Abstract
The Coriolis δ air sampler manufactured by Bertin Technologies (France) is a continuous air sampler, dedicated to outdoor monitoring of airborne spores and pollen grains. This high-volume sampler is based on patented Coriolis technology delivering a liquid sample. The air is drawn into a conical vial in a whirling type motion using suction; particles are pulled against the wall by centrifugal force. Airborne particles are separated from the air and collected in a liquid medium. This innovative solution allows rapid analysis by several techniques including PCR assay and serological assay in order to measure the antigenicity/allergenicity of pollen grains and fungal spores. Also, traditional counting of pollen grains or taxa identification by optical microscopy can be done. A study has been carried out by the Health Protection Agency (HPA), Porton Down, UK, to measure the physical efficiency of the Coriolis air sampler. The physical efficiency of the sampler for collection of micro-organism-laden particles of various sizes has been compared with that of membrane filter samplers using the techniques described by ISO 14698-1. The Coriolis was operated simultaneously with membrane filter samplers in a controlled room where they were challenged with uniform-sized particles of different diameters containing bacterial spores. For the larger particle sizes, it was found that the physical efficiency of the Coriolis was 92% for 10-μm particles. The biological performance of the Coriolis in the collection of airborne fungal spores and pollen grains was evaluated in comparison with a Hirst spore trap (one-week tape-on-drum type sampler) which is one of the most frequently used traps in the measurement of outdoor pollen grain concentrations. The advantages and limitations of both technologies are discussed. The Coriolis was operated simultaneously with a Hirst spore trap in the sampling station of Réseau National de Surveillance Aérobiologique, France (RNSA); the pollen grain and fungal spore counts were analysed by optical microscopy. The pollen grain count m−3 collected was compared for both devices. The dispersion values were obtained and statistical analysis was carried out. This study shows that the Coriolis air sampler provided equivalent recovery of pollen grain and fungal spores compared with the volumetric trap standard method (not significantly different, W test, α = 0.05). Nowadays, the French-led project, acronym MONALISA, with financial support from the European Commission––Life-Environment (LIFE05 ENV/F/000068), is testing this innovative air sampler in order to measure the antigenicity/allergenicity of the main aeroallergen particles, i.e. Betula (birch), Poaceae (grasses), Parietaria (pellitory), Olea spp (olive tree), and Artemisia (mugwort) pollen grains, and Alternaria (fungal spores) to validate a new approach of monitoring instead of quantifying pollen grains by their morphology. The robustness and efficiency of the MONALISA system is being demonstrated at a national level throughout Europe in eight different countries with different bio-climatic and topography characteristics: France, UK, Finland, Poland, Spain, Portugal, Switzerland, and Italy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bertin Technologies (France) specialises in the design and supply of innovative systems for the industrial, defence, and health sectors. For the past six years, Bertin Technologies has been developing biological air particle-collection technology and analysis systems tailored to the needs of the market. Now, Bertin Technologies is widely recognised for their cyclonic process (patented Coriolis technology) used in air samplers delivering a liquid sample into a single vial. They have manufactured a range of air-sampling equipment for indoor workplace and environmental sampling.
In the context of its aero-biocontamination environment monitoring-related activities, Bertin Technologies has developed a liquid cyclone high-volume air sampler for collection and identification of airborne bio-particles including micro-organisms such as bacteria, fungi, and pollen grains. This technology is based on a cyclone-type device that selects and captures the airborne particles and instantaneous transfer them into a liquid sample. This sample is directly compatible with all types of biological analysis (including epifluorescence microscopy, immunoassay, PCR assay, flow or solid phase cytometry, and standard culture methods). Based on the Coriolis technology, several products have been designed in order to address several market segments, such as field military aerosols collection, homeland security, and contamination control of clean rooms or industrial and environmental contamination assessment.
