Abstract
Microbial environmental monitoring represents one of the most useful methods to assess potential risks related to the integrity of cultural heritage and people’s health. The monitoring plan described in the present work is based on standardized techniques for measuring microbial air and surface contamination. Air contamination is assessed through both active and passive samplings, measuring the concentration of microbes in air (in colony forming units per cubic metre, CFU/m3) and the rate at which microorganisms settle on surfaces (expressed by the Index of Microbial Air Contamination, IMA, CFU/dm2/h). For surface contamination, two parameters are measured using nitrocellulose membranes: the Microbial Buildup (MB, the total number of microorganisms accumulated on a surface in an unknown period of time prior to the sampling) and the Hourly Microbial Fallout (HMF, the number of microorganisms that settle on a specific surface during 1 h). The monitoring plan was implemented at the Pilotta Palace in Parma, Italy, during the Correggio exhibition in 2009. Samplings were taken before and during opening times. Some microbial contamination was already detected before the arrival of visitors: air contamination mean values of 99.1 CFU/m3 and 5.2 CFU/dm2/h were recorded, while MB and HMF mean values for surfaces were 92 and 7 CFU/dm2, respectively. A significant increase was recorded in air contamination during opening times, with mean values of 323.7 CFU/m3 and 19.4 CFU/dm2/h; surface contamination values increased as well. This monitoring plan represents a contribution towards the definition of a much needed standardized methodology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In cultural heritage environments (museums, libraries, archives, exhibitions, etc.), airborne biological particles such as bacterial cells and spores, fungal spores, viruses (and all their fragments and by-products) represent a hazard both for the heritage materials, which can become deteriorated, and for operators and visitors (Apetrei et al. 2009; Caneva et al. 2007; Gallo 1993; Mandrioli and Ariatti 2001; Mandrioli et al. 2003; Mandrioli 2006; Niesler et al. 2010; Pasquariello 2006; Pasquariello et al. 2008; Tarsitani et al. 1996; Tarsitani 2007).
In any indoor environment, people represent one of the most important sources of microbial air contamination. Skin is a natural source of microorganisms, which are released into the environment through the continuous process of desquamation. The rate at which skin fragments disperse varies according to the subjects’ activity, and it has been estimated that when undressing, a person can produce up to 500,000 skin fragments, 5–10% of which may carry microorganisms (Noble and Somerville 1981). Hair is also a significant potential source of microbial contamination, and high hair densities and oily secretions favour the growth of microorganisms. Microorganisms are also introduced into the environment when people talk, cough, sneeze. The aerobiological particles present in an indoor environment can come from outside too, through air vents, windows, doors and other openings, or from any kind of contaminated material that is introduced. Particles can also come from a variety of indoor environmental fomites, such as ceiling tiles, contaminated floors and carpets, badly functioning ventilation systems and any modification or renovation of buildings that inevitably produce dust and debris and increase airborne microorganisms, especially fungal spores. Every building has its own microbial ecology, depending on its structure, finishing and furnishing materials.
Microorganisms may be found in air as single bacterial cells or spores, fungal spores, viruses, aggregates of cells, spores or viruses, or they may be carried by different substrates (e.g. skin and hair fragments, dust and debris) (Cole and Cook 1998). Depending on their size, particles settle on surfaces at different rates, and if they find favourable nutritional and environmental conditions, they may cause biodegradation of heritage materials, which in turn generates economic and cultural losses. Allergenic particles that are either inhaled or come into contact with the skin can give rise to serious problems in humans (Apetrei et al. 2009; Wiszniewska et al. 2009).
The monitoring of microbial contamination on the surface of heritage objects and in the air surrounding them, from both a quantitative and a qualitative point of view, is essential to draw a map of microbial contamination and evaluate possible risks. Such monitoring represents the basis for any further prevention strategy. Correct microbial sampling of the substrates of interest, with standardized methods and non-destructive techniques, is essential to obtain results that can be interpreted and compared. So far there have been scarce studies in the field of cultural heritage. Moreover, different methodologies and measuring techniques have been adopted, and a standardized, generally accepted methodology allowing a comparison of the results has yet to be established. As for air monitoring, both passive and active methods have been employed, with different active samplers, while for surfaces swabs, RODAC plates and nitrocellulose membranes have generally been used, without a common protocol (Brimblecombe et al. 1999; Camuffo et al. 1999; De Nuntiis et al. 2006; Gysels et al. 2004; Maggi et al. 2000; Michaelsen et al. 2009; Montemartini Corte et al. 2003; Montacutelli et al. 2000; Niesler et al. 2010; Nugari et al. 1993; Pasquariello et al. 2009; Pitzurra et al. 1999; Reddy et al. 2005; Sorlini 1993).
