Abstract
Due to spatial tumor heterogeneity and consecutive sampling errors, it is critically important to assess treatment response following antiangiogenic therapy in three dimensions as two-dimensional assessment has been shown to substantially over- and underestimate treatment response. In this study, we evaluated whether three-dimensional (3D) dynamic contrast-enhanced ultrasound (DCE-US) imaging allows assessing early changes in tumor perfusion following antiangiogenic treatment (bevacizumab administered at a dose of 10 mg/kg b.w.), and whether these changes could predict treatment response in colon cancer tumors that either are responsive (LS174T tumors) or none responsive (CT26) to the proposed treatment. Our results showed that the perfusion parameters of 3D DCE-US including peak enhancement (PE) and area under curve (AUC) significantly decreased by up to 69 and 77%, respectively, in LS174T tumors within 1 day after antiangiogenic treatment (P = 0.005), but not in CT26 tumors (P > 0.05). Similarly, the percentage area of neovasculature significantly decreased in treated versus control LS174T tumors (P < 0.001), but not in treated versus control CT26 tumors (P = 0.796). Early decrease in both PE and AUC by 45–50% was predictive of treatment response in 100% (95% CI 69.2, 100%) of responding tumors, and in 100% (95% CI 88.4, 100%) and 86.7% (95% CI 69.3, 96.2%), respectively, of nonresponding tumors. In conclusion, 3D DCE-US provides clinically relevant information on the variability of tumor response to antiangiogenic therapy and may be further developed as biomarker for predicting treatment outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Angiogenesis, the formation of new capillary blood vessels from preexisting vasculature, is necessary for tumor growth beyond 1–2 mm in diameter [1]. Given the critical role of angiogenesis in tumor growth, progression, and metastasis [2], several antiangiogenic therapies have already been approved for clinical use [3]. Combination treatments where antiangiogenic agents such as bevacizumab (a humanized anti-VEGF monoclonal antibody) are given in conjunction with chemotherapy have resulted in improved progression-free survival and overall survival in patients with metastatic colon cancer [4, 5].
Treatment response in clinical cancer trials is conventionally assessed using the Response Evaluation Criteria in Solid Tumor (RECIST) method, which is based on changes in maximum tumor diameters [6]. However, antiangiogenic agents result in acute cytostatic effects that diminish tumor blood supply, while often minimally affecting tumor size [7]. Therefore, quantifying post-treatment changes based on RECIST may not reflect tumor response, in particular at early time points. To minimize unnecessary cost and side effects in nonresponding patients, alternative methods or tools are needed to allow rapid assessment of treatment effects within the first days/weeks of treatment. Early changes in functional or molecular imaging-based biomarkers could provide such a method and could be used to predict treatment response, thereby resulting in improved cancer patient management and personalized treatments.
Several imaging approaches such as positron emission tomography (PET), dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), dynamic contrast-enhanced computed tomography (DCE-CT), and dynamic contrast-enhanced ultrasound (DCE-US) are being considered for monitoring of tumor response to novel molecularly targeted therapies such as antiangiogenic drugs [8]. While ultrasound currently does not play a significant clinical role in oncological imaging since it is not a whole body exam which is needed for traditional tumor response assessment usually several months into the treatments, it may be an attractive technique for early response assessments of target lesions that are accessible for ultrasound, such as metastases in the liver. Unlike other imaging modalities, ultrasound does not expose patients to radiation, is relatively inexpensive, is portable, and is globally available for bedside imaging. Recent commercial availability of three-dimensional (3D) DCE-US has enabled more reliably and accurate quantification of tumor blood perfusion by eliminating 2D sampling errors that can occur in longitudinal consecutive imaging applications such as treatment monitoring [9,10,11]. Tumor imaging is particularly susceptible to sampling errors due to spatial vascular heterogeneities linked to a hypoxic and necrotic microenvironment [9].
The purpose of this study was to evaluate whether 3D DCE-US imaging allows assessing early changes in tumor perfusion following antiangiogenic treatment and whether these changes could predict treatment response in two animal models of colon cancer that simulate clinical responders and nonresponders.
