Abstract
The objective of this study was to analyse the dynamics of the Alnus and Corylus pollen seasons in Poland with reference to spatial and seasonal differentiation. Aerobiological monitoring was performed in 10 cities, in 1994–2007. Five characteristics defining the pollen season were considered: 1. beginning and end dates of the season phases (5, 25, 50, 75, 95% of annual totals), 2. pollen season duration (90% method), 3. skewness and 4. kurtosis of airborne pollen curves, and 5. annual pollen totals. The beginning of the Corylus pollen season in Warsaw started on the 53rd day of a year. The Alnus pollen season started 9.5 days (SE = 1.4) later. The start of the season for both taxa was delayed by 3.3 (SE = 0.5) days for each 100 km towards the east. The Corylus pollen season lasted about 15 days longer than the Alnus season. Season duration for both taxa decreased towards the east by 3.5 days (SE = 0.7) and towards the north by 1.3 days (SE = 0.6) for each 100 km. Seasonal dynamics of both taxa are skewed to the right. In cities located west of Warsaw the dynamics are more skewed (except at Szczecin, Wroclaw). Asymmetry decreases towards the east by 0.16/100 km. Almost all kurtosis values of pollen-season dynamics were positive and higher for Alnus. Kurtosis values for both taxa increase together with delay of the pollen season beginning by 4% per day (p < 0.0001). Mean pollen total increases: for Corylus mainly towards the north (by 64%/100 km), for Alnus mainly towards the west (by 15%/100 km). Geographical location (longitude and latitude) determines: the start and duration of the pollen season, skewness of the pollen curve, and annual totals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Pollen seasons are regarded as indicators of plant phenology, ecology, and climate variability (Benninghoff 1991; Newnham 1999; Jato et al. 2002; Rodriguez-Rajo et al. 2004; Emberlin et al. 2007a). The concentration of plant pollen in the air depends on vegetative cover, plant biology, weather conditions which result from geographic locality, and geomorphology (Hyde 1959; Emberlin et al. 1994, 2000; Valencia-Barrera et al. 2001; Kornaś and Medwecka-Kornaś 2002; Reese and Liu 2005). Aerobiology deals with these relationships and also estimates the effect of spatial pattern on the dynamics of pollen seasons (Emberlin et al. 1994; Weryszko-Chmielewska et al. 2001; Garcia-Mozo et al. 2006). Valencia-Barrera et al. (2001) verified the hypothesis that pollen-season dynamics at different geographic and climatic points at the same latitude should be comparable. Such regularities with regard to the spatial pattern in forecast models could affect the efficiency of pollen season forecasts.
Knowledge of the geographic distribution of allergenic plants in a given area and knowledge of whether there is the relationship between the geographical locality and the dynamics of pollen seasons are of great importance in different disciplines, for example biogeography, ecology, meteorology, and medicine (Estrella et al. 2006).
It has been stated that in Northern and Central Europe the pollen seasons of trees flowering in early spring, for example Alnus, Corylus and Betula, are characterized by clear variability in consecutive years (Spieksma et al. 1995; Jäger et al. 1996; Frei 1998; Emberlin et al. 2007b; Smith et al. 2007).
According to many authors the air temperature in the period preceding plant pollination substantially affects the start of the Corylus and Alnus pollen seasons (Frenguelli et al. 1991, 1992; Frei 1998; Rodriguez-Rajo et al. 2004, 2006; Emberlin et al. 2007a). In Poland mean temperature in January and February decreases towards the east which is associated with a delay of thermal early spring dates (Lorenc 2005). For this reason Corylus and Alnus pollen seasons could be regarded as good models for verifying the hypothesis that variability of particular conditions during the Corylus and Alnus pollen seasons depends on geographical location.
In Poland three species of Alnus occur: A. glutinosa (L.) Gaertn., A. incana (L.) Moench, and A. viridis (Chaix) DC. Alnus is a member of the Carr forests which are regarded as related not to defined plant zones but rather to specific environments and wet alder woods. A. glutinosa occurs commonly along waterflows throughout the whole country, although as a specific lowland species occurs mainly on the Northern and Central lowlands of Poland. A. incana occurs in coniferous and mixed deciduous forests on the Southern and Central lowlands of Poland and in the lower montane forest zone up to 1,100 m a.s.l. A. viridis is known only in south-eastern Poland, in the Bieszczady mountains. Corylus occurs commonly throughout the country as one species only, C. avellana (L.), (Zając and Zając 2001).
