Abstract
The shallow Vrana Lake in Dalmatia, Croatia, is directly connected to the Adriatic Sea by the Prosika canal (0.85 km), constructed in the eighteenth century. The aim of this research was to examine the impacts of salinization on long-term changes in littoral macro-crustacean (Malacostraca) populations and assemblages in Vrana Lake and connecting canals. Benthic macroinvertebrates were sampled at seven sites during the period 2011–2020 (14 months) using a hand net (25 × 25 cm, 500 µm). During the study period, saltwater intrusions (strongest in 2012) through the Prosika Canal and site V4 caused increased salinization, resulting in a shift from normal oligohaline (0.5–5 PSU) to mesohaline (5–18 PSU) salinity. Out of a total of 18 macro-crustacean taxa identified, five widespread species—amphipods Echinogammarus stocki and Gammarus aequicauda, isopods Lekanesphaera hookeri and Proasellus coxalis, and decapod Palaemon antennarius constituted 91.6% of collected specimens (26,986). The first three brackish/marine species were significantly positively correlated with the average salinity 12 and 24 months before sampling. They disappeared or had very low abundance during the second low salinity phase (< 1–2 PSU), when freshwater/oligohaline P. coxalis and P. antennarius were the dominant macro-crustaceans. Two years of low salinity were needed for a strong population decline or disappearance of three brackish/marine species. Salinization is a major stressor in the Vrana Lake basin, and measures for its active control are urgently needed. The use of tested macro-crustacean metrics is recommended for future ecological monitoring as it could provide fast information about the effects of water management on aquatic biodiversity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Accelerated anthropogenic salinization, i.e. human caused increase of ion concentration in inland and coastal waters, has been reported in many parts of the world in the last few decades (Williams 2001; Dugan et al. 2017; Mastrocicco 2021). Due to unprecedented geographic scale at which it is occurring (Herbert et al. 2015), anthropogenic salinization now represents a widespread threat to the biodiversity, functioning, and services of fresh and brackish water ecosystems (Cañedo Argüelles 2020; Cunillera-Montcusí et al. 2022). In the Mediterranean region, which has been identified as one of the global climate change hot spots, a pronounced decrease in precipitation and increase in temperature (Giorgi 2006; Giorgi and Lionello 2008) can lead to increased salinity (Jeppesen et al. 2015). Intensification of these trends is predicted for the future (IPCC 2014; Jiménez Cisneros et al. 2014). Mediterranean coastal (tidal) and inland waters are especially sensitive to anthropogenic salinization because they are already affected by multiple other anthropogenic stressors such as land-use change, reductions of freshwater flow, organic and chemical pollution, increases in temperature, and invasive species (Herbert et al. 2015; Jeppesen et al. 2015; Martínez-Megías and Rico 2022).
In coastal systems, minimums and maximums of salinity occur as the result of the diurnal rhythm of low and high tides, after increases in freshwater discharge, and from strong winds and storm tides. Thus, coastal systems are characterized by a complex combination of fresh (0–0.5 PSU), brackish (0.5–30 PSU), and marine (> 30 PSU) conditions with specific aquatic biota. Freshwater environments are characterized by a predominance of halosensitive (stenohaline) species, which tolerate small changes of salinity. In contrast, brackish water environments, depending on prevailing salinity conditions, are characterized by various combinations of freshwater/oligohaline and more halotolerant brackish/marine euryhaline species that can tolerate large salinity variations (Williams 2001; Herbert et al. 2015; Cunillera-Montcusí et al. 2022). Salinization is especially problematic for freshwater and oligohaline (0.5–5 PSU) aquatic systems, where increasing salinity can have strong negative effects on the biota. As salinity increases, high diversity freshwater (limnic) and oligohaline assemblages will disappear and are replaced by an assemblage of euryhaline brackish/marine species with lower biodiversity (Williams 2001; Cañedo Argüelles 2020).