RNSA (Réseau National de Surveillance Aérobiologique) is the French network in charge of the air survey of biological particles and their health effects. RNSA has been collecting pollen data since 1987 in France and the French network is actually present in 70 towns. For pollen and mould collection, RNSA, in the same way as the other European networks, uses the Hirst spore trap (Hirst 1952) designed either by Burkard or Lanzoni. Beside its activity in collection, analysis, and data diffusion, RNSA still works in research and development to find new means of air monitoring. In 2004, using the Chemscan method, the RNSA tried to analyse not only pollen counts but also pollen allergenicity in the air. But the actual pollen trap was not enabled. It is for this reason that RNSA has made a partnership with Bertin Technologies to couple air collection with the Coriolis air sampler of Bertin Technologies and the know-how of RNSA in allergen recognition. With RNSA experience and taking into account papers related to this subject (D’amato 1995) this is a list of the allergenic pollen or spores which must be studied as the first step:
-
1.
pollens: Betula (birch), Poaceae (grasses), Parietaria (pellitory), Cupressus (cypress), Olea (olive tree), Artemisia (mugwort); and
-
2.
spores of Alternaria.
To study the health impact of the biological particles, RNSA has set up a clinician network.
The goals of this paper are to present the sampling efficiency of the Coriolis air sampler in the capture of airborne particles and also to compare its efficiency for spores and pollen grains counts in relation to the traditional method (Hirst trap). Taking into account the use of Coriolis to quantify aeroallergens, the efficiency of this aspect of the sampler is included in the Discussion.
2 Materials and methods
2.1 Coriolis air sampler design
The Coriolis air sampler is a cyclonic type of instrument based on the principle described by Decker et al. (1969) in which air is pulled into a conical collection sampler tangentially with a swirling buffer (Fig. 1). Airborne particles such as bacteria, pollen grains, and fungal spores are collected in this buffer which can be assayed after sampling for the presence of micro-organisms using whichever technique is thought to be appropriate.
The dimensions of the cyclone design (∅40 up to ∅50 mm) have been determined by homothetics relations and by following the geometrics of Stairmand cyclones (Leith-Licht 1972). The cyclone design is adapted to adjust from a 200 to a 630 l min−1 flow rate. An optimal air velocity for the inlet cyclone has been calculated for each configuration in order to guarantee a high separation efficiency of micronics particles.
The loss of liquid during sampling depends notably on evaporation in the cone. Under standard conditions (temperature 20°C, relative humidity 60%) evaporation is estimated at ~0.5 ml min−1. The most important effect on evaporation is air ambient humidity; the initial temperature of the liquid has been shown to have no significant effect. However, it is particularly important (up to ~2.8 ml min−1) for very hot and dry climates. In order to compensate for loss of liquid, automatic liquid injection has been designed, which allows for a longer collection time (from 10 min to several hours).
2.2 Physical collection efficiency
Coriolis technology has been compared with reference samplers by the Health Protection Agency (HPA), Porton Down, UK, in an environmental room, for evaluation of physical collection efficiency. Reference samplers were a membrane filter (Sartorius) and a cascade impactor (May 1945) as described here after.
2.2.1 Coriolis air sampler
One Coriolis air sampler (Identification No. 06 05040603) has been tested. The dimensions of the cyclone design (∅50 mm) have been determined by homothetics relations and by following geometrics of Stairmand cyclones (Leith-Licht 1972).
The sampler was operated for a period of 5 min by pulling air at a flow rate of 630 l min−1 into a cyclonic separator containing 20 ml of swirling collection fluid. After the sampling period was completed, the remaining fluid was collected, the volume measured (some volume is lost by evaporation) and it was assayed for the presence of micro-organisms.
2.2.2 Membrane filter samplers
The Coriolis was operated simultaneously with a membrane filter sampler in a controlled room, where both were challenged with uniform size particles of different diameter containing bacterial spores. The efficiency was calculated in comparison with a membrane filter sampler (0.8 μm pore size Sartorius cellulose nitrate filter) (Fig. 2), using the techniques described by ISO 14698-1 (ISO 2003).
The filters were mounted on a sterile back-up support and connected to a vacuum pump to provide a flow rate of 28 l min−1.
After sampling, the membrane filters were carefully removed and placed on an agar growth medium with the exposed side facing upwards and incubated at 37°C (±2°C) for 18 h. The colonies formed were counted. The flow rates were regularly checked using a calibrated rota meter.
2.2.3 Cascade sampler
The four-stage Cascade sampler (May 1945) was used to determine the mass mean diameters of the aerosols generated. The sampler was fitted with four new microscope slides which were ground down to a width of 25 mm in order to fit the impactor (Fig. 3).