In Poland comparative analyses of Corylus and Alnus pollen seasons at different monitoring sites have been undertaken on the basis of short studies (2–5 years), (Weryszko-Chmielewska et al. 2001; Kasprzyk et al. 2004). However, there is no comprehensive analysis of long-lasting spatial variability of Corylus and Alnus pollen-season dynamics over the whole country.
The objective of this paper is analysis of Corylus and Alnus pollen-season dynamics in Poland, on the basis of many years’ observations, with reference to seasonal and geographical differentiation.
2 Materials and methods
2.1 Aerobiological analyses
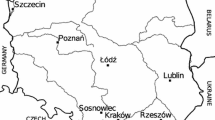
The studies were performed in 10 cities in Poland in 1994–2007 (Fig. 1). Data about the localities and climate are given in Table 1. The volumetric method with a Hirst-type trap (Lanzoni s.r.l., Burkard) was used in pollen monitoring. The trap worked continuously taking samples of airborne particles with a flow rate of 10 L min−1. The particles adhered to Melinex adhesive tape wrapped around the moving drum. Pollen grains were stained with basic fuchsin or safranin. Every week the Melinex tape was cut into seven segments corresponding to 24-h periods. For microscopical pollen grain identification the atlas of Moore et al. (1991) was used as reference book. Twelve vertical lines or four horizontal lines on each slide were analysed. Results were expressed as the number of pollen grains per cubic metre of air sampled, averaged over 24 h. In most cities the traps were installed at heights recommended by the IAA (15–25 m above ground level), (Galán Soldevilla 1998).
2.2 Statistical analysis
Generally 184 pollen seasons were analysed, comprising 94 Alnus pollen seasons and 90 Corylus pollen seasons. Five season charcteristics were studied (dates of the pollen-season phases, duration of pollen season, skewness and kurtosis of pollen curve, annual pollen totals). The abbreviations used in the models were:
-
λ—longitude (distance between a given site and Warszawa in km along longitude)
-
Φ—latitude (distance between a given site and Warszawa in km along latitude)
-
tax—taxon (Corylus and Alnus)
-
per—percentile dividing the pollen season into five phases (5 per, 25 per, 50 per (median), 75 per, 95 per)
-
st—pollen season start (day when the number of pollen grains reached 5 per)
-
year—one of the 14 seasons (this variable was considered as a random variable)
2.2.1 Dates of the pollen-season phases
The dates of consecutive phases of the pollen season were defined as the day of a year when the cumulative number of pollen grains reached a defined percentage of pollen grains sum in a given year: the season start (5%), second phase (25%), third phase (50%), fourth phase (75%), and season end (95%).
It was accepted that the error distribution was the normal distribution with zero mean and estimated variance. It was also accepted that variance of random factors (years and year*taxon) is specific for a given percentile (heteroskedastic model). Mean data presented in Fig. 2 were calculated according to the model in which geographical co-ordinates were replaced by the city name.
2.2.2 Duration of pollen season
To calculate the duration of the pollen season the 90% method was applied (the start of the season was the date when 5% of the seasonal cumulative pollen grain count was reached and the end of the season was the date when 95% of the seasonal cumulative pollen grain count was reached).
In this model the independent estimation of residual variance for both taxa was assumed.
2.2.3 Skewness of the pollen curve
The formula used was:
where M 3 = Σ (x i − mean x )3, σ 3 is the standard deviation, and n is the annual total pollen. It was observed that the skewness variability increased exponentially with delay of the season start. In the above equation, therefore, the error value is given as the normal distribution N(0, 2*exp(c*st)), where c is the factor of homoskedasticity increase. Mean data presented in Fig. 6 were calculated according to the model in which geographical co-ordinates were replaced by the city name and the season start was ignored, although the heteroscedastic model was included.