Benthic macro-crustaceans (class Malacostraca: orders Amphipoda, Decapoda, Isopoda, Mysida) are potentially good indicators of salinity changes since a wide variety of species are adapted to life in fresh, brackish, and marine waters. Thus, increased salinity from fresh to brackish water would be expected to cause significant changes in benthic macro-crustacean assemblages. Such changes have rarely been subject of long-term studies in one aquatic system (Attrill and Power 2000; Poizat et al. 2004; Boets et al. 2011). Studies of benthic macroinvertebrates in estuaries have established that rising salinity leads to expansion of brackish water and marine fauna (mostly macro-crustaceans), while limnic species retract toward head of the estuary (Attrill et al. 1996; Attrill and Power 2000; Little et al. 2017). However, our understanding of long-term changes of aquatic systems caused by salinization is limited and more such studies are needed (Cunillera-Montcusí et al. 2022). This is especially important for management of Mediterranean coastal and inland waters already heavily affected by multiple stressors and climate change (Jeppesen et al. 2015; Martínez-Megías and Rico 2022).
Many examples of Mediterranean aquatic systems exist in which salinization was identified as one of the multiple stressors (Mastrocicco 2021; Martínez-Megías and Rico 2022). The Vrana Lake in Croatia (Dalmatia region) is one of such examples. This shallow lake was artificially connected to the Adriatic Sea by the Prosika canal in the eighteenth century (Katalinić et al. 2012). In the last two decades this brackish (oligohaline) lake has experienced periods of increased salinity caused by intrusions of saltwater that entered the lake through the Prosika Canal and through permeable rock in the karst ridge. This was especially pronounced during very dry years of 2003, 2008, and 2012 (Katalinić et al. 2012; Rubinić and Katalinić 2014). However, effects of changes in salinity on aquatic biota of the Vrana Lake in Dalmatia are poorly understood.
In this study we examined long-term changes (2011–2020) of benthic macro-crustacean (Malacostraca) fauna in the Vrana Lake (littoral zone) and in connecting canals. During this period the Vrana Lake experienced the lowest and highest water levels in 74 years of water level record, with corresponding peaks of salinity and subsequent return to oligohaline conditions. The presence of both natural and artificial connections with the Adriatic Sea led to the hypothesis that more halotolerant brackish water macro-crustacean species will increase spatial distribution and abundance in Vrana Lake with increasing salinization, while oligohaline species will experience population decline and decrease in distribution. In order to test this hypothesis, long-term changes of benthic macro-crustacean assemblages, as well as population dynamics and distribution of dominant species were examined. Continuous records of water level, physical–chemical parameters at all monitoring stations were used to examine effects of salinity on spatial and temporal variations of density and distribution of dominant macro-crustacean species. Also, changes of macro-crustacean assemblages in relation to salinity were examined, as well as effects of past salinity conditions on density of dominant species and potential use of macro-crustacean metrics for ecological monitoring of salinization in the lake and its tributaries.
Materials and methods
Study area
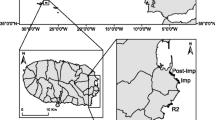
Vrana Lake (Vransko jezero) in Dalmatia has the largest surface area (31 km2) of any natural lake in Croatia (Rubinić 2014). The lake is located in the region of Ravni Kotari (North Dalmatia) between the towns of Zadar and Šibenik in close proximity to the Adriatic Sea (Fig. 1). The watershed covers 515 km2 (Stroj 2012) and land cover is predominantly agriculture and Mediterranean scrub. The watershed is underlain by Cretaceous and Eocene limestones, Cretaceous dolomites and Eocene flysch, and characterized by karst (Fritz 1984). The lake is 13.6 km long and 1.4–3.4 km wide with a NW–SE orientation parallel to the Adriatic Sea and is separated from the sea by a limestone ridge ranging from 0.8 to 2.5 km wide. Vrana Lake fills a geologic cryptodepression with the maximum depth of 5 m and an average water depth of 2 m (Šiljeg et al. 2015). The water level has significant seasonal variations (range 0.03–2.25 m a.s.l.) caused by seasonal rainfall and periodic droughts (Rubinić 2014). Salinity in the lake changes as the result of seasonal intrusion of seawater through permeable karst and Prosika canal (Rubinić and Katalinić 2014). The southern coast of Vrana Lake is mainly rocky and stony with sparse reed beds (Phragmites communis) due to the dominant direction of wind and waves, while the north and east coast has a continuous reed bed belt. The lake is one of the most important wetland areas on Croatian part of eastern Adriatic coast and due to its high importance for biodiversity it was protected as Nature Park, Natura 2000 and Ramsar site (Katalinić et al. 2012).