The slides were covered with a gel prepared from a mixture of 5 g bovine skin gelatin (Type B, Sigma) and 10 ml glycerol made up to 100 ml with distilled water. This solution was heated twice to 120°C in an oven before being applied thinly to the microscope slides to avoid microbiological contamination. After sampling, the gel was dissolved in 5 ml warm sterile distilled water, diluted, and spread on tryptone soya agar (TSA) plates. The sampling rate was controlled by placing a 17.5 l min−1 critical orifice between the sampler and the vacuum source. The mass mean diameter was calculated by plotting the cumulative percentage collected against the 50% cut off of each stage on probability table graph paper. The mass mean diameter was read off the plot at the 50% probability value.
2.2.4 Test micro-organisms
Suspensions of washed Bacillus atrophaeus spores (NCTC 10073; ca 1 × 105 colony-forming units (CFU) per ml (CFU ml−1)) were prepared in 0%, 0.05%, 0.1%, and 5.0% solutions of potassium iodide (KI) in 80% aqueous ethanol. The size of the particles produced depended on the concentration of the KI in the suspension.
2.2.5 Environmental room
The room used was 28 m3 in volume with an optional flow of horizontal clean air supplied through a bank of HEPA filters. Microbial aerosols were generated in still air inside the chamber as described below. At the end of the sampling period, the room was vented by supplying a horizontal flow of clean air for 10 min. After re-setting of the samplers another microbial cloud containing bacterial spores was generated and the experiment was repeated. The ability to flush the room with clean air to remove airborne organisms allowed a large number of bacterial aerosol challenges to be carried out over relatively short periods.
The spinning top aerosol generator STAG Mark 2 (Bristol Industrial and Research Associates, Portishead, Bristol, UK) (Fig. 4) was used to generate particles of different sizes containing viable Bacillus atrophaeus spores. This was done by preparing suspensions (ca 105 CFU ml−1) of the spores in 0–7% (w/v) solutions of potassium iodide (KI) in 80% aqueous ethanol. The suspensions were injected at a rate of approximately 1 ml min−1 into the STAG, operating at 48,000 rpm, using a peristaltic pump. The size of the particles produced depended on the concentration of KI in the suspension. The liquid associated with the generated droplets evaporated completely in less <1 s at the test relative humidity (40–60%). The concentration of spores in the suspension was low enough to ensure that the vast majority of particles were mono-dispersed.
2.2.6 Microbiological assays
2.2.6.1 Media
Tryptone soya agar plates (90 mm) for use in all the physical efficiency testing were provided by Biomerieux.
2.2.6.2 Microbiological assays
All plates used in the samplers and those used for the cellulose nitrate filters were incubated at 37°C for up to 18 h before counting the colonies. The cascade sampler slides were placed into sterile 50 ml falcon tubes with 5 ml sterile distilled water, vortex mixed, and diluted via serial dilution in sterile distilled water before being plated (0.2 ml) on to TSA plates. All liquid samples from the Coriolis air sampler (0.2 ml) were plated out on to the correct agar plates for the test carried out and were incubated at 37°C for up to 18 h before counting the colonies. The volume of the samples was measured from the difference in weight of the collection tube before and after the sample had been collected.
2.2.7 Test procedures
2.2.7.1 Physical efficiency
The aerosols were generated from the various spore suspensions by the STAG Mark 2 as described above. The samplers were arranged in a semi-circle at a distance of 1 m from the STAG in the environmental room. The STAG was 1 m off the floor and a fan was operated immediately below the STAG at a distance of 0.4 m from the floor. The heads of all the filter samplers were level with the Coriolis inlet. The nebulisation, sampling regimes, and fan speed were chosen after conducting a number of trial and error preliminary experiments. Conditions were chosen so that a reasonable number of colony-forming units (25–250 per plate) would be produced following incubation. The nebulisation was started remotely 5 s before the sampling process was started. The nebulisation and sampling times were 5 min.
When the cascade impactor was used, the flow rate was controlled at 17.5 l min−1 by insertion of a critical orifice in the line between the sampler and the vacuum source.