2.2.4 Kurtosis of the pollen curve
The formula used was:
where M j = Σ(x i − mean x )j, σ 4 is the standard deviation, and n is the annual pollen total
It was accepted that the effect of season start date on kurtosis value was non-linear. The heteroscedastic model was adapted from the above-mentioned model.
2.2.5 Annual pollen totals
The annual pollen total is a sum of the daily pollen concentration values.
This model was calculated separately for Corylus and Alnus, assuming that the number of pollen grains was given as the Poisson distribution. The effect of season start date on seasonal pollen-grain number was analysed by use of two models (for Corylus and Alnus, respectively):
and
It was accepted that expected values were presented with 95% confidence intervals (CI). Statistical analysis was performed by use of SAS software and the MIXED and NLMIXED procedures (Littell et al. 2006).
3 Results
In Warszawa the Corylus pollen season start was on the 53rd day of the year (14 March). The start of the Alnus season occurred about 9.5 days later (SE = 1.4). The date of the season start for both taxa was delayed by 3.3 days (SE = 0.5 day) for each 100 km eastward (Figs. 2, 3).
In the next phases of the season (25, 50, 75, and 95 percentiles) the importance of longitude gradient disappeared (Fig. 2). The date of the season start could fluctuate by two and a half months whereas the date of the season end fluctuated by about a month and a half only (Table 2).
The duration of the Alnus pollen season in Warszawa was about 25 days (Fig. 4). Generally in Polish cities Corylus pollen seasons were about 15 days longer (t 130 = 5.22; p < 0.0001). The duration of the seasons decreased by about 3.5 days (SE = 0.7) for every 100 km move to the east of Warszawa. Change of latitude by 100 km to the north was accompanied by reduction of the season by 1.3 days (SE = 0.6).
Duration of the seasons changed according to a calendar year (variance of this factor is 136 (SE = 59)). It seems that the duration of the pollen season is more variable for Corylus than for Alnus (residual variance for Corylus is 135 (SE = 29), for Alnus it is 87 (SE = 15)). The difference between the duration of the seasons resulting from geographical gradients and taxonomical positions disappears if the dates of the season starts are incorporated in the model (Fig. 5). Such a statistical model indicates that delay of the beginning of the pollen season by one day causes reduction of this season by 0.8 days (t 12 = 14.07; p < 0.0001).
The dynamics of pollen seasons of both taxa are largely skewed to the right. The course of the Alnus pollen season is more skewed to the right (0.81; CI: 0.20–1.40) than the course of the Corylus pollen season (0.21; CI: −0.338–0.81), (t 12 = 2.339; p = 0.0340), (Fig. 6).
The expected value of Alnus dynamics skewness, calculated on the basis of the statistical model which includes the season start date and geographical position of the studied site, is 0.86 (95%CI: 0.11; 1.61). For Corylus the value (0.17; 95% CI: −0.53; 0.88) is smaller (t 25 = 1.87; p = 0.0739). The ten-day-later start of the season affects the increase in skewness by 0.09 units (t 146 = 0.97; p = 0.3331). If we compare those two taxa taking into consideration the fact that the Alnus pollen season starts later than the Corylus pollen season, the asymmetry differences disappear. The delay of the season start affects skewness factor variability. For a delay of the season start by one day the residual variance increases 1.045 times (SE = 1.006; Z = 7.82; p < 0.001). The variability of the season skewness depends on the year studied. The variance value is 0.74 (SE = 0.4; Z = 1.86; p = 0.0314). Skewness of the pollen season depends on longitude (Fig. 7). In cities located to the west of Warszawa the course of the pollen seasons is more skewed to the right (except for Szczecin and Wrocław). Skewness decreases towards the east by 0.16 (95% CI: −0.32; 0.003) units for each 100 km.
Almost all pollen seasons are characterized by positive values of kurtosis (Fig. 8). Kurtosis values calculated for Alnus pollen are greater by 14.5 (95% CI: 5.2; 23.7) units than those for Corylus pollen. Statistical analysis did not reveal an effect of geographical position on the kurtosis value (longitude t 13 = 0.17; p = 0.8660, latitude t 13 = 0.08; p = 09403).