As a contribution to the standardization of monitoring methodologies, we propose an environmental monitoring plan for cultural heritage environments based on environmental sampling experiences in fields where the risk of contamination or infection is high (e.g. health care environments, food industry, space industry) (Albertini et al. 2009; Castiglia et al. 2008; Guarnieri et al. 1997; Pasquarella et al. 2009; Pitzurra et al. 1997a, b; Pitzurra et al. 2007; Poletti et al. 1999). This plan was implemented at the Pilotta Palace in Parma during the exhibition of works by the Renaissance painter Antonio Allegri (1489–1534), better known as Correggio, held there between September 20, 2008 and January 25, 2009. Together with Raphael, Michelangelo, Leonardo and Titian, Correggio represents the height of the Italian Renaissance movement. The exhibition was an international event which attracted about 250,000 visitors.
2 Materials and methods
2.1 Microbial environmental monitoring
The microbial environmental monitoring plan proposed here is based on air and surface samplings.
2.1.1 Air sampling
Microbial air sampling is carried out as both active sampling, in order to measure the concentration of microorganisms in the air, and passive sampling, in order to measure the rate at which viable particles settle on surfaces (ISO 14698-1 2003).
Active sampling is performed using DUO SAS 360 sampler (International PBI, Milan, Italy), with a flow rate of 180 l/min and a suction volume of 500 l. The sampler is placed one metre above the floor in the monitored room and about one metre away from any major physical obstacle. To sample the air coming from the heating, ventilation and air conditioning (HVAC) system, the sampler is placed at a distance of 30 cm from the air grille. The results are expressed as colony forming units per cubic metre (CFU/m3). A Hirst spore trap (Burkard) with a flow rate of 10 l/min is also used for direct detection of fungal spores at the microscope, both viable and non-viable, and for the evaluation of the temporal distribution of the particulate; the count is performed according to the methods defined by UNI 11108/04, Italian Organization for Standardization.
Petri dishes with 9 cm diameter are used for passive sampling to determine the Index of Microbial Air Contamination (IMA) (Pitzurra et al. 1997a; Pasquarella et al. 2000). This value corresponds to the number of CFU counted on a lidless Petri dish previously exposed for air particle collection according to the 1/1/1 scheme (for 1 h, 1 m above the floor and about one metre away from walls or any major obstacles). The results of IMA are expressed as CFU/dm2/h (Pasquarella et al. 2000).
Tryptic Soy Agar is used for bacteria isolation, with incubation at 36 ± 1°C for 48 h, while Sabouraud Dextrose Agar with chloramphenicol is used for fungi isolation, with incubation at 22 ± 1°C for 120 h.
2.1.2 Surface sampling
Microbial surface sampling is carried out in a non-destructive way using nitrocellulose membrane filters (Sartorius AG, Goettingen, Germany) with 47 mm diameter. Microbial Buildup (MB) and Hourly Microbial Fallout (HMF) are measured (Pitzurra et al. 1997b, Poletti et al. 1999).
The MB indicates how many microorganisms accumulated on a certain surface during an indefinite period of time prior to the sampling. Data are collected by pressing a nitrocellulose membrane on the surface for 30 s with finger tips protected by sterile gloves. The HMF corresponds to the number of microorganisms that fall on a certain surface during the period of 1 h. Data are collected by leaving a nitrocellulose membrane on the surface for 1 h. Once the samples have been collected, the nitrocellulose membranes are transferred to Petri dishes containing Tryptic Soy Agar, for bacteria isolation, and Sabouraud Dextrose Agar with chloramphenicol, for fungi isolation, with incubation at 36 ± 1°C for 48°C and 22 ± 1°C for 120 h, respectively. MB and HMA are expressed in CFU/dm2.