The aerosol concentration and total CFU collected were calculated as follows:Filter samplers:
Coriolis air sampler:
The physical efficiency percentage is determined by:
2.3 Biological collection efficiency
2.3.1 Coriolis air sampler sampling
The Coriolis δ air sampler is completely dedicated to outdoor air monitoring of airborne spores and pollen grains. This air sampler offers a wide range of settings (air flow rate up to 300 l min−1, collection time) that can easily be adjusted by use of an ergonomic display and key-pad. The standard model is available with a 250 ml sampling liquid tank in a weather-proof metal case mounted on a tripod. The equipment is connected to a wind-vane that keeps the air inlet pointing into the wind (Fig. 5). The dimensions of the cyclone (∅40 mm) were determined by homothetics relationships and following the geometrics of Stairmand cyclones (Leith-Licht 1972). This cyclone design is adapted for a 200–350 l min−1 flow rate. An optimal air velocity in the inlet cyclone has been calculated for this configuration in order to guarantee a high separation efficiency of micronic particles. For this study, an air flow rate of 200 l min−1 was selected and the time period for sampling was 6 h (from 10:00 am to 16:00 pm).
The Coriolis air sampler was located in a garden, 5 m above ground level, in an urban area (St Genis l’Argentière, France), 480 m a.s.l. The climate is temperate, South-West winds are predominant. All around, there are meadows and some forest with hazel, birch, ash, alder, oak, and beech. During 2007, daily samples of air were collected from March to September. The sampling ran from 10 am to 16 pm. Before sampling started, tests were carrying out to ensure:
-
1.
a full sampling liquid tank;
-
2.
no presence of air bubbles in the continuous injection tube;
-
3.
settings: 200 l min−1 of air flow and auto mode of injection.
At the end of the 6 h, the cone was taken off and the equipment was switched off. The pollen grains were concentrated in the liquid cone.
The liquid was measured in the laboratory. An average of 13.22 ml of final liquid was obtained, for all tests carried out. Five aliquots of 1.5 ml were put in a freezer, at −18°C for ELISA tests. When the liquid sample had been centrifuged for 15 min at 4,000 rpm, the supernatant was extracted and the residue was homogenized by vortex mixing and 100 μl of the residue was placed on each of three identified slides. Each slide was left on the bench to dry, then one drop of stained gelled liquid was put on it. The rest of the residue was kept in an Eppendorf tube in the freezer.
Seven horizontal lines were read with an optical microscope. For recognition of pollen, ×400 magnification was applied. Pollen count and identification were analysed, trying to classify the different types. The objective was positioned over the top left hand corner, and moved in 3-mm distances. All the horizontal lines should be read (52 mm, the size of the cover slip), and the operator must repeat this operation every millimetre at 3, 6, 9, 12, 15, 18 and 21 mm.
2.3.2 Hirst spore trap
The Hirst spore trap (Hirst 1952) is the standard sampler for recording the atmospheric concentration of pollen grains and fungal spores. It is a suction sampler based on the basic principle of impaction, with a 2 × 14 mm intake orifice through which the sampled air is impacted on to a collection surface moving at 2 mm h−1. These samplers can run for a week or can incorporate a daily mechanism. The air flow is 10 l min−1.
The Hirst samplers used by RNSA were designed by Burkard or Lanzoni. They have the same sampling features and consist essentially of three units—the impactor unit with an adhesive-coated transparent tape placed on a drum rotating once a week, the wind-vane mounting, and the motor housing. A vane tail keeps the cylindrical housing facing the wind. The motor housing contains a vacuum pump and equipment for measuring the rate of suction.
In the weekly sampler, the tape is mounted on the drum. The tape is 336 mm long and correspond with the 7 days of the week. Once the tape is on the drum the adhesive must be applied with the aid of a brush. If Vaseline is used the person who is mounting the tape applies it by slightly and continuously turning the drum without stopping, ensuring that a very fine and uniform layer is obtained. If silicone fluid is used the operator should let the brush slide slowly, once only, and exclusively in one direction. In this case, the thickness of the layer of silicone does not matter, because within 30 s the tape dries and is ready to be used.
As the drum rotes at a 2 mm per hour, and the Melinex tape is 336 mm long, the operator must cut, in the laboratory, seven 48 mm pieces (each one corresponds to one day) with the aid of a transparent ruler. These pieces of tape are mounted on the slides with a mounting medium (Galán 2007; Spanish Aerobiology Network REA Management and Quality Manual). The most frequently used mounting media are: glycerol jelly, poly(vinyl alcohol), and poly(vinyl lactophenol). It is important to add phenol to this media to prevent microbial growth. In pollen grain collection, basic fuchsin is always used to stain the samples.