The kurtosis value increases exponentially with delay of the season start (Fig. 9). Differences between season starts result in differences between kurtosis for both taxa. If the pollen seasons of Corylus and Alnus start simultaneously, the differences between kurtosis will be 2.9 (95% CI: 1.2; 4.9). The date of the season start has a substantial effect on the variability of kurtosis. A one-day delay of the season start causes the increase in residual variance by 6.6% (Fig. 9).
The two taxa studied differ with regard to two characteristics of the pollen seasons—annual pollen totals and direction of spatial gradient. The annual total of Alnus pollen grains is several times greater than the annual total of Corylus pollen grains.
For Corylus the annual pollen total increases mainly towards the north (each 100 km towards the north is accompanied by an increase in the number of pollen grains by 64% (t 75 = 223; p = 0.0001). To some extent pollen total increases towards the east also, 2% for each 100 km (t 75 = 6.57; p < 0.0001), (Fig. 10). For Alnus the direction from the east to the west is a dominant and vertical gradient. The pollen concentration decreases by 15% for each 100 km towards the east (t 78 = 103.15; p < 0.0001).
Mean annual totals of Alnus and Corylus pollen grains in 10 cities in 1994–2007. Slanting lines are isolines of expected pollen totals which result from the statistical model. The values of these isolines are defined by the numbers on the top and right of the graphs. Next to the city names the observed mean annual totals are presented
4 Discussion
One of the most important objectives of aerobiological studies is to analyse variability of occurrence of consecutive pollen-season phases, especially the season start. Results of these studies are useful for agriculture, ecology, and allergology to define a threshold value of pollen allergens in patients (Frenz 2000; Garcia-Mozo et al. 2002; Vázquez et al. 2003; Orlandi et al. 2006).
Studies performed in other European countries indicate that differences between the starts of the pollen seasons of Alnus and Corylus in consecutive years can range from two weeks to a month and a half (Weryszko-Chmielewska et al. 2001; Kasprzyk et al. 2004; Piotrowska 2004; Piotrowicz and Myszkowska 2006; Emberlin et al. 2007a; Smith et al. 2007). This difference between pollen-season start dates can be explained as the effect of meteorological conditions, especially air temperature, in the period preceding the pollen season (Frenguelli et al. 1991; Frei 1998; Rodriguez-Rajo et al. 2004). In Poland, thermal conditions at the beginning of a year vary substantially; this results in variability of season starts and difficulty of forecasting for the coming year.
The earlier start of the pollen season in the western part of Poland could be associated with typical distribution of January and February isotherms and calendar early spring dates (Lorenc 2005). Mean temperatures in January and February decrease by about 4°C from the west to the east. Dates of thermal early spring are different, e.g. the 20th January in the western part of Poland and the 15th of March in the eastern part of Poland, which proves our hypothesis about the direction of pollen-season start variability.
The spatial relationship results reported in our paper agree with those of Weryszko-Chmielewska et al. (2001). Evaluation of the course of the pollen seasons in Szczecin, Warszawa, and Lublin reveals there is a relationship between geographical location and the start of the season. In Szczecin the Corylus pollen season started about 2–3 weeks earlier than in Lublin and the Alnus pollen season 1 week earlier.
Smith et al. (2007), investigating the relationship between the geographical position of sites located far away from each other and the beginning of the Alnus pollen seasons in 1996–2005, stated that pollen seasons started earlier in Worcester (England) (approx. 30th day of a year) than in Poznań (Poland), (approx. 39th day of a year). In the Mediterranean region the variability of Alnus pollen seasons is smaller. This resulted from greater stability of thermal conditions before the pollen season and from the occurrence of only one species of Alnus (A. glutinosa). Studies performed in Spain and Italy showed that the start of the season could differ by about two weeks among sites (Jato et al. 2000; Rodriguez-Rajo et al. 2004).
Kasprzyk et al. (2004), studying the dynamics of pollen seasons in 1995–1996 along a south–north transect in Poland, stated that there is the relationship between the latitude of the studied site and the start of the pollen season. It was also stated that the Alnus and Corylus pollen seasons started earliest in Poznań and latest in Gdańsk and that for Corylus the differences were clearer.
