Abstract
The objective of this research was to investigate the prevalence and distribution of airborne and waterborne fungi and actinomycetes along the main stream of the Nile river during April to July, 2005. Air and water samples were collected at eight sites within a ~50 km stretch of the river. The distribution and prevalence of air and water microorganisms varied with location. The highest counts of airborne fungi (516 CFU/p/h) and actinomycetes (222 CFU/p/h) were detected at suburban sites near cultivated areas. However, the highest counts of waterborne fungi (56.4 CFU/ml) and actinomycetes (15.4 CFU/ml) were detected at Al-Galaa (city centre) and Kafr-El-elwe (south Cairo), respectively. A total of 1,816 fungal colonies (943 isolates from air and 873 from water samples) belonging to 27 genera were identified. Aspergillus, Alternaria, Cladosporium, and yeasts were the predominant fungal types in both air and water environments. Dreschlera, Emericella, Nigrospora, Spicaria, Stachybotrys, and Verticillium were only detected in the air, and Epicoccum, Philaphora, Phoma and Ulocladium were only detected in the water. Mycotoxin-producing fungi represented by Aspergillus flavus, Aspergillus parasiticus, Penicillium, Fusarium, and Trichoderma were found in the air and water environments. Significant differences (P ≤ 0.05) were found between fungal populations in air and water at different sampling sites. No significant differences (P ≥ 0.05) were found between waterborne actinomycetes. Sampling location, human activity, and pollution load are the main factors affecting the variability and biodiversity of microorganisms in different microenvironments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The Nile river is the main drinking water resource for the Egyptian people (Abdel Shafy and Aly 2002), providing ~97% of their water demand. The problem of water quality is especially acute for developing nations, where up to 90% of cities discharge their untreated sewage into rivers and streams. The problem of air pollution is continuing to grow more acute in the developing world where populations are expanding rapidly and rapid industrialization is coupled with increasing use of motor vehicles. The air conditions and fresh water quality in the area around the Nile river have been severely affected . The impact of pollution in Egypt appears in all environmental media, i.e., air, water, and soil. The potential damaging effects of pollution are numerous, and include adverse human health and environmental effects.
Materials accumulate at the water–air interface of the Nile river, or any water body, and form a surface film. This film consists of a complex of organic matter and microorganisms, some of which may be harmful (Geldreich 2001). Aspergillus, Cladosporium, Epicoccum, Penicillium, Fusarium, and Mucor are the most dominant fungal genera in treated and untreated water (Kinsey et al. 1999). Many other fungal genera have been isolated from water in Europe including Acremonium, Absidia, Beauveria, Monilia, Mortierella, Rhizopus, and Stemphylium (Tothova 1999). Moreover, actinomycete concentrations have been reported to be higher in river water and lake sediments than in lake water bodies (Willoughby 1969). Levels of actinomycetes in water in Texas, ~7 × 105 CFU/ml (Silvey and Wyatt 1971), were higher than those found in the UK, 10–200 CFU/ml (Burman 1973), and Finland, 200 CFU/ml (Seppänen and Jokinen 1969).
Airborne fungi and actinomycetes originate from different environments, for example soil, plants and water. Wind storms spread dust clouds, mould spores, pollen, and organic materials from plants and animals. Fungi and actinomycetes in the aquatic environment may be transferred to air by wave action and wind. In contrast, airborne microorganisms may settle on the water surface. The prevalence and distribution of air and waterborne flora is highly variable between different locations depending on meteorological, topographical, and human activities (Lacey 1981; Su et al. 2001 and Mitakakis and Guest 2001). Airborne fungal spore concentrations depend on various factors, for instance, time of day, meteorological and seasonal climatic factors and type of vegetation (Pepeljnjak and Segvic 2003). Abdel Hameed (2005) found airborne fungi at mean values of 71, 64, and 175 CFU/p/h in urban and vegetable and chamomile growing areas, respectively. Cladosporium, Alternaria, Aspergillus, and Penicillium are the dominant fungal genera in the air environment (Khan et al. 1999).