Materials and methods
Subcutaneous colon cancer xenografts in mice and antiangiogenic treatment
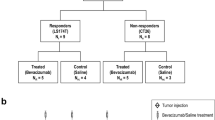
All experimental procedures involving laboratory animals were approved by the Institutional Animal Care and Use Committee. Forty female nude mice (Charles River, Wilmington, MA; 6–8 weeks old; 20–25 g) were used for inducing the colon cancer models. The human colon cancer cell line LS174T (ATCC, Manassas, VA), which has been shown to be sensitive to the treatment of VEGF pathway blockade [12], was used to simulate clinical responders, while the murine colon cancer cell line CT26.WT (ATCC, Manassas, VA), which has been shown to be resistant to VEGF pathway blockade [13, 14], was used to simulate clinical nonresponders. Both cell lines were grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY), supplemented with 10% fetal bovine serum (Gibco), penicillin (50 U/ml), and streptomycin (50 µg/ml) at 37 °C in a humidified 5% CO2 atmosphere. At 70–80% confluence, the tumor cells were collected following trypsinization, and 4 × 106 LS174T cells or 1 × 106 CT26 cells suspended in 50 μl phosphate-buffered saline (PBS) and 50 μl Matrigel (BD Biosciences, San Jose, CA) were injected subcutaneously on the right lower hind limb of all nude mice. Both LS174T and CT26 tumors were allowed to grow for 10 days after cancer cell injection to reach 6–15 mm (mean, 10 mm) in maximum diameter, which was measured by using an electronic caliper available on the ultrasound system. The average tumor volume doubling time for LS174T colon cancer tumors was 3.7 days and 3 days for CT26 tumors. Mice with either tumor type were randomized into (1) the treatment group (n = 10 from each cell type; total, 20 tumors); and (2) the control group (n = 10 each; total, 20 tumors). The clinically used antiangiogenic agent bevacizumab (Avastin, Genentech, South San Francisco, CA) diluted in sterile saline was administered by means of intravenous injection on days 0 (baseline), 3, and 7 at a dose of 10 mg/kg b.w. (corresponding to a fluid volume of 10 μl/g b.w.) for animals in the treatment group (including responders and nonresponders). Since tumors in nude mice grow faster than those in patients, in general bevacizumab dosing regimen is different in mice than in humans. The dosing regimen used in this study was based on previous animal studies [15,16,17,18]. Mice in the control group received the same volume of sterile saline only with the same timing and dosing schedule used for the treatment group (Fig. 1).
Summary of experimental study design of 3D DCE-US imaging in both responding (LS174T) and nonresponding (CT26) tumors. Ten days after tumor cell injection, 3D DCE-US scans (green arrows) were performed prior to treatment (baseline scan at day 0), and at subsequent days 1, 3, 7, and 10 after treatment (yellow arrows) using either bevacizumab (treated mice) or saline (control mice). All mice were sacrificed at day 10 (red arrows) and tumors were excised for histologic analysis. (Color figure online)
Monitoring antiangiogenic treatment with 3D DCE-US imaging
For all imaging procedures, mice were anesthetized with inhalation of 2% isoflurane in room air (administered at 2 L/min). Mice were placed prone on a heated imaging stage in order to maintain constant body temperature for the entire duration of the experiments. A 27G needle catheter (Vevo MicroMarker; VisualSonics, Toronto, Canada), which attached to an infusion pump (Kent Scientific, Torrington, CT), was placed into one of the two tail veins for contrast agent injection. Successful placement was confirmed before contrast agent injection each time by observing blood filling into the catheter tube. All animals were imaged with 3D DCE-US imaging on days 0, 1, 3, 7, and 10 using a clinical ultrasound scanner (EPIQ 7; Philips Healthcare, Andover, MA) coupled to a clinical matrix array transducer (X6-1; Philips). The transducer was held in a fixed position with a clamp to minimize motion artifacts. To reduce artifacts in the near-field zone of the clinical transducer, a customized standoff ultrasound gel was placed on the skin of all mice. The distance between the transducer and the center of the tumor was set at 3 cm. In all tumors, 3D DCE-US imaging was performed in power modulation contrast imaging mode using the following imaging setting: center frequency, 3.2 MHz; mechanical index, 0.09; volume rate, 1 Hz; dynamic range, 52 dB; focus length, 5 cm. Tumor growth was monitored at each time by using electronic calipers measurements available on the ultrasound system. The greatest longitudinal, transverse, and anteroposterior dimensions of each tumor were measured in grayscale imaging. Tumor volumes were determined by using the following formula: volume = π/6 × L × W × H, where L is length, W is width, and H is height.