Newnham (1999), analysing the pollen seasons of grasses in eight sites in New Zealand, reported the occurrence of a statistically significant correlation between the start of the pollen season and maximum concentration, and latitude. Disappearance of the effect of the latitude gradient during the pollen season is evidence of some regularity in the occurrence of the final phase of the season. The date of the season was characterized by low variability. On the basis of this variability the end of the season could be predicted. We did not observe any extension of the final phase. However, it was not supported in mountain sites (Zakopane, Rabka) where the extended final phase was accepted as the effect of redeposition and long-distance transport (Kasprzyk et al. 2004). The 90% method used in this paper to define the duration of the pollen season excluded single pollen grains observed when the pollen season was over. It was also found that in Poland Corylus pollen occurred in the air earlier than Alnus pollen, which was consistent with results given for Worcester (England) (Emberlin et al. 2007b).
The earlier start of the pollen season in the western part of Poland was indicative of reduction of the duration of the season according to longitude. The important finding is that the delay of the season start reduced its duration. This could result from plant vital functions and physiological processes associated with elongation of the day and the relative amount of sunshine (Kopcewicz 2007). These results are very important for predicting the end of the pollen season and the occurrence of allergic symptoms in patients.
Piotrowska (2004) found that the duration of the Alnus and Corylus pollen seasons in Lublin (Poland) and in Skien (Norway) was similar (5–6 weeks). Kasprzyk et al. (2004) reported slight differences in the duration of the Corylus pollen season at sites situated along a south–north transect whereas for Alnus there were no differences. Results obtained by Weryszko-Chmielewska et al. (2001) have shown that the longest Corylus pollen season was noted in Szczecin, and the shortest Alnus pollen season in Warszawa. According to the results for Worcester the Alnus seasons were shorter than the Corylus seasons (15 and 26 days respectively), (Emberlin et al. 2007b). In Ourense (Spain) the Corylus pollen season was longer, by 10 days, than the Alnus season (Rodriguez-Rajo et al. 2004); in Zagreb (Croatia) it was longer by 52 days (Peternel et al. 2007).
The shape of a pollen curve results from plant biology. Early spring trees respond to any increase in temperature by flowering and releasing pollen. Pollen seasons then last for a few days (Suszka 1980; Faegri and Iversen 1989; Weryszko-Chmielewska and Piotrowska 2007). After the seasonal concentration peak, pollen concentration decreases gradually (right-skewed asymmetry) which could be associated with pollen transport in the air (including long-distance transport) and redeposition (Szczepanek 2003). The length of time of pollen-grain occurrence in the atmosphere depends on its size and weight, and on weather conditions. The size of both the studied taxa pollen grains is similar (mean weight of one pollen grain is 9.37 mμg for Alnus glutinosa and 9.45 mμg for Corylus avellana; Dyakowska 1959). It was found that one of the longest-occurring pollen grains in the air was that of Alnus pollen (about 410 s) (Dyakowska 1959; Faegri and Iversen 1989). Moreover, Alnus released pollen gradually from consecutive inflorescences which caused gradual disappearance of the pollen after the concentration peak (Szczepanek 2003). Therefore the Alnus pollen curve was strongly skewed to the right, as reported in this paper.
The significant relationship between skewness and both longitude and season start found here was probably associated with higher temperature in the thermal early spring and the spring in the western part of Poland (Lorenc 2005). Similar results were obtained by Tedeschini et al. (2006), who compared skewness values of Platanus pollen curves in chilly and warm years.
The kurtosis value correctly describes the process whereby pollen reaches its maximum daily concentration. In our study all kurtosis values were positive and higher for Alnus, which means that Alnus pollen reached its maximum concentration more quickly. This involved the occurrence of shorter pollen seasons with higher annual concentrations.
The lack of a significant relationship between kurtosis and geographical position indicated that reaching of maximum concentration values depended on taxon rather than on various weather conditions occurring in the different regions (Tedeschini et al. 2006). Delay of the start of the pollen season for both taxa caused reduction of season duration and an increase in the kurtosis value. Pollen of the studied plants reached maximum concentration faster, irrespective of geographical position.