Fungi and actinomycetes alter the taste and odour of water. Actinomycetes and fungi cannot be excluded as possible pathogens (Muittari et al. 1980). Exposure to fungi and actinomycetes can lead to allergic respiratory symptoms in sensitive persons (Lacey and Crook 1988). Alternaria spore concentrations may be responsible for increasing levels of respiratory diseases (Corden and Millington 2001).
The authors were aware that airborne and waterborne fungi and actinomycetes were poorly studied in Egypt, particularly along the Nile river ecosystem. Study of airborne and waterborne microorganisms at as many geographical sites as possible is important in understanding the distribution and prevalence of microorganisms in relation to the location, the microenvironment, and human activity. The objective of this study was to investigate the distribution and prevalence of fungi and actinomycetes in air (1 m above the water surface) and water environments at eight trapping sites within a ~50-km stretch of the Nile river, to study the biodiversity of fungi, and to monitor mycotoxin-producing fungi in both air and water environments.
2 Materials and methods
2.1 Locations description
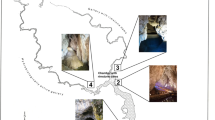
Sampling of air and water was performed at eight sites within a ~50 km stretch along the Nile river (Fig. 1). The estimated distance between each sampling site averaged 4–6 km, and the sites were representative of different human activity, lifestyle, and microenvironment. The main property and activity of each sampling site are shown in Table 1.
2.2 Sampling and isolation methods
Air and water sampling was performed twice per month during the period between April and July 2005 (warm months), the time when environmental conditions help microbial growth. Two consecutive samples were collected during every sampling event, a total of eight samples were collected at every sampling site. The gravitational method was used to collect airborne fungi and actinomycetes (Pelczar et al. 1993). Petri dishes, two replicates, containing rose bengal streptomycin agar and starch casein agar were exposed to the air for 10–15 min, for counting of airborne fungi and actinomycetes, respectively. Petri dishes were placed at a height of ~1 m above the water surface.
Water samples were obtained at a depth of ~30 cm below the water surface, using sterile glass bottles. One millilitre of water sample was spread–plated onto replicate plates containing rose bengal streptomycin agar and starch casein agar for counting waterborne fungi and actinomycetes, respectively. Air and water samples were taken between 9 am and 4 pm at approximately one-half of the width of the Nile river. Water temperature and relative humidity ranged between 23 and 25°C and 6.8 and 7.2, respectively, whereas atmospheric temperature varied between 25 and 32°C.
Air and water-inoculated plates were incubated for 5–7 and 7–15 days at 28°C for counting of fungi and actinomycetes, respectively. The resulting colonies were counted and the mean count from the consecutive samples was calculated. The counts are expressed as colony-forming units per plate per hour (CFU/p/h) and colony-forming units per millilitre (CFU/ml) for airborne and waterborne microorganisms, respectively. Fungal colonies were isolated, purified, and identified to the genus level, with the exception of Aspergillus genus, which was identified to the species level. Identification was performed mainly on the basis of macro and micro-morphological features, and reverse and surface colouration of colonies on the different media (Ellis 1976; Barnett 1972; Singh et al. 1991; Barnett and Hunter 1999).
2.3 Statistical analysis
The Mann–Whitney U test was used to ascertain the significance of differences between mean values of two independent groups for non-parameter distribution. A probability less or equal to 0.05 (P ≤ 0.05) was considered significant.
3 Results
The mean counts of airborne and waterborne fungi and actinomycetes at different sampling sites along the Nile river are shown in Figs. 2 and 3, respectively. Airborne fungi and actinomycetes varied within 135.6–516 CFU/p/h and 46–222 CFU/p/h, respectively. The highest counts of airborne fungi (516 CFU/p/h) and actinomycetes (222 CFU/p/h) were detected at the Al-Qanater Al-khairyia and Imbaba sites, respectively. Slightly higher counts of airborne actinomycetes were also found in Tanash (180 CFU/p/h) and Al-Qanater Al-khairyia (167.6 CFU/p/h) (Fig. 2). On the other hand, the counts of waterborne fungi and actinomycetes ranged between 8–56.4 and 9.2–15.4 CFU/ml, respectively. The highest counts of waterborne fungi (56.4 CFU/ml) and actinomycetes (15.4 CFU/ml) were detected at the Al-Galaa and Kafr-Elelwe sites, respectively (Fig. 3).