All ultrasound examinations were performed by one radiologist (with 10 years experience in contrast-enhanced ultrasound), who was blind to the treatment status. Three-dimensional DCE-US imaging data sets were acquired by using the bolus technique which is based on the wash-in and wash-out kinetics of contrast microbubbles after a single intravenous bolus injection [19]. FDA-approved perflutren microbubbles (Definity; Lantheus Medical Imaging, Inc., North Billerica, MA) were used (diluted 1:4 in sterile 0.9% saline solution after activation) and a 120-μl suspension was administered within a 5-s bolus (at a constant injection rate of 24 μl/s) by using the infusion pump. Three-dimensional DCE-US data sets were acquired in real time continuously for 4 min in each mouse through a built-in Digital Navigation Link connecting the ultrasound system directly to custom in-house MeVisLab modules (MeVis Medical Solutions AG, Bremen, Germany) written in C++ [20].
Image analysis of 3D DCE-US data sets
Image analysis was performed by one reader with 6 years of DCE-US experience in random order. The reader was blinded to the treatment information and types of animal (responder vs. nonresponder). All 3D DCE-US imaging data sets were analyzed with a custom software which was developed by using the software package MeVisLab [21]. A volume of interest (VOI) was manually contoured covering the whole tumor visualized on sagittal, longitudinal, and coronal planes. Voxel intensities were then linearized to obtain an approximate linear depiction of the backscattered signal intensity, and a time-intensity curve (TIC) was obtained from the average voxel intensities within the VOI. A first-pass kinetics analysis was performed to obtain perfusion parameters by fitting a lognormal perfusion model to the TIC [19, 22]. The following perfusion parameters were calculated as previously described [9, 23, 24] from the fitted TIC [22]: peak enhancement (PE, arbitrary units, a.u.), area under the curve (AUC, a.u.), and time to peak (TTP, seconds). PE was defined as the maximum increase in the signal intensity following bolus injection of the contrast agent. AUC was defined as the total area under the curve of the fitted TIC. Both PE and AUC are proportional to blood volume. TTP was defined as the time interval from the beginning of enhancement to the peak of the fitted curve and is generally related to blood flow. Tumor volumes and all 3D DCE-US perfusion parameters after treatment were normalized to the baseline values to show percent changes.
Ex vivo analysis of the tumor neovasculature
At day 10, all mice were euthanized after the imaging exam, and tumors were surgically removed and fixed in 4% paraformaldehyde and PBS solution for 24 h. Tumors were then cryopreserved in a 30% sucrose and PBS solution (Sigma-Aldrich, St Louis, Mo). Tissue samples were placed in optimum cutting temperature (OCT, Fisher Scientific, Pittsburgh, Pa) and then sectioned into 10 μm slices for immunofluorescence staining. For neovasculature analysis, slices were stained for the vascular endothelial marker CD31. Standard methods were used for immunofluorescence staining [25]. Briefly, tumor slices were rinsed with PBS for 10 min to remove remaining OCT and permeabilized in 0.5% Triton-X 100 for 10 min. Tissue sections were blocked in a solution that contained 3% goat serum (Sigma, St Louis, MO), 3% bovine serum albumin (Sigma, St Louis, MO), and 3% donkey serum (Sigma, St Louis, MO) for up to 5 h at room temperature to remove nonspecific staining. A rat anti-mouse CD31 antibody, with 1:100 dilution (eBioscience, San Jose, CA), was used as primary antibody to incubate the tumor specimens overnight at 4 °C. After rinsing with PBS, a secondary antibody (1:250 Alexa Fluor 488 donkey anti-rat IgG; Invitrogen, Grand Island, NY) was added to make the primary antibody visible. Samples were mounted by using ProLong Gold (BioGenex, San Ramon, CA). Fluorescent microscopy was performed by using a LSM510 metaconfocal microscope (Zeiss, Maple Grove, MN) and a high-resolution digital camera (AxioCam MRc, Bernried, Germany) under 200-fold magnification. The percentage area of neovasculature per slice was quantified by using Image J software (National Institutes of Health, Bethesda, MD) as the average value from 5 randomly selected fields of view (single field of view area, 0.19 mm2).