2.2 Microbial environmental monitoring at the Correggio exhibition
The study was carried out at the Pilotta Palace in Parma, during the Correggio exhibition, on January 12, 2009. The Pilotta Palace, whose construction began in 1583, during the final years of Ottavio Farnese’s rule (1547–1586), consists in a group of buildings in the historic centre of the city. Its name comes from the pilotta, i.e. the glove worn at the time by the players of a ball game similar to Basque pelota. The building currently houses the Archaeological Museum, the Palatina Library, the Bodonian Museum, the Farnese Theatre, the National Gallery and the Toschi State School of Art.
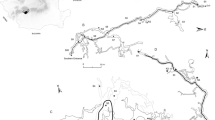
Seven rooms were monitored before and during opening times (Fig. 1). Microbial air samplings were performed with DUO SAS 360, in order to evaluate the number of CFU/m3, and Petri dishes, in order to determine the IMA. A Burkard sampler was located in room 7 to collect fungal spores and pollens over a period of 1 week.
Surface samplings to determine MB and HMA were performed on three surfaces (two furniture items and a marble statue) when visitors were absent. Two surfaces—the marble statue and an urn—were also sampled when visitors were present.
Both bacterial and fungal contaminations were assessed for air, while only bacterial contamination was assessed for surfaces. Microclimatic data—environmental temperature (°C) and relative humidity (%)—were also acquired, as recorded by the centralized system.
2.2.1 Statistical analysis
SPSS 17 (Statistical Package for Social Sciences) was used for statistical evaluations. Analysis of variance (ANOVA) was used to establish significant differences between variables. A P-value below 0.05 was considered statistically significant.
3 Results
Table 1 shows all the single values and the descriptive statistics referring to microbial air contamination in the monitored rooms, with and without visitors present.
The values for the total microbial load with no visitors present ranged from 70 to 110 CFU/m3, with a mean value of 99.1 CFU/m3, while the IMA varied between 1.6 and 11 CFU/dm2/h, with a mean value of 5.2 CFU/dm2/h. With visitors present, the microbial charge ranged from 190 to 420 CFU/m3, with a mean value of 323.7 CFU/m3, while the IMA varied between 4.8 and 36.2 CFU/dm2/h, with a mean value of 19.4 CFU/dm2/h (Table 1). A statistically significant increase for the total microbial charge was observed during opening hours, for both CFU/m3 values (P = 0.0001) and the IMA (P = 0.009). Air bacterial contamination increased significantly, for both CFU/m3 values (P = 0.0001) and the IMA (P = 0.0001), while the increase of fungi was only significant for CFU/m3 values (P = 0.002). CFU/m3 value increases ranged from a minimum of 1.8 times (room 7) to a maximum of 4.6 times (room 3); IMA value increases, on the other hand, ranged from a minimum of 0 times (rooms 2 and 6) to a maximum of 11.5 times (room 1).
Microscopic examination of slides from the Burkard spore trap revealed the presence of Alternaria spp. spores during the whole week, except on Tuesday; the highest value (8 spores/m3) was recorded on Saturday. Particulate was always present.
As for surface contamination, the total microbial contamination with no visitors present ranged from 5 to 184 CFU/dm2 for the MB and from 0 to 17 CFU/dm2 for the HMF; with visitors present, the MB rose from 5 to 46 CFU/dm2 and the HMF went from 17 to 35 CFU/dm2 (Table 2).
As regard temperature, the mean (±SD) values were 14.7°C (±1.07) with no visitors present and 15.5°C (±0.71) with visitors present; as for humidity, the mean values were 39.8% (±1.83) with no visitors present and 38.7% (±1.43) with visitors present. The monitored rooms were not heated since the authorization to the installation of a heating system was not granted by the Cultural Heritage Bureau in order to protect the integrity of the building architecture.
No correlation was found between microclimatic parameters and air microbial contamination.
