circulating progenitor cells and endothelial colony-forming cells are decreased in preeclampsia. Reprod Sci 24(7):1088–1096. https://doi.org/10.1177/1933719116678692
Solomon I, O'Reilly M, Ionescu L, Alphonse RS, Rajabali S, Zhong S, Vadivel A, Shelley WC, Yoder MC, Thébaud B (2016) Functional differences between placental micro- and macrovascular endothelial colony-forming cells. Stem Cells Transl Med 5(3):291–300. https://doi.org/10.5966/sctm.2014-0162
Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC (2004) Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104(9):2752–2760
Prasain N, Meador JL, Yoder MC (2012) Phenotypic and functional characterization of endothelial colony forming cells derived from human umbilical cord blood. J Vis Exp 62:3872. https://doi.org/10.3791/3872
Zhang H, Tao Y, Ren S, Liu H, Zhou H, Hu J, Tang Y, Zhang B, Chen H (2017) Isolation and characterization of human umbilical cord-derived endothelial colony-forming cells. Exp Ther Med 14(5):4160–4166. https://doi.org/10.3892/etm.2017.5035
Bompais H, Chagraoui J, Canron X, Crisan M, Liu XH, Anjo A, Tolla-Le Port C, Leboeuf M, Charbord P, Bikfalvi A, Uzan G (2004) Human endothelial cells derived from circulating progenitors display specific functional properties compared with mature vessel wall endothelial cells. Blood 103(7):2577–2584
Javed MJ, Mead LE, Prater D, Bessler WK, Foster D, Case J, Goebel WS, Yoder MC, Haneline LS, Ingram DA (2008) Endothelial colony forming cells and mesenchymal stem cells are enriched at different gestational ages in human umbilical cord blood. Pediatr Res 64(1):68–73. https://doi.org/10.1203/PDR.0b013e31817445e9
Baker CD, Ryan SL, Ingram DA, Seedorf GJ, Abman SH, Balasubramaniam V (2009) Endothelial colony-forming cells from preterm infants are increased and more susceptible to hyperoxia. Am J Respir Crit Care Med 180(5):454–461. https://doi.org/10.1164/rccm.200901-0115OC
Safranow K, Kotowski M, Lewandowska J, Machalińska A, Dziedziejko V, Czajka R, Celewicz Z, Rudnicki J, Machaliński B (2012) Circulating endothelial progenitor cells in premature infants: is there an association with premature birth complications? J Perinat Med 40(4):455–462. https://doi.org/10.1515/jpm-2011-0199
Borghesi A, Massa M, Campanelli R, Bollani L, Tzialla C, Figar TA, Ferrari G, Bonetti E, Chiesa G, de Silvestri A, Spinillo A, Rosti V, Stronati M (2009) Circulating endothelial progenitor cells in preterm infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med 180(6):540–546. https://doi.org/10.1164/rccm.200812-1949OC
Muñoz-Hernandez R, Miranda ML, Stiefel P, Lin RZ, Praena-Fernández JM, Dominguez-Simeon MJ, Villar J, Moreno-Luna R, Melero-Martin JM (2014) Decreased level of cord blood circulating endothelial colony-forming cells in preeclampsia. Hypertension 64(1):165–171. https://doi.org/10.1161/HYPERTENSIONAHA.113.03058
Grundmann M, Haidar M, Placzko S, Niendorf R, Darashchonak N, Hubel CA, von Versen-Höynck F (2012) Vitamin D improves the angiogenic properties of endothelial progenitor cells. Am J Physiol Cell Physiol 303(9):C954–962. https://doi.org/10.1152/ajpcell.00030.2012
von Versen-Höynck F, Brodowski L, Dechend R, Myerski AC, Hubel CA (2014) Vitamin D antagonizes negative effects of preeclampsia on fetal endothelial colony forming cell number and function. PLoS ONE 9(6):e98990. https://doi.org/10.1371/journal.pone.0098990