Statistical analysis
The nonparametric paired-samples Wilcoxon rank test was used to compare the changes in tumor volume and perfusion parameters (PE, AUC and TTP) following treatment. The nonparametric Mann–Whitney U test was used to assess the statistical significance in tumor volume, perfusion parameters, and ex vivo percentage area of neovasculature between treated and control groups. To assess whether relative changes in the perfusion parameters of 3D DCE-US at day 1 compared to baseline could predict treatment response at a later time point (day 10), perfusion parameters were compared to the relative change of tumor volume at day 10 with tumor progression defined as a fourfold or more increase in tumor volume from day 0 to day 10. The 95% confidence intervals (CI) were constructed with exact method due to small sample sizes. A P value of <0.05 was considered statistically significant. All analyses were performed by using IBM SPSS statistics software version 22 (IBM Corp, Chicago, IL).
Results
Antiangiogenic therapeutic effects on tumor growth
No significant differences were noted between control and treated tumors at day 0 for both the responder (LS174T, P = 0.247) and nonresponder (CT26, P = 0.218) groups. For the responder group, the tumor volume of saline-treated mice increased significantly (by 543%; P = 0.005) when compared to bevacizumab-treated mice (by 83%; P = 0.005). On days 3, 7, and 10, the relative increase in tumor volume (P < 0.001) in bevacizumab-treated mice was significantly smaller than saline-treated mice; no significant difference was observed at day 1 (P = 0.631).
In contrast, in the nonresponder group, tumor volumes in both bevacizumab-treated (by 887%) and saline-treated (by 990%) mice significantly increased (P < 0.05) at 1, 3, 7 and 10 days. There were no significant differences in relative increases of tumor volumes compared to baseline between bevacizumab-treated and saline-treated nonresponding tumors at any time point (P from 0.280 to 0.912) (Fig. 2).
Assessment of tumor perfusion with 3D DCE-US
Longitudinal changes in 3D DCE-US perfusion parameters after bevacizumab treatment are summarized in Fig. 3. In the responder group, bevacizumab-treated tumors showed a significant decrease in the 3D DCE-US perfusion parameters PE and AUC on all days as compared with day 0 (P = 0.005), while saline-treated tumors had no significant change in AUC at any time point (P from 0.153 to 0.878) and in PE at the first three time points (P from 0.102 to 0.540); PE significantly decreased at day 10 (P = 0.005), possibly due to tumor necrosis. Overall, relative changes in PE and AUC were significantly decreased in bevacizumab-treated tumors as compared with saline-treated tumors on days 1, 3, 7, and 10 (P < 0.05). There were no significant differences in TTP in bevacizumab- or saline-treated mice at any time point (P from 0.393 to 0.912).
Bar graph summary of both 3D DCE-US values, AUC (a, c) and PE (b, d), obtained in responding and nonresponding tumors, treated either with bevacizumab (white) or saline only (black). Note that signal decrease in nonresponding tumors and control-treated responding tumors at later time points is likely due to tumor necrosis in these fast growing tumors. *P < 0.001, **P = 0.001. Bars represent mean values; error bars = standard deviations
In contrast, in the nonresponder group, no significant differences in relative changes of all 3D DCE-US perfusion parameters were observed (PE, AUC, and TTP) in bevacizumab- versus saline-treated tumors at any time point (for PE: P ranged from 0.280 to 0.912; for AUC: P from 0.075 to 1.000; for TTP: P from 0.089 to 0.796) (Fig. 4).
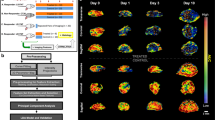
Representative volume rendered 3D DCE-US imaging data sets at peak enhancement in a responding and nonresponding tumor imaged over a 10-day treatment course. In the responding tumor, 3D DCE-US signal already substantially decreased one day after administration of bevacizumab and remained low during the subsequent days. Note that while tumor volume slightly increased in responding tumor, 3D DCE-US imaging signal remained low over time. Scale bar 10 mm
Prediction of treatment response
When AUC decreased by 45% or more at day 1 following initiation of bevacizumab treatment, treatment response could be predicted in 100% (95% CI 69.2, 100%) of responding and in 86.7% (95% CI 69.3, 96.2%) of nonresponding tumors. All tumors (95% CI 86.8, 100%) progressed by day 10 when AUC decreased by less than 45% at day 1.
At a 50% or more decrease in PE at 1 day following initiation of bevacizumab treatment, a treatment response could be predicted in 100% both in responding (95% CI 69.2, 100%) and nonresponding (95% CI 88.4, 100%) tumors. All tumors with a less than 50% decrease in PE progressed (95% CI 88.4, 100.0%).