Alnus and Corylus, as anemophilous plants, produce a large amount of pollen (Szczepanek 2003). However, comparison of their annual totals reveals values were higher for Alnus. Piotrowska (2008) reported that Alnus inflorescences contained more flowers and produced approximately twice as much pollen as Corylus inflorescences. Alnus inflorescences are flabby and exposed to the action of the wind, which favours pollen release and distribution. Pollen concentration in a given season depends on many factors (weather conditions in the current and preceding years, season start, plant vital functions, taxon, and vegetative cover in the studied area). Pollen concentration varies substantially, depending on calendar year (Spieksma et al. 1995; Jato et al. 2002).
Some authors have indicated the alternate occurrence of high and low concentrations in consecutive years for Alnus (Pidek 2007); however, we did not observe such a relationship. To find the relationship between annual totals and a geographical position was difficult, although we succeeded in finding the relationship between annual totals and longitude and latitude. The strongest relationship was found for Corylus, and north was the dominant direction. This did not result directly from the distribution of Corylus sites in Poland. The effect of Alnus site numbers on the curve shape of the expected pollen-grain number was also not univocal. It could be suggested that long-distance transport and the dominant wind direction (western wind) resulted in the higher concentration of Alnus pollen in the north-eastern region of Poland.
5 Conclusions
-
1.
The duration of the pollen season of both studied taxa in Poland statistically depended on longitude and latitude, whereas dates of consecutive season phases and the skewness of the pollen curve depended on longitude only. Annual total of pollen grains depended also on longitude and latitude, although there are different patterns for Corylus (annual totals increase in the northern direction) and Alnus (annual totals increase in the eastern direction).
-
2.
The variability gradient of charcteristics defining the pollen season, which were significantly dependent on longitude, was concurrent with the shape of temperature isolines in January and February in Poland, and with the start of the thermal early spring.
-
3.
Factors which modify the effect of geographical position on pollen-season dynamics include year and taxon. Significant dependence on geographical position was found for pollen phases, season duration, skewness, kurtosis, and annual totals. Delay of the start of the pollen season significantly affected season duration and the skewness and kurtosis values of the pollen curve. The date of the start of the pollen season could be a good indicator of these charcteristics.
References
Benninghoff, W. S. (1991). Aerobiology and its significance to biogeography and ecology. Grana, 30, 9–15.
Dyakowska, J. (1959). Podręcznik palinologii. Warszawa: Wydawnictwo Geologiczne.
Emberlin, J., Jäger, S., Dominguez-Vilches, E., Galán Soldevilla, C., Hodal, L., Mandrioli, P., et al. (2000). Temporal and geographical variations in grass pollen seasons in areas of western Europe: An analysis of season dates at sites of the European pollen information system. Aerobiologia, 16, 73–379.
Emberlin, J., Jones, S., Bailey, J., Caulton, E., Corden, J., Dubbels, S., et al. (1994). Variation in the start of the grass pollen season at selected sites in the United Kingdom 1987–1992. Grana, 33, 94–99.
Emberlin, J., Laaidi, M., Detandt, M., Jäger, S., Myszkowska, D., Nolard, N., et al. (2007a). Changement climatique et évolution du contenu pollinique de l’air dans sept pays européens: Exemple du bouleau Climate change and evolution of the pollen content of the air in seven European countries: The example of Birch. Revue Française D’allergologie Et D’immunologie Clinique, 47, 57–63.
Emberlin, J., Smith, M., Close, R., & Adams-Groom, B. (2007b). Changes in the seasons of the early flowering trees Alnus spp and Corylus spp in Worcester, United Kingdom, 1996–2005. International Journal of Biometeorology, 51, 181–191.
Estrella, N., Menzel, A., Krämer, U., & Behrendt, H. (2006). Integration of flowering dates in phenology and pollen counts in aerobiology: Analysis of their spatial and temporal coherence in Germany (1992–1999). International Journal of Biometeorology, 5, 49–59.
Faegri, K., & Iversen, J. (1989). Textbook of pollen analysis. In K. Faegri, J. Iversen, P.E. Kaland, & K. Krzywiński (Eds.), (IV edn.). New York: Wiley & Sons.
Frei, T. (1998). The effects of climate change in Switzerland 1969–1996 on airborne pollen quantities from hazel, birch and grass. Grana, 37, 172–179.