Reading from the whole of the sample in four horizontal lines for a sample of 360 min, the microscope must be at ×400 magnification. The adjustment for the position of the microscope was done at the top corner, on the left of the slide. From the time of drum replacement and drum rotation, the time the Coriolis was started was located and the objective was moved forward by the corresponding number of millimetres to reach the hour. The objective should be positioned forward to X = 5 mm, and the analyst can read one horizontal line of 12 mm (=6 h). After having moved the objective forward by 3 mm, the analyst can read the three other horizontal lines of 12 mm (corresponding to 8, 11, and 14 mm of the slide).
2.3.3 Microscopic assays
The pollen taxon is determined by use of an identification key. A CD “The pollen content of the air”, made by RNSA, enables identification of pollen from the main taxa.
In the living pollen grain, the wall is made up of two layers; the outer layer is called the exine and is composed of a very unusual substance, sporopollenin. The inner layer, or intine, is made up of cellulose and is very similar in construction to an ordinary plant cell wall. Under the light microscope, the exine can be observed and bears all the morphological characteristics. For identification of any grain, there are three features to note:
-
aperture type and number;
-
shape and size of grains; and
-
exine sculpturing.
The apertures are generally thin or missing parts of the exine through which the pollen tube emerges at germination on a stigma.
2.3.4 Statistical methods
The Mann–Whitney (Wilcoxon) W test (nonparametric test) was performed to compare medians rather than means. The W test was used to determine whether differences between the medians obtained from both methods (Hirst spore trap and Coriolis air sampler) were significant or not significant. The results are regarded as significant at P < 0.05. Statistical analysis was performed with the software Stagraphics Centurion XV.II (Statpoint).
3 Results
3.1 Physical collection efficiency of the Coriolis air sampler
3.1.1 Experimental
The results from physical sampling studies using the Coriolis air sampler are shown in Graph 1 and in Table 1. The results of this part of the study show that the sampler had a physical sampling efficiency of between 40% and 50% for the two lowest particles sizes and a sampling efficiency of 84% for 4.6-μm particles and 92% for the 10-μm particles.
3.2 Biological performance of the Coriolis air sampler
3.2.1 Pollen grain concentrations
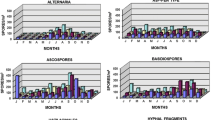
Daily amounts of pollen grains and spores were expressed as an average number of particles m−3. Total pollen grains and spores were expressed as in index. The total quantity of all pollen grain and spore counts is larger for the Coriolis air sampler, but for some species the total number is larger for the Hirst spore trap than for the Coriolis air sampler from March to September 2007. The statistical comparison W test (Table 2) with a P-value >0.05 led to the conclusion that the medians of the two populations are not significantly different. The Hirst trap and the Coriolis air sampler gave equivalent results for pollen grain concentrations during the period studied.
To demonstrate the biological efficiency of the Coriolis air sampler, the temporal distribution of pollen concentrations (pollen grain per cubic meter) was represented for the two traps, first for Betula pollen grain (Graph 2) and second for the all pollen grains (Graph 3). The results obtained show a similar level of pollen concentration for specific species and total pollen grains, and curves with peaks on the same days.
3.2.2 Pollen grain diversity
By comparing the number of different pollen types in the samples we observed higher diversity for the Coriolis air sampler than for the Hirst spore trap (Graph 4). During the different pollen seasons we detected an average of 10.3 different pollen types for the Coriolis air sampler, with a maximum of 24 taxa, compared with 8.3 for the Hirst spore trap, with a maximum of 14.
4 Discussion
The results from the physical sampling studies on the Coriolis air sampler are shown in Graph 1 and in Table 1. The results of this part of the study show that the Coriolis air sampler had a physical sampling efficiency of between 40% and 50% for the two lowest particles sizes and a sampling efficiency of 84% for 4.6-μm particles and 92% for the 10-μm particles. We have verified that the theoretical performance calculated by Bertin and based on a dry cyclone design was similar to the experimental one. For particle diameters above 10 μm (diameter range for pollen grains is between 10 and 120 μm), the sampling efficiency is stable and superior to 90% with a 40 mm cyclone diameter.