4 Discussion
Cultural heritage conservation is only one of the many fields where microorganisms, whether viable or not viable, can cause damage to objects or affect people’s health. Hospitals, food processing plants, pharmaceutical plants, electronic-components factories, the space industry and space vehicles are other examples of potentially vulnerable environments (Alava et al. 1996; Cobo and Concha 2007; Dancer 2009; EudraLex 2008; Jarvis 2007; Lelieveld et al. 2003; Pierson 2001). When dealing with these issues, the main goals are detecting and evaluating microbial contamination, in both a quantitative and a qualitative way, identifying critical control points and defining tools to reduce microbial contamination and prevent associated risks. A reliable measuring method is essential to study the situation and judge the effectiveness of any preventive measure that may have been adopted. In some cases, sampling methods are interchangeable from one field to another, whereas in other cases, they need to be specifically adapted. Over the last few years, the need for a standardized and generally accepted methodology has grown in all fields where a risk can derive from the presence of microorganisms.
Several studies have been carried out on the quantitative and qualitative evaluation of the environmental microbial flora in museums. The results, however, are often difficult to compare because different methodologies and techniques have been used and sampling parameters have not been standardized.
As for microbiological air sampling, the two commonly used methods, active and passive sampling, are often employed using different parameters. Issues affecting active sampling relate to the use of different samplers, different collection times and different volumes of sampled air. All these factors can strongly affect the comparison of results and need to be standardized. The physical and biological efficiency of commercially available air samplers varies, and the related parameters have not been certified for all devices. Since this implies a varying performance when collecting microorganisms, only the results obtained using exactly the same device are comparable. We used DUO SAS sampler, with a certified efficiency d50 of 1.45, which is the cut-diameter or aerodynamic diameter above which the collection efficiency of the impactor approaches 100%. This sampler allowed us to simultaneously sample bacteria and fungi. A volume of 500 l has been considered adequate for an acceptable density of microorganisms on the collection plate. For passive sampling, we applied the parameters to determine the Index of Microbial Air Contamination (IMA), which has been standardized and validated in several environments (Albertini et al. 2009; Castiglia et al. 2008; Pasquarella et al. 2009; Pitzurra M et al. 1997a; Pitzurra et al. 2007).
Active and passive samplings have often been considered as alternatives, and their relative advantages and disadvantages are strongly debated (Pasquarella et al. 2008). We advocate the simultaneous use of both, since, as shown in the ISO 14698-1 norm, they have different functions: active sampling provides information about the concentration of viable particles in the air, whereas passive sampling provides a measure of how biocontamination of air contributes to biocontamination of surfaces. Our experience confirms the usefulness of having such converging information. In the present study, for example, sometimes an increase in CFU/m3 values was recorded without a parallel increase in the IMA, particularly for fungi. A possible explanation could be that some of the spores were too buoyant to settle effectively on the collection plates or that the presence of air flows or air eddies hindered the deposition of airborne particles. Our study confirms that settle plates appear to be less sensitive in collecting fungi than active samplers, which are known to retrieve a broader spectrum of fungi and generally show a better picture of airborne fungal species. In a recent study performed in a food processing plant, an air sampler recovered eleven fungal species which had not been captured by the settle plates (Asefa et al. 2009). The settle plates, on the other hand, recovered two organisms (Fusarium spp. and Penicillum jensenii) that had not been captured by the air sampler. The plates and the air sampler also gave similar qualitative information regarding the dominant airborne fungi, irrespective of spore size. The authors concluded that even though it is important for allergists to detect the existence and concentration of each and every fungal species,—and the active sampler is better at this than settle plates— the plates can provide important information regarding the dominant airborne fungal spores, which can fall on and contaminate food products. This concept could be applied to the field of cultural heritage. Indeed, the sensitivity of settle plates could be improved by increasing exposition time, as recommended for the pharmaceutical industry in the European Commission Guide to good manufacturing practice (EudraLex 2008), where threshold values are given for both active (CFU/m3) and passive samplings (settle plates/9 cm in diameter/4-h exposition).
The Burkard spore trap allows to quickly detect fungal spores, both viable and non-viable. This is crucial, since non-viable particles too can pose a risk to heritage objects and people’s health.