Attar A, Monabati A, Parsanezhad ME (2017) Endothelial progenitor cell subsets and preeclampsia: findings and controversies. J Chin Med Assoc 80(10):615–622. https://doi.org/10.1016/j.jcma.2017.06.013
Ligi I, Simoncini S, Tellier E, Vassallo PF, Sabatier F, Guillet B, Lamy E, Sarlon G, Quemener C, Bikfalvi A, Marcelli M, Pascal A, Dizier B, Simeoni U, Dignat-George F, Anfosso F (2011) A switch toward angiostatic gene expression impairs the angiogenic properties of endothelial progenitor cells in low birth weight preterm infants. Blood 118(6):1699–1709. https://doi.org/10.1182/blood-2010-12-325142
Sipos PI, Rens W, Schlecht H, Fan X, Wareing M, Hayward C, Hubel CA, Bourque S, Baker PN, Davidge ST, Sibley CP, Crocker IP (2013) Uterine vasculature remodeling in human pregnancy involves functional macrochimerism by endothelial colony forming cells of fetal origin. Stem Cells 31(7):1363–1370. https://doi.org/10.1002/stem.1385
Sipos PI, Bourque SL, Hubel CA, Baker PN, Sibley CP, Davidge ST, Crocker IP (2013) Endothelial colony-forming cells derived from pregnancies complicated by intrauterine growth restriction are fewer and have reduced vasculogenic capacity. J Clin Endocrinol Metab 98(12):4953–4960. https://doi.org/10.1210/jc.2013-2580
Gui J, Rohrbach A, Borns K, Hillemanns P, Feng L, Hubel CA, von Versen-Höynck F (2015) Vitamin D rescues dysfunction of fetal endothelial colony forming cells from individuals with gestational diabetes. Placenta 36(4):410–418. https://doi.org/10.1016/j.placenta.2015.01.195
Kim H, Prasain N, Vemula S, Ferkowicz MJ, Yoshimoto M, Voytik-Harbin SL, Yoder MC (2015) Human platelet lysate improves human cord blood derived ECFC survival and vasculogenesis in three dimensional (3D) collagen matrices. Microvasc Res 101:72–81. https://doi.org/10.1016/j.mvr.2015.06.006
Reid E, Guduric-Fuchs J, O'Neill CL, Allen LD, Chambers SEJ, Stitt AW, Medina RJ (2018) Preclinical evaluation and optimization of a cell therapy using human cord blood-derived endothelial colony-forming cells for ischemic retinopathies. Stem Cells Transl Med 7(1):59–67. https://doi.org/10.1002/sctm.17-0187
Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC (2005) Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood 105(7):2783–2786
Huang L, Critser PJ, Grimes BR, Yoder MC (2011) Human umbilical cord blood plasma can replace fetal bovine serum for in vitro expansion of functional human endothelial colony-forming cells. Cytotherapy 13(6):712–721
Lacaud G, Kouskoff V (2017) Hemangioblast, hemogenic endothelium, and primitive versus definitive hematopoiesis. Exp Hematol 49:19–24. https://doi.org/10.1016/j.exphem.2016.12.009
Muñoz-Chápuli R, Carmona R, Guadix JA, Macías D, Pérez-Pomares JM (2005) The origin of the endothelial cells: an evo-devo approach for the invertebrate/vertebrate transition of the circulatory system. Evol Dev 7(4):351–358
Funding
This work was supported by the Spanish Ministry of Science, Innovation and Universities under Grants BFU2017-83907-P, Instituto de Salud Carlos III-TERCEL network under Grant RD16/0011/0030; Consejería de Salud and Consejería de Economía y Conocimiento, Junta de Andalucía, under Grants PC0066‐2017/0034 and UMA18-FEDERJA-146, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Díaz del Moral, S., Barrena, S., Muñoz-Chápuli, R. et al. Embryonic circulating endothelial progenitor cells. Angiogenesis 23, 531–541 (2020). https://doi.org/10.1007/s10456-020-09732-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-020-09732-y