Results from the Mann–Whitney U test showed there were significance differences (P ≤ 0.05) between airborne fungi at the Imbaba, Tanash, and Al-Qanater Al-khairyia sites compared with the other sampling sites (Table 2). The distribution of waterborne fungi was shifted toward the Al-Galaa site (P ≤ 0.05). Significant differences were found between airborne actinomycete counts, with actinomycete populations shifted toward suburban sites in north Cairo (Table 3). Moreover, significant differences were found between waterborne fungi at the Imbaba and Tanash sites compared with the other sampling sites. In contrast, the distribution of waterborne actinomycete populations was identical (P ≥ 0.05) at the sampling sites (Table 3).
A total of 1,816 fungal colonies (943 from air and 873 from water samples) were identified (Table 4). Aspergillus, Alternaria, Cladosporium, Penicillium, and yeasts were the predominant fungal genera in air and water at different sampling sites. Aspergillus and yeasts were the predominant genera in the air and water environments. Aspergillus varied between 1.88 and 44.9% in air and between 1.88 and 12.9% in water. Yeasts varied between 2.79 and 24.87% and between 21.5 and 90.56% in air and water, respectively. Dreschlera, Verticillium, Stachybotrys, Nigrospora, and Spicaria were only found in the air environment, Epicoccum, Philaphora, Phoma, and Ulocladium were only found in the water environment (Table 4). The potential mycotoxin-producing fungi, including Aspergillus flavus, Aspergillus parasiticus, Aspergillus niger, Fusarium, Penicillium, Trichoderma, Alternaria, and Stachybotrys were detected at different levels in both air and water samples (Table 4).
4 Discussion
Limited studies have been carried-out on fungi and actinomycetes in air and water along the river streams nationally and internationally. In this study air and water samples were collected during warm months when the number of microorganisms in the water surface can vary. A variety of environmental factors, including climate, affect the distribution of fungi among specific sources. Some fungi produce spores in response to specific climatic factors and most can produce spores over a range of temperatures (15–30°C). Spore concentrations usually increase through the summer months (AAAI 1993). The maximum airborne fungal spore counts have been found during warm months (Abdel Hameed 2005). Moreover, Ren et al. (2001) concluded that increased temperature and suitable relative humidity might lead to higher microfungi counts.
The gravitational method was used to isolate airborne spores because of its practical usage and low cost. Moreover, it is easy to use in the middle of rivers. On the other hand, it gives a rough approximation of the counts (Pelczar et al. 1993) and its reliability is affected by the size and the shape of the particles and the motion of the surrounding air (Reponen et al. 2001).
It is well documented that fungi can alter the taste and odour of water. The occurrence and variation of waterborne fungi have been globally studied in Japan (Tojo et al. 1992), Taiwan (Ann and Ko 1985), India (Khuble 1991), and Egypt (El Hissy et al. 1994). In contrast, few studies have been carried-out on actinomycetes, despite the serious odour and taste problems caused by these organisms (Paterson 1971). Therefore, examination of airborne and waterborne fungi and actinomycetes along an aquatic system helps to determine air and water quality and to understand their relationships with different human activities and microenvironments.
Fungi and actinomycetes are widespread in the air environment; they originate from different sources such as soil, plants, and water. In this study airborne and waterborne microbial counts and types varied between different sites. This variation may be attributed to many factors including character of geographical location, human activity in the region, and plant-growing areas (Lacey 1981 and Pasanen 1992). The results from this study agree with those of Abdel Hameed (2005) who found airborne fungi at mean values of 71, 64, and 175 CFU/p/h in urban and vegetable and chamomile-growing areas, respectively. Fungal and actinomycete spores in the aquatic environment may become airborne through water droplets released as a wave breaks or as airborne bubbles rise to the surface and burst out of the water stream (Lacey 1981). In contrast airborne spores may reach water by deposition and rain washing-out action. Water movement is considered an effective factor causing variations of waterborne microorganisms. These variations were confirmed by Smither-Kopperl et al. (1998) who reported that fungal spores were dispersed rapidly by moving water. Hasnain et al. (1984) stated that basidiospores and ascospores may be elevated near standing or running water. Peciulyte et al. (2000) concluded that counts of waterborne fungi varied between different locations. Awad et al. (2006) found fungal spores in rain water in the range 200–240 CFU/ml. This confirms that rain is one way microorganisms reach water bodies.