Vrana (Vransko) Lake Nature Park (grey borders) and seven sampling sites shown as red circles. Yellow triangles represent three coastal monitoring sites (Croatian Waters-CW, 2002–2020), which are the same as sampling sites K, V1 and V3, while green triangles represent open water monitoring sites (Nature Park-NP, 2008–2020). Black triangles represent three gauging stations. Upper inset map shows the position of the Vrana Lake in Croatia. The lower inset map shows the position of four sampling and three monitoring sites near the Prosika canal. (Sampling sites: K-Kotarka canal, V1-Crkvine, V2-Drage, V3-Prosika-lake, V4-Jugovir, P1-Prosika canal-1, P2-Prosika canal-2)
The Vrana Lake watershed is hydrologically complex (Rubinić 2014). Water is supplied to the lake by a system of drainage canals and underground springs within the watershed. Discharge from the canals is strongly influenced by the seasonal precipitation, high in winter and low in summer months (Rubinić and Katalinić 2014). Two largest tributaries draining into the lake are the Kotarka Canal and Lateral Canal (Fig. 1). Additionally, Lake Vrana is hydrologically connected to the Adriatic Sea through permeable karst in the limestone ridge and the artificial Prosika Canal. The most important natural karst connection with the sea is Jugovir (site V4) at the southwest side of the lake (Fig. 1). Lake water flows to the Adriatic Sea through the Jugovir and Prosika Canal when water levels are greater than 45 cm at gauging station G2 and sea water flows into the lake when water levels are below 45 cm, depending on the tide (Rubinić personal communication). The 0.85 km long Prosika Canal was constructed in the eighteenth century where the limestone ridge was the narrowest. Channelization of tributaries, water extraction from the canals and main springs for water supply and irrigated agriculture (Rubinić 2014), as well as extensive historical land use change in the watershed all contributed to reduction of freshwater inflow to the lake, especially in summer months. In last few decades these changes were combined with climate change induced warming, more frequent droughts, and relative sea level rise all contributing to more intensive salinization of the lake (Rubinić and Katalinić 2014).
Hydrological and water quality data
Water level data were collected from three gauging stations operated by the Croatian Meterological and Hydrological Services (Fig. 1). Station G1 is located on the Kotarka Canal at the Pakoštanski Bridge (300 m upstream from the mouth) at the northern end of the lake and has a continuous record since 1947. Station G2 is located in the lake close to Prosika Canal and has operated since 1948 but is lacking data for 2017 and part of 2018 and 2020. Station G3 is located in the Adriatic Sea close to the mouth of the Prosika Canal and has operated since 1986.
Croatian Waters, the national agency for water monitoring, provided physical and chemical composition data for three monitoring locations along the coast of the lake, which are the same as macroinvertebrate sampling sites K, V1, and V3 (Fig. 1). Surface water parameters were measured at each location and water samples were collected monthly or every two months during the period 2002–2020. Croatian Waters used standard analytical methods to assess surface water quality (ISO norms). For the purposes of this study, we used five parameters: water temperature (°C), pH, specific conductivity (μS/cm), dissolved oxygen (mg O2/L) and oxygen saturation (%). Additionally, the Vrana Lake Nature Park monthly measured six parameters (water temperature, oxygen concentration and saturation, specific conductivity, salinity and pH) using a WTW probe at three open water locations (V5-V7) in the period 2008–2020. Missing values of conductivity and salinity at Croatian Waters monitoring sites (K, V1 and V3) were replaced by measurements in corresponding month from our own measurements during sampling or from Natural Park monitoring dataset.
Specific conductivity data were used to calculate practical salinity units (PSU) using the equation (Eq. 1) of Wagner et al. (2006).
In this equation, Kn are a set of empirically derived constants and R is the ratio of the specific conductivity to the reference conductivity of 53,087 μScm−1. Five salinity classes were distinguished, each defined by a range of salinity, according to the ‘Venice System’ (Caspers 1959): freshwater (0–0.5 PSU), oligohaline (0.5–5 PSU), mesohaline (5–18 PSU), polyhaline (18–30 PSU) and euhaline (30–40 PSU).