Ex vivo quantitative immunofluorescence analysis of tumor neovasculature
In responding tumors, the percentage area of neovasculature was significantly (P < 0.001) decreased in bevacizumab-treated (1.04 ± 0.43%) versus saline-treated tumors (4.95 ± 0.35%). In contrast, in nonresponding tumors, there were no significant differences (P = 0.796) in the percentage area of neovasculature between bevacizumab-treated (5.05 ± 0.64%) and saline-treated tumors (4.96 ± 0.63%; Fig. 5).
Immunofluorescence analysis of the tumor neovasculature in responding and nonresponding tumors, either treated with bevacizumab or saline (control). a Representative tissue micrographs show tumor neovasculature (green) which substantially decreased in responding tumor compared to nonresponding tumor; blue DAPI-stained cell nuclei. Scale bar 100 μm. b Bar graphs summarize quantitative results of the measured percentage area of neovasculature. *P < 0.001. Bars represent mean values; error bars = standard deviations. (Color figure online)
Discussion
In this study, the 3D DCE-US perfusion parameters PE and AUC were found sensitive to longitudinal treatment response following antiangiogenic therapy in a mouse model simulating clinical responders and nonresponders. In responding tumors, PE and AUC substantially decreased within 1 day following a single dose of bevacizumab, whereas no significant changes in PE and AUC were noted at any time point in the nonresponding tumors. Tumor responds to antiangiogenic therapy was further confirmed by tumor volume assessed at the end of the treatment. In addition, results indicate that the decrease in both PE and AUC by day 1 following treatment is predictive for treatment outcomes at the end of the treatment cycle.
Over the past decade, DCE-US has been increasingly investigated in preclinical [24, 26, 27] and clinical [28, 29] studies to depict therapy-induced changes in tumor perfusion. However, a major drawback of conventional DCE-US to date is its limitation to 2D, which results in limited perfusion assessments to a single 2D plane, and results in longitudinal sampling errors. Studies using 2D DCE-US to image breast cancer in mice have in fact demonstrated that slight millimeter-sized deviations in transducer positioning can lead to substantial errors in estimation of tumor perfusion, ranging from 6.4 to 40.3% [9]. In clinical studies, inconsistent results have been reported on the use of 2D DCE-US to monitor tumor response during antiangiogenic therapy [28, 30]. One study found that changes in three perfusion parameters (AUC, time to peak [TTP], and slope of wash-in) of 2D DCE-US 15 days after treatment initiation were significantly associated with progression-free survival in patients with advanced hepatocellular carcinoma treated with sorafenib [28]. In contrast, a different study found that perfusion parameters (AUC, TTP and MTT) were not associated with progression-free survival in patients with metastatic renal cell carcinoma treated with sunitinib [30]. In the latter study, the authors concluded that sampling errors were likely contributing to poor reproducibility of the measurements. These further highlight the need for 3D DCE-US for monitoring treatment response [31].
The recent availability of 3D matrix array transducers with volumetric contrast-mode imaging has potentiated 3D DCE-US as a robust technique for assessment of tumor perfusion without sampling errors [10]. A recent study demonstrated that 3D DCE-US allows quantification of early treatment effects [10] and that substantial decreases in tumor perfusion following antiangiogenic treatment can be detected with perfusion parameters [10, 11]. In addition, quantified perfusion parameters were found to correlate well with ex vivo quantification of vascular densities in histologic specimens [10, 11]. However, whether these early changes of tissue perfusion parameters from 3D DCE-US could also predict treatment response remained unknown. Furthermore, it remained unknown whether there are dynamic changes of tumor perfusion in both responders and nonresponders during longitudinal antiangiogenic therapy. In this study, the potential of 3D DCE-US for monitoring longitudinal treatment response of antiangiogenic therapy in a more complex animal model setup was assessed in which two animal models of colon cancer were used to simulate clinical responders and nonresponders. Our results showed that PE and AUC values on 3D DCE-US substantially decreased in the responding tumors 24 h after treatment initiation, while no significant changes in PE and AUC were noted at any time point in the nonresponding tumors. Also, an early decrease in both PE and AUC was highly predictive of later time point treatment response assessed at day 10.