In this study airborne fungi and actinomycete spores were higher at suburban sampling sites (Imbaba, Tanash, and Al-Qanater Al-khairyia) than other sites, where different types of vegetation and many agricultural activities are present. Damped wood panels and agricultural waste water are considered the main reasons for slightly elevated counts of waterborne actinomycetes in Kafr El-elwe. The elevated count of waterborne fungi at the Al-Galaa site (city centre) is attributed to extensive human activity, heavy traffic, and dead organic matter reaching the water body. Burman (1973) reported that under normal conditions it is possible that actinomycetes are washed into water from soil, so that the relative abundance of Streptomyces species in the River Thames, UK, is the same as the abundance in the surrounding soil. In this study airborne actinomycetes were found in lower counts than fungi. Because actinomycetes are smaller in size, they are more difficult to transport as aerosols and their settling velocity is slower than for fungi.
Discovery of the occurrence of fungi in a particular environment contributes to extending information about species diversity. In this study the fungal genera identified agree with results obtained by Hassin (1993) who found that Aspergillus, Penicillium, Rhizopus, Fusarium, Trichoderma, Cladosporium, Scopularopsis, Alternaria, and Paecilomyces were the dominant fungal genera in the Nile river at Dakahlia governorate (East Delta). Cladosporium, Alternaria, Penicillium, and Aspergillus were reported to be the dominant fungal genera in the atmosphere, and their concentrations differed from place to place, because of local environmental variables and human activities (Shelton et al. 2002). Alternaria, Aspergillus, and Penicillium are the predominant fungal genera in the air of Kuwait (Moustafa and Kamel 1976) and Nigeria (Lawande and Onyemehkwe 1984). Alternaria, Aspergillus, Penicillium, and Cladosporium were the dominant fungal isolates in urban and rural areas (Abdel Hameed 2005). The frequent detection of these organisms in an environment is attributed to their ready dissemination into the air from many sources including vegetation and urbanization activities. In this study Stachybotrys was only recorded in air; it is a toxic and allergenic fungus (Johanning et al. 1996) and its presence in air is being debated (Etzel et al. 1998). Epicoccum, Philaphora, Phoma, and Ulocladium were only found in water; this may be attributed to their hydrophilic properties (Kowalski 2000).
Surveys of fungi in drinking water have recovered many different taxa including Aspergillus flavus (Goncalves et al. 2006) and Aspergillus fumigatus (Anaissie et al. 2003). Paterson et al. (1997) reported that many fungi producing aflatoxins are found in water. Aflatoxins are carcinogenic to animals and humans and can cause acute or chronic intoxication and damage to human food and animal feed products (Marasas and Nelson 1987). Mycotoxins can be produced by Aspergillus flavus and Aspergillus parasiticus (IARC 1982) and ochratoxin A is produced by Aspergillus ochraceous (Bayman et al. 2002). Air may contain fungi (Fusarium, Aspergillus, and Trichoderma) that produce mycotoxins. Some mycotoxins, in low concentrations, may cause gastrointestinal disorders and damage to haemopoietic and genital systems (Mandrioli et al. 2003).
Many of the air and waterborne fungi detected may be pathogenic to aquatic and terrestrial plants and may cause diseases in humans. Mucor, Stemphylium, Fusarium, and Alternaria are plant pathogens. Fusarium species are common plant pathogens. They have a fairly wide host range and are known to cause vascular wilt in tomatoes and other crops (Richardson et al. 1988). Outdoor allergens play a role in allergic rhinitis in humans (Burge and Rogers 2000). Many of the fungi detected, for example Aspergillus, Cladosporium, Alternaria, Fusarium, Mucor, and Penicillium are allergenic agents (Chin-Shan et al. 1995). Elevated concentrations of Cladosporium are usually associated with respiratory symptoms and increased concentrations of Cladosporium and Penicillium cause allergic diseases (Li et al. 1995). Actinomycete spores cause inflammatory disorders and pulmonary alveolitis (Reponen et al. 2001). Some streptomycetes are causative agents of potato scab that cause a significant financial loss to agricultural products (Takeuchi et al. 1996).