Frenguelli, G., Bricchi, E., Romano, B., Minigrucci, G., Ferranti, P., & Antognozzi, E. (1992). The role of air temperature in determining dormancy release and flowering of Corylus avellana L. Aerobiologia, 8, 415–418.
Frenguelli, G., Spieksma, F. T., Bricchi, E., Romano, B., Mincigrucci, G., Nikkels, A. H., et al. (1991). The influence of air temperature on the starting dates of the pollen season of Alnus and Populus. Grana, 30, 196–200.
Frenz, D. A. (2000). Interpreting atmospheric pollen count for use in clinical allergy: Spatial variability. Annals of Allergy, Asthma & Immunology, 84, 481–491.
Galán Soldevilla, C. (1998). Pollen sampling. In P. Mandrioli, P. Comtois, & V. Levizzani (Eds.), Methods in aerobiology (pp. 76–81). Bologna: Pitagora Editrice.
Garcia-Mozo, H., Galán, C., Aira, M. J., Belmonte, J., Diaz de la Guardia, C., Fernández, D., et al. (2002). Modeling start of oak pollen season in different climate zones in Spain. Agricultural and Forest Meteorology, 110, 247–257.
Garcia-Mozo, H., Galán, C., & Vázquez, L. (2006). The reliability of geostatistic interpolation in olive field floral phenology. Aerobiologia, 22, 97–108.
Hyde, H. A. (1959). Atmospheric pollen in relation to land use. Nature, 183, 1694–1695.
Jäger, S., Nilsson, S., Berggren, B., Pessi, A. M., Helander, M., & Ramfjord, H. (1996). Trends of some airborne tree pollen in the Nordic countries and Austria, 1980–1993. Grana, 35, 171–178.
Jato, V., Frenguelli, G., Rodriguez, F. J., & Aira, M. J. (2000). Temperature requirements of Alnus pollen in Spain and Italy (1994–1998). Grana, 39, 240–245.
Jato, V., Rodriguez-Rajo, F. J., Mendez, J., & Aira, M. J. (2002). Phenological behaviour of Quercus in Ourense (NW Spain) and its relationship with atmospheric pollen seasons. International Journal of Biometeorology, 46, 176–184.
Kasprzyk, I., Uruska, A., Szczepanek, K., Latałowa, M., Gaweł, J., Harmata, K., et al. (2004). Regional differentiation in the dynamice of the pollen seasons of Alnus, Corylus and Fraxinus in Poland (preliminary results). Aerobiologia, 20, 141–151.
Kopcewicz, J. (2007). Rozwój generatywny. In J. Kopcewicz & S. Lewak (Eds.), Fizjologia roślin (pp. 520–555). Warszawa: Wydawnictwo Naukowe PWN.
Kornaś, J., & Medwecka-Kornaś, A. (2002). Geografia roślin. Warszawa: Wydawnictwo Naukowe PWN.
Littell, R., Milliken, G., Stroup, W., Wolfinger, R., & Schabenerge, O. (2006). SAS for mixed models (2nd ed.). Cary, NC: SAS Press.
Lorenc, L. (2005). Atlas klimatu Polski. Warszawa: IMGW.
Moore, P. D., Webb, J. A., & Collinson, M. E. (1991). Pollen analysis. Oxford: Blackwell.
Newnham, R. M. (1999). Monitoring biogeographical response to climate change: The potential role of aerobiology. Aerobiologia, 15, 87–94.
Orlandi, F., Lanari, D., Romano, B., & Fornaciari, M. (2006). New model to predict the timing of olive (Olea europea) flowering: A case study in central Italy. New Zealand Journal of Crop and Horticultural Science, 34, 93–99.
Peternel, R., Milanović, S. M., Hrga, I., Mileta, T., & Čulig, J. (2007). Incidence of Betulaceae pollen and pollinosis in Zagreb, Croatia, 2002–2005. Annals of Agricultural and Environmental Medicine, 14, 87–91.
Pidek, A. (2007). Nine-year record of Alnus pollen deposition in the Roztocze region (SE Poland) with relation to vegetation data. Acta Agrobotanica, 60(2), 57–64.