In parallel with DCE-US, 3D ultrasound molecular imaging using molecularly targeted contrast microbubbles is emerging as a promising tool to assess expression levels of angiogenic markers such as VEGFR2 for monitoring antiangiogenic therapy [32]. A recent study has shown that both 3D ultrasound molecular imaging with clinical grade VEGFR2-targeted microbubbles and 3D DCE-US with FDA-approved nontargeted microbubbles could provide complementary molecular and functional in vivo information on antiangiogenic treatment effects [11]. Also, early changes in molecular ultrasound imaging signal were highly predictive of later treatment outcomes in another study in a mouse colon cancer xenograft model [33]. Our findings are complementary to these results and demonstrate for the first time that early decrease in PE and AUC values obtained by 3D DCE-US can be predictive of treatment response following several treatment administration cycles. Since the use of ultrasound contrast agents is widely available and approved for clinical use globally, the use of 3D DCE-US for treatment monitoring as described in our study is readily translatable to patients.
Several limitations of this study need to be acknowledged. First, the number of animals in each group was small. However, since strong changes in perfusion parameters were observed in bevacizumab-treated versus saline-treated mice in responding tumors, additional animals were not warranted after statistical analysis. Second, a murine colon cancer model which is known to be refractory to bevacizumab treatment was used to simulate clinical nonresponding tumors. This animal model does not exactly mimic human tumor behavior as tumor resistance often develops during antiangiogenic treatment in patients, besides inherent resistance [3]. Also, subcutaneous colon cancer models may be limited in terms of modeling the tumor microenvironment of metastases in the liver or other organs and longitudinal scanning was limited to 10 days due to rapid tumor growth with outgrowth of control tumors in our model tumors. Therefore, future longer-term longitudinal studies in orthotopically implanted cancer or in transgenic mice along with early clinical trials in patients are warranted to confirm the results of this study. Finally, 3D DCE-US imaging results were only compared with the percentage area of neovasculature in this study as bevacizumab has a direct antiangiogenic effect and ultrasound perfusion parameters depend on the number of perfused vessels. Previous studies have demonstrated increased hypoxia following antiangiogenic therapy in human colon cancer xenografts in mice following bevacizumab treatment [15, 18]; also, bevacizumab has been shown to upregulate apoptosis executioner protein cleaved caspase-3 and to downregulate both antiapoptotic protein Bcl-2 and tumor cell proliferation marker p-Erk expressions in human squamous cell carcinoma xenografts in mice [34].
Conclusions
In conclusion, our results suggest that 3D DCE-US is sensitive to early tumor response after antiangiogenic treatment, and changes in quantitative parameters are predictive of treatment response in an animal model of human colon cancer. Since 3D DCE-US provides a robust and reproducible longitudinal imaging platform, clinical studies are under way to test whether this technique can be clinically translated and allows detection of early treatment changes in patients undergoing antiangiogenic and other therapies.
References
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186. doi:10.1056/NEJM197111182852108
Folkman J (2002) Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29(6 Suppl 16):15–18. doi:10.1053/sonc.2002.37263
Shojaei F (2012) Anti-angiogenesis therapy in cancer: current challenges and future perspectives. Cancer Lett 320(2):130–137. doi:10.1016/j.canlet.2012.03.008
Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M, Zaniboni A, Tonini G, Carlomagno C, Allegrini G, Chiara S, D’Amico M, Granetto C, Cazzaniga M, Boni L, Fontanini G, Falcone A (2015) FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 16(13):1306–1315. doi:10.1016/S1470-2045(15)00122-9
Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, von Moos R, Vieitez JM, Bouche O, Borg C, Steffens CC, Alonso-Orduna V, Schlichting C, Reyes-Rivera I, Bendahmane B, Andre T, Kubicka S, Investigators MLS (2013) Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 14(1):29–37. doi:10.1016/S1470-2045(12)70477-1
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Hlatky L, Hahnfeldt P, Folkman J (2002) Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn’t tell us. J Natl Cancer Inst 94(12):883–893
Marcus CD, Ladam-Marcus V, Cucu C, Bouche O, Lucas L, Hoeffel C (2009) Imaging techniques to evaluate the response to treatment in oncology: current standards and perspectives. Crit Rev Oncol Hematol 72(3):217–238. doi:10.1016/j.critrevonc.2008.07.012
Hoyt K, Sorace A, Saini R (2012) Quantitative mapping of tumor vascularity using volumetric contrast-enhanced ultrasound. Investig Radiol 47(3):167–174. doi:10.1097/RLI.0b013e318234e6bc
Wang H, Hristov D, Qin J, Tian L, Willmann JK (2015) Three-dimensional dynamic contrast-enhanced US imaging for early antiangiogenic treatment assessment in a mouse colon cancer model. Radiology 277(2):424–434. doi:10.1148/radiol.2015142824
Wang H, Lutz AM, Hristov D, Tian L, Willmann JK (2017) Intra-animal comparison between three-dimensional molecularly targeted US and three-dimensional dynamic contrast-enhanced US for early antiangiogenic treatment assessment in colon cancer. Radiology 282(2):443–452. doi:10.1148/radiol.2016160032
Varey AH, Rennel ES, Qiu Y, Bevan HS, Perrin RM, Raffy S, Dixon AR, Paraskeva C, Zaccheo O, Hassan AB, Harper SJ, Bates DO (2008) VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer 98(8):1366–1379. doi:10.1038/sj.bjc.6604308
Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, Chorianopoulos E, Liesenborghs L, Koch M, De Mol M, Autiero M, Wyns S, Plaisance S, Moons L, van Rooijen N, Giacca M, Stassen JM, Dewerchin M, Collen D, Carmeliet P (2007) Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 131(3):463–475. doi:10.1016/j.cell.2007.08.038
Xu L, Stevens J, Hilton MB, Seaman S, Conrads TP, Veenstra TD, Logsdon D, Morris H, Swing DA, Patel NL, Kalen J, Haines DC, Zudaire E, St Croix B (2014) COX-2 inhibition potentiates antiangiogenic cancer therapy and prevents metastasis in preclinical models. Sci Transl Med 6(242):242ra284. doi:10.1126/scitranslmed.3008455
Ren Y, Fleischmann D, Foygel K, Molvin L, Lutz AM, Koong AC, Jeffrey RB, Tian L, Willmann JK (2012) Antiangiogenic and radiation therapy: early effects on in vivo computed tomography perfusion parameters in human colon cancer xenografts in mice. Investig Radiol 47(1):25–32. doi:10.1097/RLI.0b013e31823a82f6
Heijmen L, Punt CJ, Ter Voert EG, de Geus-Oei LF, Heerschap A, Bussink J, Sweep CG, Zerbi V, Oyen WJ, Span PN, Boerman O, van Laarhoven HW (2013) Monitoring the effects of bevacizumab beyond progression in a murine colorectal cancer model: a functional imaging approach. Investig New Drugs 31(4):881–890. doi:10.1007/s10637-012-9920-9
Zhang HP, Shi QS, Li F, Liu L, Bai M, Gu JY, Wu Y, Du LF (2013) Regions of interest and parameters for the quantitative analysis of contrast-enhanced ultrasound to evaluate the anti-angiogenic effects of bevacizumab. Mol Med Rep 8(1):154–160. doi:10.3892/mmr.2013.1499
Heijmen L, Ter Voert EG, Punt CJ, Heerschap A, Oyen WJ, Bussink J, Sweep CG, Laverman P, Span PN, de Geus-Oei LF, Boerman OC, van Laarhoven HW (2014) Monitoring hypoxia and vasculature during bevacizumab treatment in a murine colorectal cancer model. Contrast Media Mol Imaging 9(3):237–245. doi:10.1002/cmmi.1564
Greis C (2011) Quantitative evaluation of microvascular blood flow by contrast-enhanced ultrasound (CEUS). Clin Hemorheol Microcirc 49(1–4):137–149. doi:10.3233/CH-2011-1464
Schlosser J, Kirmizibayrak C, Shamdasani V, Metz S, Hristov D (2013) Automatic 3D ultrasound calibration for image guided therapy using intramodality image registration. Phys Med Biol 58(21):7481–7496. doi:10.1088/0031-9155/58/21/7481
Heckel F, Schwier M, Peitgen H-O (2009) Object oriented application development with MeVisLab and Python. GI Jahrestag 154:1338–1351
Strouthos C, Lampaskis M, Sboros V, McNeilly A, Averkiou M (2010) Indicator dilution models for the quantification of microvascular blood flow with bolus administration of ultrasound contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control 57(6):1296–1310. doi:10.1109/TUFFC.2010.1550