For surface sampling, the commonly used methods (RODAC plates, moistened swab, moistened sponge, rinse, etc.) cannot be used to sample the surface of artworks as they all are destructive methods. In addition, some of them are very difficult to standardize. A sampling system based on nitrocellulose membranes, which have already been tested in other critical environments (Guarnieri et al. 1997; Poletti et al. 1999; Pitzurra et al. 2007; Castiglia et al. 2008; Tarsitani et al. 1996), could be employed in cultural heritage conservation (Pitzurra et al. 1999; Pasquariello et al. 2008). With nitrocellulose membranes, two parameters can be measured, i.e. MB and HMF, which provide different types of information. The former reveals the microbial contamination of the surface at a specific time, while the latter gives a direct indication of how many microorganisms are likely to deposit onto a critical surface. The HMF informs about the likelihood of airborne contaminants settling on a specific surface. Different microclimatic conditions, in particular ventilation, can justify different levels of contamination on several surfaces. Therefore, each surface of interest should be studied specifically also considering factors which may affect deposition (e.g. ventilation), since it is difficult to extrapolate the data obtained on one surface to other surfaces.
In our study, the proposed monitoring plan was applied during the Correggio exhibition in Parma, an international event with very large attendance. The extreme importance and success of that event (on the day of the monitoring, 1,772 people visited the exhibition), however, caused some organizational and logistic problems which led to a limited number of samples being collected. This is an aspect that will have to be taken into consideration when monitoring events that might attract large numbers of people.
In this study, the microbial monitoring only aimed at providing a quantitative evaluation, without any qualitative analysis. However, the Burkard spore trap revealed the presence of Alternaria spp., which is a recognized allergenic (Anderssen et al. 2003).
Even though limited in number, the results obtained by our study provide some interesting information. Some contamination was already detected before the arrival of visitors. During the opening to the public, a statistically significant increase in all monitored rooms was observed. The highest values were registered in rooms 4 and 5, which were the most visited: room 5 hosted the Virgin of St. Jerome, Correggio’s most famous painting and room 4 hosted the Head of Christ, a painting which aroused great curiosity and interest because of its questionable ascription to Correggio. These findings suggest that the microbial contamination of a room may be associated with the number of visitors and the amount of time they spend in the room. We were not able to measure the exact number of visitors per room. Indeed, this is an aspect that needs further investigation, as the information it could provide could be used to regulate the access of visitors according to the internal volume of rooms.
The increase in the microbial charge during the opening hours was essentially due to the increase in the bacterial load. A slight increase in the fungal load was recorded too. In one room (room 6), the fungal load actually decreased, both in active and in passive samplings, thus showing that for the most part people disperse bacteria.
5 Conclusions
Our proposal for a microbial monitoring in cultural heritage environments represents a contribution for an integrated approach to evaluate the microbial environmental quality in cultural heritage (for air: active and passive samplings; for surface: MB and HMF); it is also a contribution towards the definition of a standardized method for the evaluation of microbial environmental contamination, from both a static and a dynamic perspective.
Appropriate sampling methods are essential for the subsequent cultural analysis, but are also important for molecular analyses, which are showing promising results in the study of environmental microorganism diversity (Michaelsen et al. 2009; Pangallo et al. 2009; Portillo et al. 2009; Ranalli et al. 2005; Saiz-Jimenez and Gonzalez 2007). The implementation of our environmental monitoring scheme during the Correggio exhibition has demonstrated its feasibility, but a much wider collection of comparable data on microbial environmental contamination—regarding different locations, including old and modern structures, different seasons, different periods of time, different operational conditions, with the parallel study of the microclimatic conditions is needed to understand the circulation and spread of microorganisms and the baseline contamination characterizing cultural heritage environments. Once these data are available, understanding of microorganism-related phenomena in cultural heritage environments will improve, and it will become possible to define reliable threshold contamination values and appropriate preventive measures. In this regard, environmental microbial monitoring represents a fundamental aid to identify potentially risky situations and to define and implement effective preventive measures aimed at protecting both the heritage materials and the health of operators and visitors. Microbial environmental monitoring, therefore, can be regarded as an integral part of a broader quality management system for the field of cultural heritage conservation.
References
Alava, J. I., de Urbina, O., Solozabal, R., & Valero, J. M. (1996). Biodeterioration of electronic circuits by bacteria, fungi and algae. Materials and Design, 17, 19–21. doi:10.1016/0261-3069(96)00023-4.