Much basic research is still needed on fungi and actinomycetes to understand their ability to survive and proliferate in different environments and to elucidate factors that control their dispersal in air and water environments. A database of air and waterborne fungi and actinomycetes in different parts of the Nile river in different seasons should also be produced in the future.
5 Conclusion
Airborne and waterborne fungi and actinomycetes varied between sites depending on geographical location, human activity, and local and intermediate sources. Aspergillus, Alternaria, yeasts, and Penicillium were the dominant fungal genera in both air and water environments. Airborne and waterborne fungi along the Nile river shifted toward the Al-Qanater Al-khairyia and Al-Galaa sites, respectively. The distribution of waterborne actinomycetes was identical between locations. Fungi producing mycotoxins (Aspergillus flavus, Aspergillus fumigatus, and Aspergillus parasiticus), Fusarium, and Alternaria were detected in water samples. Evaluation of airborne fungal and actinomycete spores may be useful allergologically and ecologically. Two unanswered questions are raised by this study:
-
1
Is there any process for removal of mycotoxins from drinking water during treatment?
-
2
Is there any limit value for mycotoxins in water if they are detected to be present?
Further studies are needed to evaluate mycotoxin concentrations and types in surface water and air environments.
References
AAAI. (1993). Pollen and spore report. American academy of allergy and clinical immunology. Milwaukee, WI.
Abdel Hameed, A. A. (2005). Vegetation: A source of air fungal biocontaminant. Aerobiologia, 21, 53–61.
Abdel Shafy, H. I., & Aly, R. O. (2002). Water issue in Egypt: Resources, pollution and protection endeavors. Central European Journal of Occupational and Environmental Medicine, 8(1), 3–21.
Anaissie, E. J., Stratton, S. L., Dignani, M. C., Lee, C. K., Summerbell, R. C., Rex, J. H., et al. (2003). Pathogenic molds (including Aspergillus species) in hospital waste distribution systems: A 3-year prospective study and clinical implications for patients with hematologic malignancies. Blood, 101(7), 2542–2546.
Ann, P. J., & KO, W. H. (1985). Variants of Phytophthora cinnamoni extend the known limits of the species. Mycologia, 77, 946–950.
Awad, A. A., Green, C. F., & Gibbs, Sh. G. (2006). Rainfall and its effect on ambient airborne fungi in Giza, Egypt. Journal of Environmental Science, 32(5), 275–292.
Barnett, H. L. (1972). Illustrated Genera of Imperfect Fungi. Minneapolis, Minn, USA: Burgess Publishing Company.
Barnett, H. L., & Hunter, B. B. (1999). Illustrated genera of imperfect fungi (4th ed., p. 218). St. Paul, Minnesota, USA: APS press.
Bayman, P., Baker, J., Boster, M., Michailides, T. X., & Mohoney, N. (2002). Ochratoxin producing by the Aspergillus ochraceous group and Aspergillus alliaceus. Applied and environmental microbiology, 68, 2328–2329.
Burge, H. A., & Rogers, C. A. (2000). Outdoor allergens. Environmental Health Perspectives, 108, 653–659.
Burman, N. P. (1973). The occurrence and significance of actinomycetes in water supply. Society for Applied Bacteriology Symposium Series, 2, 219–230.
Chin-Shan, L., Li-Yuan, H., Chen-Cheng, C., & Kue-Hsiung, H. (1995). Fungus allergens inside and outside the residences of atopic and control children. Archives of Environmental Health, 50(1), 38–43.
Corden, J. M., & Millington, W. M. (2001). The long term trends and seasonal variations of the aeroallergen Alternaria in Derby UK. Aerobiologia, 17, 127–136.
Ellis, M. B. (1976). More dematiaceous hyphomycetes. Kew, Surry, England: Common Wealth Mycol. Inst.
El Hissy, F. T., Khallil, A. M., & Ali, E. H. (1994). Aquatic phycomycetes from Egyptian soil. Microbiological Research, 149, 271–282.
Etzel, R. A., Montana, E., Sorenson, W. G., Kullman, G. J., Allan, T. A., & Dearborn, D. G. (1998). Acute pulmonary hemorrhage in infants associated with exposure to Stachybotrys atra and other fungi. Archives of Pediatrics and Adolescent Medicine, 152, 757–762.