Quaia E (2011) Assessment of tissue perfusion by contrast-enhanced ultrasound. Eur Radiol 21(3):604–615. doi:10.1007/s00330-010-1965-6
Wang JW, Zheng W, Liu JB, Chen Y, Cao LH, Luo RZ, Li AH, Zhou JH (2013) Assessment of early tumor response to cytotoxic chemotherapy with dynamic contrast-enhanced ultrasound in human breast cancer xenografts. PLoS ONE 8(3):e58274. doi:10.1371/journal.pone.0058274
Obermueller E, Vosseler S, Fusenig NE, Mueller MM (2004) Cooperative autocrine and paracrine functions of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in the progression of skin carcinoma cells. Cancer Res 64(21):7801–7812. doi:10.1158/0008-5472.CAN-03-3301
Chen Y, Han F, Cao LH, Li C, Wang JW, Li Q, Zheng W, Guo ZX, Li AH, Zhou JH (2015) Dose-response relationship in cisplatin-treated breast cancer xenografts monitored with dynamic contrast-enhanced ultrasound. BMC Cancer 15:136. doi:10.1186/s12885-015-1170-8
Baetke SC, Rix A, Tranquart F, Schneider R, Lammers T, Kiessling F, Lederle W (2016) Squamous cell carcinoma xenografts: use of VEGFR2-targeted microbubbles for combined functional and molecular US to monitor antiangiogenic therapy effects. Radiology 278(2):430–440. doi:10.1148/radiol.2015142899
Zocco MA, Garcovich M, Lupascu A, Di Stasio E, Roccarina D, Annicchiarico BE, Riccardi L, Ainora ME, Ponziani F, Caracciolo G, Rapaccini GL, Landolfi R, Siciliano M, Pompili M, Gasbarrini A (2013) Early prediction of response to sorafenib in patients with advanced hepatocellular carcinoma: the role of dynamic contrast enhanced ultrasound. J Hepatol 59(5):1014–1021. doi:10.1016/j.jhep.2013.06.011
Hoyt K, Umphrey H, Lockhart M, Robbin M, Forero-Torres A (2015) Ultrasound imaging of breast tumor perfusion and neovascular morphology. Ultrasound Med Biol 41(9):2292–2302. doi:10.1016/j.ultrasmedbio.2015.04.016
Williams R, Hudson JM, Lloyd BA, Sureshkumar AR, Lueck G, Milot L, Atri M, Bjarnason GA, Burns PN (2011) Dynamic microbubble contrast-enhanced US to measure tumor response to targeted therapy: a proposed clinical protocol with results from renal cell carcinoma patients receiving antiangiogenic therapy. Radiology 260(2):581–590. doi:10.1148/radiol.11101893
Lassau N, Bonastre J, Kind M, Vilgrain V, Lacroix J, Cuinet M, Taieb S, Aziza R, Sarran A, Labbe-Devilliers C, Gallix B, Lucidarme O, Ptak Y, Rocher L, Caquot LM, Chagnon S, Marion D, Luciani A, Feutray S, Uzan-Augui J, Coiffier B, Benastou B, Koscielny S (2014) Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: the French multicenter support for innovative and expensive techniques study. Investig Radiol 49(12):794–800. doi:10.1097/RLI.0000000000000085
Wang H, Kaneko OF, Tian L, Hristov D, Willmann JK (2015) Three-dimensional ultrasound molecular imaging of angiogenesis in colon cancer using a clinical matrix array ultrasound transducer. Investig Radiol 50(5):322–329. doi:10.1097/RLI.0000000000000128
Zhou J, Wang H, Zhang H, Lutz AM, Tian L, Hristov D, Willmann JK (2016) VEGFR2-targeted three-dimensional ultrasound imaging can predict responses to antiangiogenic therapy in preclinical models of colon cancer. Cancer Res 76(14):4081–4089. doi:10.1158/0008-5472.CAN-15-3271
Wang Y, Dong L, Bi Q, Ge X, Zhang X, Wu D, Fu J, Zhang C, Wang C, Li S (2011) Beyond antiangiogenesis: intratumorally injected bevacizumab plays a cisplatin-sensitizing role in squamous cell carcinomas in mice. Chemotherapy 57(3):244–252. doi:10.1159/000326485
Acknowledgements
We would like to thank Philips for providing the EPIQ7 US system. We would also like to thank the China Scholarship Council and Program for New Century Excellent Talents in University for providing funding for Dr. Jianhua Zhou to study abroad at Stanford University.
Funding
This research was supported by the NIH R01 CA155289 Grant (JKW) and NIH R01 CA195443 (JKW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhou, J., Zhang, H., Wang, H. et al. Early prediction of tumor response to bevacizumab treatment in murine colon cancer models using three-dimensional dynamic contrast-enhanced ultrasound imaging. Angiogenesis 20, 547–555 (2017). https://doi.org/10.1007/s10456-017-9566-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-017-9566-5