Field sampling and sample processing
Benthic macroinvertebrate samples were collected using a hand net (25 × 25 cm, 500 µm) at seven sites (Fig. 1) from March 2011 to December 2020. There were 14 separate sampling events (months) at five sites (K, V1-V4) in the 7 years between 2011 and 2020 as sampling was not conducted in 2012, 2013 and 2015. At five sites, five months were sampled in 2011 (3, 5, 7, 10, 12), two months (5, 10) in 2014 and three months (3, 10, 12) in 2020, while in the rest 4 years (2016–2019) sampling was conducted once per year. At site P1 sampling was conducted from 2016 to 2020, while at site P2 from 2014 to 2020, seven times at both sites. At sites V1, V2, V3 and V4, located along south lake coast, samples were collected in deeper areas of the littoral zone, at site K in shallow accessible parts on the right bank of the Kotarka Canal (200 m upstream from the mouth). At sites P1 (lake side) and P2 (sea side, i.e. mouth of the canal) the whole width of the Prosika Canal was sampled. Prior to macroinvertebrate sampling water temperature, dissolved oxygen concentration and saturation, specific conductivity, salinity and pH were measured using a WTW probe. In 2011, 2014, and 2016, three quantitative replicate samples of macroinvertebrates (3 × 0.0652 m−2) were collected. From 2017 through 2020, five quantitative replicate samples (5 × 0.0652 m−2), were collected at each site. From 2016 to 2020 additional qualitative samples (kick samples for 5 min) were collected on submerged parts of the aquatic and land plants. Densities (ind. m−2) were calculated from quantitative samples by dividing number of collected individuals by sample area and averaged for replicate samples. Most samples were collected on stony substrate (mostly microlithal + mesolithal, at V3 mesolithal + macrolithal), except at site K where substrate was different and consisted of mud, fine sand, and parts of dead plants (mostly reed). Macroinvertebrate samples were fixed in the field with 96% ethanol and later processed in the laboratory, where all macroinvertebrates were separated from the samples using stereomicroscope.
Macro-crustacean specimens (class Malacostraca) were identified using determination keys for different groups: Amphipoda (Pinkster 1993; Bellan Santini et al. 1982, 1993), Decapoda (Froglia 1978; Tzomos and Koukouras 2015), Isopoda (Argano 1979; Henry and Magniez 1983; Jacobs 1987; Poore 2001; Kemp et al. 2020), and Mysida (Wittmann et al. 2016).
Statistical analyses
The relationship between salinity and average density of macro-crustacean species per site was established with Spearman correlation. All sites were combined to calculate spatial–temporal correlations between salinity and other four physical–chemical parameters measured during sampling and the density of five dominant crustacean species. Temporal correlation analyses (density vs. salinity) could be conducted at four sample locations (K, V1, V2, V3), due to availability of continuous salinity data, whereby for site V2 salinity data from nearby monitoring site V6 were used. In these analyses, salinity in the month of sampling (0), and average salinities for 6, 12, and 24 months before sampling were used. Due to multiple testing, Bonferroni correction was applied for significance of these correlations. Spearman correlation coefficients (rs) in correlation matrix were calculated using the ‘Hmisc’ package (Harrell 2023) in R (R Core Team 2023).
Benthic macro-crustacean assemblages were analysed using different multivariate analyses. Prior to analyses, the abundance matrix (average per site-month-year) was square root transformed to reduce effects of dominant species, followed by calculation of similarity matrix using the Bray–Curtis index. Analyses were done in two ways: (1) using only quantitative samples and (2) using all (quantitative + qualitative) samples. Since similar results were obtained by both methods, results were shown only for analyses with all samples. Differences between sites and salinity periods were visualized using non-parametric multi-dimensional scaling (nMDS) and tested with one or two-factorial PERMANOVA (factors were fixed) in PRIMER 6 + PERMANOVA (2009 PRIMER-E Ltd.). Due to multiple testing for each site separately, Bonferroni correction was applied for significance of one-factorial PERMANOVA tests. Indicator species analysis was used to examine how the relative abundance and occurrence of the five dominant species contribute to differences between salinity periods. The Indicator Value (IndVal) index was used, which combines a species' relative abundance (specificity) with its relative frequency of occurrence in a group of samples (fidelity) (Dufrêne and Legendre 1997; Legendre and Legendre 2012).