Albertini, R., Sansebastiano, G.,E., Mariotti, F., et al. (2009). Air microbiological monitoring in a centralized pharmacy compounding unit for cytotoxic drugs. Narbonne, MicrobAERO 2009 Colloque National “Microbiologie des Aerosols”. P1.
Anderssen, M., Downs, S., Mitakakis, T., Leuppi, J., & Marks, G. (2003). Natural exposure to Alternaria spores induces allergic rhinitis symptoms in sensitized children. Pediatric Allergy & Immunology, 14, 100–105. doi:10.1034/j.1399-3038.2003.00031.
Apetrei, I. C., Draganescu, G. E., Popescu, I. T., et al. (2009). Possible cause of allergy for the librians: Books manipulation and ventilation as sources of fungus spores spreading. Aerobiologia, 25, 159–166. doi:10.1007/s10453-009-9121-y.
Asefa, D. T., Langsrud, S., Gjerde, R. O., et al. (2009). The performance of SAS-super-180 air sampler and settle plates for assessing viable fungal particles in the air of dry-cured meat production facility. Food Control, 20, 997–1001. doi:10.1016/j.foodcont.2008.11.011.
Brimblecombe, P., Blades, N., Camuffo, D., et al. (1999). The indoor environment of a modern museum building, The Sainsbury Centre for Visual Arts, Norwich, UK. Indoor Air, 9, 146–164.
Camuffo, D., Brimblecombe, P., Van Grieken, R., et al. (1999). Indoor air quality at the Correr Museum, Venice, Italy. The Science of the Total Environment, 236, 135–152. doi:10.1016/S0048-9697(99)00262-4.
Caneva, G., Nugari, M. P., & Pasquariello, G. (2007). L’aerobiologia applicata alla conservazione dei beni culturali. Dossier Bollettino ICR- Nuova Serie, No. 14.
Castiglia, P., Liguori, G., Montagna, M. T., et al. (2008). Italian multicenter study on infection hazards during dental practice: Control of environmental microbial contamination in public dental surgeries. BMC Public Health, 8, 187–193. doi:10.1186/1471-2458-8-187.
Cobo, F., & Concha, A. (2007). Environmental microbial contamination in a stem cell bank. Letters in Applied Microbiology, 44, 379–386. doi:10.1111/j.1472-765X.2006.02095.x.
Cole, E. C., & Cook, C. E. (1998). Characterization of infectious aerosols in health care facilities: An aid to effective engineering controls and preventive strategies. American Journal of Infection Control, 26, 453–464. doi:10.1016/S0196-6553(98)70046-X.
Dancer, S. J. (2009). The role of environmental cleaning in the control of hospital-acquired infection. The Journal of Hospital Infection, 73, 378–385.
De Nuntiis, P., Vitelli, L., Guaraldi, C., Mandrioli, P., Monco, A., & Salvi, A. (2006). Progetto Musa: Musei & ambiente. In: C. Sabbioni, F. Persia, L. Castelletti (Eds.), Biologia e archeobiologia nei beni culturali. Conoscenze, problematiche e casi studio (pp. 248–259). Como: AIAR e Musei Civici.
EudraLex. (2008). The rules governing medicinal products in the European Union. Volume 4. EU guidelines to good manufacturing practice. Medicinal products for human and veterinary use. Annex 1. Manufacture of sterile medicinal products. Brussels, Belgium.
Gallo, F. (1993). Aerobiological research and problems in libraries. Aerobiologia, 9, 117–130. doi:10.1007/BF02066253.
Guarnieri, V., Gaia, E., Batocchio, L., et al. (1997). New methods for microbial contamination monitoring: An experiment on board the MIR orbital station. Acta Astronautica, 40, 195–201. doi:10.1016/S0094-5765(97)00102-1.
Gysels, K., Delalieux, F., Deutsch, F., et al. (2004). Indoor environment and conservation in the Royal Museum of Fine Arts, Antwerp, Belgium. Journal of Cultural Heritage, 5, 221–230. doi:10.1016/j.culher.2004.02.002.
ISO 14698-1. (2003). Cleanrooms and associated controlled environments—Biocontamination control. Part 1. General principles and methods.