Geldreich, E. E. (2001). Microbial water quality concerns for water supply use. Environmental Toxicology and Water Quality, 6(2), 209–223.
Goncalves, A. B., Paterson, R. R., & Lima, N. (2006). Survey and significance of filamentous fungi from tap water. International Journal of hygiene and environmental health, 209, 257–264.
Hasnain, S. M., Newhook, F. J., Wilson, J. D., & Corbin, J. B. (1984). First report of Ganoderma allergenicity in New Zealand. The New Zealand Journal of Science, 27, 261.
Hassin T. A. (1993). Ecological and physiological studies on fungi present in water and its relation to pollutants in Dakahlia province. M.Sc thesis, Mansoura Univ., Faculty of Science, Bot. Dept.
IARC. (1982). The evaluation of the carcinogenic risk of chemical to humans. IARC Monograph supplement, Vol 4. Lyon, France: International Agency for Research on cancer.
Johanning, E., Biagini, R., Hull, D., Morey, P., Jarvis, B., & Landsbergis, P. (1996). Health and immunology study following exposure to toxigenic fungi (Stachybotrys chartarum) in water damaged office environment. International Archives of Occupational and Environmental Health, 68, 207–218.
Khan, Z. U., Khan, M. A. Y., Chady, R., & Sharma, P. N. (1999). Aspergillus and other moulds in the air of Kuwait. Mycopathologia, 146, 25–32.
Khuble, R. D. (1991). An ecological study of water molds of forest soils of kumaun Himalaya, India. Tropical Ecology, 32, 127–135.
Kinsey, G. C., Paterson, R. R., & Kelley, J. (1999). Methods for the determination of filamentous fungi in treated and untreated waters. Journal of Applied Microbiology, 85, 2145–2245.
Kowalski WJ. 2000. Indoor mold growth: health hazards and remediation. The Pennsylvania State Univ., Dept. of architectural Engineering. Sept. 2000-HVAC- heating/piping/air conditioning engineering.
Lacey, J. (1981). The aerobiology of conidial fungi. Biology of conidial fungi, 1, 373–416.
Lacey, J., & Crook, B. (1988). Review: fungal and actinomycete spores as pollutants of the workplace and occupational allergens. The Annals of Occupational Hygiene, 32(4), 515–533.
Lawande, R. V., & Onyemehkwe, G. C. (1984). Airborne fungi during harmatten in Zaria, Nigeria. Annals of Allergy, 52, 47–49.
Li, C. S., Hsy, L. Y., Chou, C. C., & Hsieh, K. H. (1995). Fungus allergens inside and outside the residence of atpoic and control children. Archives of Environmental Health, 50, 38–43.
Mandrioli, P., Caneva, G., & Sabbioni, C. (2003). Cultural heritage and aerobiology. Dordercht, Boston, London: Kluwer Academic Publishers.
Marasas, W. F. O., & Nelson, P. C. (1987). Mycotoxicology. University Park, PA: Pennsylvania State University.
Mitakakis, T. Z., & Guest, D. I. (2001). A fungal spore calendar for the atmosphere of Melbourne, Australia for the year 1993. Aerobiologia, 17, 171–176.
Moustafa, A. F., & Kamel, M. S. (1976). A study of fungal populations in the atmosphere of Kuwait. Mycopathologia, 59(1), 29–35.
Muittari, A., Kuusisto, P., Virtanen, P., Sovijarvi, A., Gronroos, P., Harmoinen, A., et al. (1980). An epidemic of extrinsic allergic alveolitis caused by tape water. Clinical allergy, 10, 79–90.
Pasanen, A. L. (1992). Airborne mesophilic fungal spores in various residential environments. Atmospheric Environment, 25A(2), 459–462.
Paterson, R. A. (1971). Lacustrine fungal communities. In J. Cairns Jr (Ed.), The structure and function of freshwater microbial communities. American Microscopical Society Symposium. Research Division Monograph 3 (pp. 209–218). Blacksburg, VA: Virginia Polytechnic Institute and State University.
Paterson, R. R. M., Kelley, J., & Gallagber, M. (1997). Natural occurrence of aflatoxins and Aspergillus flavus link in water. Letters in Applied Microbiology, 25, 435–436.