In our case, for specificity (Akj), Nindividualskj represents the mean abundance of species j across the months within a cluster (i.e., salinity period) and Nindividuals+k is the sum of the mean abundances of species j within salinity periods. For fidelity (Bkj), Nmonthkj is the number of months in salinity period k where species j was present and Nmonthk+ is the total number of months in that period. IndVal indices were calculated for the five dominant species in each group of months (salinity period), separately for each site, as well as for the five sites combined together which were sampled in all four salinity periods (K, V1-V3). IndVal calculation was performed using the 'labdsv' package (Roberts 2023) in R (R Core Team 2023). Significance (permutation) tests for the association between a species and a salinity period were conducted using the 'signassoc' function from the 'indicspecies' package (De Cáceres and Legendre 2009).
Six macro-crustacean metrics were calculated (EsGaLh-a, EsGaLh-d, %EsGaLh, PcPa-d, PcPa-a, %PcPa; a-abundance, d-density, %-percentage of summed abundance in total abundance of all macroinvertebrates), for each site and month using two different combinations of five dominant macro-crustacean species (see caption of Table 1 for species abbreviations). The relationship between physical–chemical parameters and six macro-crustacean metrics was established with Spearman correlation.
Results
Water level and salinity
Water level in the lake varies on an annual basis with maximums during the wet season (January–April) and minimums during the dry season (August–October) (Fig. 2a). Relative average monthly sea levels at gauging station G3 showed greater variability, with one or more peaks in different months, mostly increasing at the end of a year (often with strongest peaks in autumn). During the time period from 2002 through 2020, 11 years had minimum average monthly lake water levels fall below average monthly sea levels. In seven of these years (2002, 2003, 2010, 2015, 2017, 2019, 2020) lake water levels were briefly (1–3 months) and only slightly below relative sea levels during the driest months of the year. However, during the intensive drought period in 2007/2008, lake levels were a minimum of 24 cm below sea level for 7 months. During the longest drought in 2011/2012, lake levels were for 12 months with a minimum of 33 cm below sea level. These two drought periods were associated with the most intensive and longest salinization of the lake. The highest maximum salinity values ever recorded at all lake sites (from 12.2 PSU at V1 to 18.3 PSU at V7) occurred in 2012 during the period from September to November (Fig. 2b). Salinity in the Kotarka Canal never exceeded 5 PSU during drought periods.
Temporal changes of water level and salinity in the period 2002–2020: a average monthly water levels at gauging stations G1 (Kotarka canal), G2 (Vrana Lake-Prosika) and G3 (Prosika-sea); b monthly values of salinity in the Kotarka canal (K) and at three sites in the Vrana Lake (V1, V3, V6; sites V5 and V7 are omitted for clarity, for site V6 data are available for period 2008–2020)
During the 8 years when minimum average monthly water levels did not fall below relative sea levels, salinity was usually below 2 PSU at lake sites (V1, V3, V5-V7), with slight increase during summer/autumn months. Exceptions were 2009, 2010 and 2013 when salinities in the lake were higher (2–5 PSU) and decreased after previous salinity peaks in 2008 and 2012. In the same years, the Kotarka Canal (K) had higher annual oscillations than the lake sites, lower salinity during the winter/spring months and higher salinity (2–4 PSU) during summer/autumn months. Salinity maximums for the lake sites usually followed one to 2 months after maximums for Kotarka (K). Statistically significant negative correlations between water level at gauging station G1 and salinity at all monitoring sites were established, with stronger negative correlation at monitoring site in the Kotarka Canal, than those in the lake (Spearman correlations, Kotarka (K): rs = − 0.86, p < 0.001; lake sites V1-V6: rs = − 0.4 to − 0.59, p < 0.001).
In order to relate salinity changes with population changes of dominant macro-crustaceans, several different salinity periods could be distinguished between 2011 and 2020: 2011—increasing salinity (sampled in 5 months Mar–Dec), 2012—peak salinity (not sampled), 2013—decreasing salinity (not sampled), 2014—first low salinity period (< 2 PSU) after peak salinity (sampled in May and Oct), 2015 to first half of 2020—second low salinity period (sampled five times from Sep 2016 to Mar 2020), second half of 2020—second period of increasing salinity (sampled twice in Oct and Dec 2020; Fig. 3). In 2020, salinity started to increase above 2 PSU at the beginning of August and peaks were in October/November (Fig. 2b).