Jarvis, W. R. (2007). The inanimate environment. In W. R. Jarvis (Ed.), Bennett & Brachman’s hospital infections (5th ed., pp. 275–301). Philadelphia: Lippincott Williams & Wilkins.
Lelieveld, H. L. M., Mostert, M. A., Holah, J., & White, B. (2003). Hygiene in food processing. Woodhead Publishing Limited, CRC.
Maggi, O., Persiani, A. M., Gallo, F., et al. (2000). Airborne fungal spores in dust present in archives: Proposal for a detection method, new for archival materials. Aerobiologia, 16, 429–434. doi:10.1023/A:1026522826477.
Mandrioli, P. (2006). Aerosol biologico: fattore di rischio per la conservazione del patrimonio artistico e culturale. In C. Sabbioni, F. Persia, & L. Castelletti (Eds.), Biologia e archeobiologia nei beni culturali. Conoscenze, problematiche e casi studio (pp. 101–111). Como: AIAR e Musei Civici.
Mandrioli, P., & Ariatti, A. (2001). Aerobiology: Future course of action. Aerobiologia, 17, 1–10. doi:10.1023/A:1007602928928.
Mandrioli, P., Caneva, G., & Sabbioni, C. (2003). Cultural heritage and aerobiology. Methods and measurement techniques for biodeterioration monitoring. Dordrecht: Kluwer.
Michaelsen, A., Piñar, G., Montanari, M., & Pinzari, F. (2009). Biodeterioration and restoration of a 16th-century book using a combination of conventional and molecular techniques: A case study. International Biodeterioration & Biodegradation, 63, 161–168. doi:10.1016/j.ibiod.2008.08.007.
Montacutelli, R., Maggi, O., Tarsitani, G., & Gabrielli, N. (2000). Aerobiolgical monitoring of the “Sistine Chapel”: Airborne bacteria and microfungi trends. Aerobiologia, 16, 441–448. doi:10.1023/A:1026525432412.
Montemartini Corte, A., Ferroni, A., & Salvo, V. S. (2003). Isolation of fungal species from test samples and maps damaged by foxing, and correlation between these species and the environment. International Biodeterioration & Biodegradation, 51, 167–173. doi:10.1016/S0964-8305(02)00137-3.
Niesler, A., Gorny, R. L., Wlazlo, A., et al. (2010). Microbial contamination of storerooms at the Auschwitz-Birkenau Museum. Aerobiologia, 26, 125–133. doi:10.1007/s10453-009-9149-z.
Noble, W. C., & Somerville, D. A. (1981). Microbiology of the human skin. London: Lloyd-Luke.
Nugari, M. P., Realini, M., & Roccardi, A. (1993). Contamination of mural paintings by indoor airborne fungal spores. Aerobiologia, 9, 131–139.
Pangallo, D., Chovanova, K., Simonovicova, A., & Ferianc, P. (2009). Investigation of microbial community isolated from indoor artworks and air environment: Identification, biodegradative abilities, and DNA typing. Canadian Journal Microbiology, 55, 277–287.
Pasquarella, C., Albertini, R., Dall’Aglio, P., et al. (2008). Il campionamento microbiologico dell’aria: lo stato dell’arte. Igiene e Sanità Pubblica, 64, 79–120.
Pasquarella, C., Albertini, R., Saccani, E., Ugolotti, M., Mariotti, F., Boccuni, C., et al. (2009). Air microbiological control in an operating theatre: The monitoring plan at the University Hospital in Parma. Narbonne, MicrobAERO 2009 Colloque National “Microbiologie des Aerosols”. 1 F.
Pasquarella, C., Pitzurra, O., & Savino, A. (2000). The index of microbial air contamination. Journal of Hospital Infections, 46, 241–256. doi:10.1053/jhin.2000.0820.
Pasquariello, G. (2006). L’Aerobiologia applicata alla conservazione dei beni culturali negli ambienti confinati. In: C. Sabbioni, F. Persi, L. Castelletti (Eds.), Biologia e Archeologia nei Beni Culturali. Conoscenze, problematiche e casi di studio (pp. 125–141). AIAR e Musei Civici, Comune di Como. Como.