Piotrowicz, K., & Myszkowska, D. (2006). The start date, end and duration of the hazel pollen seasons on the background of climatic changes in Krakow. Alergologia Immunologia, 3(3–4), 86–89.
Piotrowska, K. (2004). Comparison of Alnus, Corylus and Betula pollen counts in Lublin (Poland) and Skien (Norway). Annals of Agricultural and Environmental Medicine, 11, 205–208.
Piotrowska, K. (2008). Ecological features of flowers and the amount of pollen released in Corylus avellana (L.) and Alnus glutinosa (L.) Gaertn. Acta Agrobotanica, 61(1), 33–39.
Reese, C. A., & Liu, K. (2005). A modern pollen rain study from the central Andes region of South America. Journal of Biogeography, 32, 709–718.
Rodriguez-Rajo, F. J., Dopazo, A., & Jato, V. (2004). Environmental factors affecting the start of pollen season and concentrations of airborne Alnus pollen in two localities of Galicia (NW Spain). Annals of Agricultural and Environmental Medicine, 11, 35–44.
Rodriguez-Rajo, F. J., Valencia-Barrea, R. M., Vega-Maray, A. M., Suarez, F. J., Fernandez-Gonzales, D., & Jato, V. (2006). Prediction of airborne Alnus pollen concentration by using Arima models. Annals of Agricultural and Environmental Medicine, 13, 25–32.
Smith, M., Emberlin, J., Stach, A., Czarnecka-Operacz, M., Jenerowicz, D., & Silny, W. (2007). Regional importance of Alnus pollen as an aeroallergen: A comparative study of Alnus pollen counts from Worcester (UK) and Poznań (Poland). Annals of Agricultural and Environmental Medicine, 14, 123–128.
Spieksma, F. T. M., Emberlin, J., Hjelmroos, M., Jäger, S., & Leuschner, R. M. (1995). Atmospheric birch (Betula) pollen in Europe: Trends and fluctuations in annual quantities and starting dates of the seasons. Grana, 34, 51–57.
Suszka, B. (1980). Rozmnażanie generatywne: Olsza Alnus Mill. In A. Białobok (Ed.), Nasze drzewa leśne (99–120). Warszawa, Poznań: PAN.
Szczepanek, K. (2003). Wytwarzanie i rozprzestrzenianie spor i ziarn pyłku. In S. Dybova-Jachowicz & A. Sadowska (Eds.), Palinologia (pp. 16–28). Kraków: Wydawnictwo Instytutu Botaniki PAN.
Tedeschini, E., Rodriguez-Rajo, F. J., Caramiello, R., Jato, V., & Frenguelli, G. (2006). The influence of climate changes in Platanus spp pollination in Spain and Italy. Grana, 45, 222–229.
Valencia-Barrera, R. M., Comtois, P., & Fernández-González, D. (2001). Biogeography and bioclimatology in pollen forecasting. Grana, 40(4–5), 223–229.
Vázquez, L. M., Galán, C., & Domínguez, E. (2003). Influence of meteorological parameters on Olea pollen concentration in Córdoba (South-western Spain). International Journal of Biometeorology, 48, 83–90.
Weryszko-Chmielewska, E., & Piotrowska, K. (2007). Cechy morfologiczne kwiatów i pyłku wybranych roślin alergennych. In E. Weryszko-Chmielewska (Ed.), Aerobiologia (pp. 95–137). Lublin: Wydawnictwo Akademii Rolniczej w Lublinie.
Weryszko-Chmielewska, E., Puc, M., & Rapiejko, P. (2001). Comparative analysis of pollen count of Corylus, Alnus and Betula in Szczecin, Warszawa and Lublin (2000–2001). Annals of Agricultural and Environmental Medicine, 8, 235–240.
Zając, A., & Zając, M. (Eds.). (2001). Distribution atlas of vascular plants in Poland. Kraków: Institute of Botany, Jagiellonian University and Foundation of Jagiellonian University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Myszkowska, D., Jenner, B., Puc, M. et al. Spatial variations in the dynamics of the Alnus and Corylus pollen seasons in Poland. Aerobiologia 26, 209–221 (2010). https://doi.org/10.1007/s10453-010-9157-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-010-9157-z