The Hirst spore trap is a reference method in aerobiology (Hirst 1952). Comparison of the results for spores/pollen grains concentration from the Coriolis air sampler and the Hirst spore trap shows that the Coriolis air sampler obtains temporal distribution comparable with that of the Hirst spore trap and statistical comparison (W test, alpha:0.05) confirms that the medians of the two populations are not significantly different. The Coriolis air sampler performed with equivalent sampling efficiency for spores and pollen grains during the period studied in relation to the traditional method (Table 2). The results are better for pollen grain concentrations, particularly when pollen grains are present in important amounts. It is important to point out the better diversity of the Coriolis air sampler than Hirst spore trap. The Coriolis air sampler is a continuous air sampler dedicated to outdoor monitoring of airborne spores and pollen grains.
The information provided by monitoring airborne spores and pollen grain cannot be ignored by the allergist or by allergic individuals who must respond, but there are important divergences observed with clinical observations which justify a new approach to monitoring airborne allergens. The French-led project MONALISA with financial support from the European Commission-LIFE is testing this innovative air sampler in order to measure the antigenicity/allergenicity of the main aeroallergens particles, i.e. Betula (birch), Poaceae (grasses), Parietaria (pellitory), Olea (olive tree), Artemisia (mugwort), and Alternaria (fungal spores) to validate a new monitoring approach instead of quantifying pollen grains by their morphology. The robustness and efficiency of the MONALISA system is being demonstrated at a national level throughout Europe in eight different countries with different bio-climatic and topography characteristics: France, UK, Finland, Poland, Spain, Portugal, Switzerland, and Italy. Among others, this program confirms the divergences between airborne spores and pollen grain count, with airborne allergens load (Thibaudon and Sindt 2008). Those open results will lead to continued studies to ensure enhancement of the capacity for air-quality assessment and the delivery of better information for clinicians and atopic people.
This innovative solution based on patented Coriolis technology delivering a liquid sample into a single vial enables rapid analysis by several techniques including PCR and serological assay (ELISA) in order to measure pollen grain and fungal spore antigenicity/allergenicity. Traditional counting of pollen grains or species identification by optical microscopy can also be done. A new approach could be also carried out with Coriolis cyclonic dry sampling versus cyclonic wet sampling.
The monitoring of spores and pollen grains is of direct interest to aerobiology, medical health, forestry, and plant science, for providing time-to-time data, determining rate of allergenicity in air, or pollen grain count and spore concentrations.
References
Decker, H. M., Buchanan, L. M., Frisque, D. E., Filler, M. E. & Dahlgren, C. M. (1969). Advances in large-volume air sampling. Contamination Control, August, 13–17.
ISO. (2003). ISO 14698-1, Clean rooms and associated controlled environments—bio contamination control. Part 1: General principles and methods. International Organization for Standardization Geneva, Switzerland
D’amato, G. (1995). Pollen allergy in the Mediterranean area. Revue Francaise d’Allergologie, 38, 5160–5162.
Leith-Licht (1972). The collection efficiency of cyclone type particle collectors: A new theoretical approach. AIChE Symp. Ser. Vol. 68, no. 126, pp. 196–206.
Galán M. et al. (2007). Spanish Aerobiology Network (REA)—Management and Quality Manual Servicio de publicaciones de la Universidad de Córdoba.
Hirst, J. M. (1952). An automatic volumetric spore traps. Annals of Applied Biology, 39, 257–265.
May, K. R. (1945). The cascade impactor: An instrument for sampling coarse aerosols. Journal of Scientific Instruments, 22, 187–195.
Thibaudon, M., & Sindt, C. (2008). Measure of airborne tree pollen allergens (birch, olive). Revue française d’allergologie et d’immunologie clinique, 48(3), 179–186.
Acknowledgements
This study was partially supported by the European Community (DG, Belgium) with the Life-Environment financial instrument.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carvalho, E., Sindt, C., Verdier, A. et al. Performance of the Coriolis air sampler, a high-volume aerosol-collection system for quantification of airborne spores and pollen grains. Aerobiologia 24, 191–201 (2008). https://doi.org/10.1007/s10453-008-9098-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-008-9098-y