Peciulyte, D., Veistyte, L., Juozaitis, A., Lugauskas, A., Mordas, G., Ceburnis, D., et al. (2000). Water to air transfer of microorganisms from the Baltic Sea and the Curonian lagoon. Environmental and Chemical physics, 22(1), 13–18.
Pelczar, M. J., Chan, E. C. S., & Krieg, N. R. (1993). Microbiology concepts and applications (p. 966). New York, USA: International Ed, McGraw–Hill Inc.
Pepeljnjak, S., & Segvic, M. (2003). Occurrence of fungi in air and on plants investigation of different climatic regions in Croatia. Aerobiologia, 19, 11–19.
Ren, P., Jankun, T. M., Belanger, K., Bracken, M. B., & Leaderer, B. P. (2001). The relation between fungal propagules in indoor air and home characteristics. Allergy, 56, 419–424.
Reponen, T., Willeke, K., Grinshpun, S. A., & Nevalanen, A. (2001). Biological particles sampling. In P. Baron & K. Willeke (Eds.), Aerosol measurement, principles, techniques and applications (2nd ed.). New York: Wiley.
Richardson, S. E., Bannatyne, R. M., Summerbell, R. C., Milliken, J., Gold, R., & Weitzman, S. S. (1988). Disseminated Fusarium infection in the immunocompromised host. Reviews of infectious diseases, 10, 1171.
Seppänen P., & Jokinen S. (1969). On the actinomycetes causing odors and tastes in blue green algae blooms. pp. 69–87. In: H. Dahlström & I. Kangas (Ed.), Limnologi-Symposion 1968-Suomen Limnolginen yhdistys, Helsink.
Silvey, J. K. G., & Wyatt, J. T. (1971). The interrelationship between freshwater bacteria, algae and actinomycetes in Southwestern reservoirs. In J. Cairns Jr (Ed.), The structure and function of freshwater microbial communities. American Microscopical Society Symposium. Research Division Monograph 3 (pp. 249–275). Blacksburg, VA: Virginia Polytechnic Institute and State University.
Singh, K., Frisvd, J. C., Thrane, U., & Mathur S. B. (1991). An Illustrated Manual on Identification of some Seed-borne Aspergilli, Fusaria, Penicillia and their mycotoxins. Danish Government Institute of Seed Pathology for Developing Countries, Ryvangs Alle 78 DK-2990 Hellerup: Denmark.
Shelton, B. G., Kirkland, K. H., Flanders, W. D., & Morris, G. K. (2002). Profiles of airborne fungi in buildings and outdoor environments in the United States. Applied and Environmental Microbiology, 68, 1743–1753.
Smither-Kopperl, M.L., Charudattan, R., Berger, R.D. (1998). A model system to investigate the spread of Plectosporium tabacinum disease on hydrilla. Phytopathology 88 (Suppl.):S83.
Su, H. J., Wu, P. C., Chen, H. L., Lee, F. C., & Lin, L. L. (2001). Exposure assessment of indoor allergens, endotoxins and airborne fungi for homes in southern Taiwan. Environmental Research A, 85, 135–144.
Takeuchi, T., Sawada, H., Tanaka, F., & Matsuda, I. (1996). Phylogenetic analysis of Streptomyces spp causing p-potato scab based on 16 S RNA sequences. International Journal of systematic bacteriology, 16, 525–540.
Tojo, M., Nakazono, E., Hotta, K., & Ichitani, T. (1992). Seasonal variations of Pythium spp in vegetable field soil. Proceedings of the Kansai Plant Protection Society, 34, 11–16.
Tothova, L. (1999). Occurrence of microscopic fungi in the Slovak section of the Danube River. Biologia, 54, 379–385.
Willoughby, L. G. (1969). A study of the aquatic actinomycetes of Blelhamtarn. Hydrobiologia, 34, 465–483.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel Hameed, A.A., El Hawarry, S. & Kamel, M.M. Prevalence and distribution of airborne and waterborne fungi and actinomycetes in the Nile river. Aerobiologia 24, 231–240 (2008). https://doi.org/10.1007/s10453-008-9101-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-008-9101-7