Pasquariello, G., Moroni, C., Architrave, R., & Tarsitani, G. (2008). Il rischio biologico nelle attivita’ di conservazione e restauro dei beni culturali. In: VI Congresso Nazionale IGIIC—Lo Stato dell’Arte 6—Spoleto, Rocca Albornoziana, 2–4 ottobre 2008 (pp. 125–132).
Pasquariello, G., Moroni, C., Architrave, R., & Tarsitani, G. (2009). Esperienze di indagini aerobiologiche per la valutazione del rischio microbiologico nelle operazioni di conservazione e restauro dei beni culturali. European Journal of Aerobiology and Environmental Medicine, V(1), 29–31.
Pierson, D. L. (2001). Microbial contamination of space craft. Gravitational and Space Biology Buletinl, 14, 1–6.
Pitzurra, L., Bellezza, T., Giammarioli, M., Giraldi, M., Sbaraglia, G., Spera, G., et al. (1999). Microbial environmental monitoring of the refectory in the monastery of St. Anna in Foligno, Italy. Aerobiologia, 15, 203–209. doi:10.1023/A:1007667213499.
Pitzurra, O., Hennlich, W., Pasquarella, C., Gantenbein-Demarchi, C., Ortaggi, G., et al. (2007). The EUREKA Project MEMFOODIN. Microbial environmental monitoring in the food industry. GIT Laboratory Journal Europe, 11(12), 40–45.
Pitzurra, M., Savino, A., & Pasquarella, C. (1997a). Il Monitoraggio Ambientale Microbiologico. Annali di Igiene, 9, 439–454.
Pitzurra, M., Savino, A., Pasquarella, C., & Poletti, L. (1997b). A new method to study the microbial contamination on surfaces. Hygiene Medizine, 22, 77–92.
Poletti, L., Pasquarella, C., Pitzurra, M., & Savino, A. (1999). Comparative efficiency of nitrocellulose membranes versus RODAC plates in microbial sampling on surfaces. Journal of Hospital Infections, 41, 195–202.
Portillo, M. C., Saiz-Jimenez, C., & Gonzales, J. M. (2009). Molecular characterization of total and metabolically active bacterial communities of “white colonizations” in the Altamira Cave, Spain. Research in Microbiology, 160, 41–47. doi:10.1016/j.resmic.2008.10.002.
Ranalli, G., Alfano, G., Belli, C., et al. (2005). Biotechnology applied to cultural heritage: Biorestoration of frescoes using viable bacterial cell and enzymes. Journal of Applied Microbiology, 98, 73–83. doi:10.1111/j.1365-2672.2004.02429.x.
Reddy, M. K., Suneela, M., Sumathi, M., & Reddy, R. C. (2005). Indoor air quality at the Salarjung Museum, Hyderabad, India. Environmental Monitoring and Assessment, 105, 359–367. doi:10.1007/s10661-005-4344-z.
Saiz-Jimenez, C., & Gonzalez, J. M. (2007). Aerobiology and cultural heritage: Some reflections and future challenger. Aerobiologia, 23, 89–90. doi:10.1007/s10453-007-9059-x.
Sorlini, C. (1993). Aerobiology: General and applied aspects on the conservation of art works. Aerobiologia, 109–115.
Tarsitani, G. (2007). Qualità dell’aria di ambienti di conservazione e salute degli operatori: criteri per la valutazione dei rischi. Bollettino ICR—Nuova serie—N. 14.
Tarsitani, G., Fusillo, C., Micali, O., et al. (1996). Contaminazione microbiologica del libro e rischi per i lettori. Annali di Igiene, 8, 65–70.
Wiszniewska, M., Walusiak-Skorupa, J., Pannenko, I., et al. (2009). Occupational exposure and sensitization to fungi among museum workers. Occupational Medicine, 59, 237–242. doi:10.1093/occmed/kqp043.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pasquarella, C., Sansebastiano, G.E., Saccani, E. et al. Proposal for an integrated approach to microbial environmental monitoring in cultural heritage: experience at the Correggio exhibition in Parma. Aerobiologia 27, 203–211 (2011). https://doi.org/10.1007/s10453-010-9189-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-010-9189-4