Average densities (a–e) or average abundance (f) of five dominant species of benthic macro-crustaceans in 14 sampling months (March 2011–December 2020) at seven study sites: a Echinogammarus stocki, b Gammarus aequicauda, c Lekanesphaera hookeri, d Proasellus coxalis, e Palaemon antennarius-density, f P. antennarius-abundance. At sites P1 and P2 sampling was conducted in seven months (May 2014–Dec 2020) (n.s. – not sampled in 2012, 2013 and 2015)
Site Jugovir (V4) is the most important natural connection between Vrana Lake and the Adriatic Sea. In July, October, and December of 2011, and again in October of 2020 salinity at V4 increased to between 30 and 40 PSU. In October of 2020, salinity also increased to greater than 30 PSU in the Prosika Canal (P1, not measured in 2011). In both years, the salinity increase occurred when lake levels were lower than sea level and that salinity was driven by saltwater intrusion.
Diversity and distribution of benthic macro-crustaceans
In a total of 26,986 macro-crustacean specimens collected, 18 taxa were identified (13 species and 5 genus) from four orders within Class Malacostraca (Table 1). Amphipods were the order with the most taxa (10), whereas orders Isopoda, Mysida, and Decapoda all had less than five. The greatest number of taxa (15) were found at the connection of the Prosika Canal (P2) with the Adriatic Sea, whereas all other sites had 5–7 taxa.
Five widespread species, amphipods Echinogammarus stocki G. Karaman, 1970 and Gammarus aequicauda (Martynov 1931), isopods Lekanesphaera hookeri (Leach 1814) and Proasellus coxalis (Dollfus 1892), and decapod Palaemon antennarius H. Milne Edwards, 1837 constituted 91.6% of collected specimens. These five species were found at most of the seven sampling sites. Amphipod E. stocki was never found at Kotarka (K) and the isopod P. coxalis was extremely rare, with only one recorded specimen at site P1, and was never found at site P2. Amphipods Niphargus sp. and Orchestia sp. were found only at lake sites (V1-V4) and at Kotarka (K). All other species were found infrequently only at site P2, except Melita palmata (Montagu 1804) also found at V4 in Oct and Dec 2011, and typically were associated with higher salinities (Table 1).
All of the five more abundant species found in the lake system can tolerate salinities ranging from the lower limit of oligohaline to marine conditions. The lowest median (or average) values of salinities (Table 1), were observed for the decapod P. antennarius and the isopod P. coxalis, and the other three dominant species (E. stocki, G. aequicauda, L. hookeri), had medians and averages of salinity in upper oligo- to mesohaline class.
Benthic macro-crustaceans were found in almost all samples from all seven studied sites, with the exception of March 2011 and September 2016, when they were not present in the benthic samples at site V1. Of the five dominant macro-crustacean species, the decapod shrimp P. antennarius was found for the first time in September 2016 in the Kotarka Canal (Fig. 3), where it already reached the highest recorded density of 235 ind. m−2. By October 2017, P. antennarius had appeared at three new sites (V3, V4 and P1), and by March 2019 it was recorded at all seven study sites. When found, P. antennarius was usually much more abundant in qualitative samples collected on aquatic/terrestrial plants in the littoral (average for all samples at all sites: 38.0), than in quantitative samples collected on stony substrate (avg: 7.5). Thus, for P. antennarius, density as well as average abundance for both qualitative and quantitative samples were used in further analyses (Fig. 3 e, f).
Population changes of dominant macro-crustaceans and salinity
The first density peaks of E. stocki, G. aequicauda and L. hookeri at the lake sites (V1-V4) occurred in May or July 2011 (Fig. 3a, b, c), during first period of increasing salinity and the second density peaks occurred in May or Oct 2014, first period of low salinity. During the second low salinity period from September 2016 to March 2020, the average total densities of E. stocki and L. hookeri at four lake sites decreased to 1.9–2.9 ind. m−2 or 27–290 times lower than in other three periods (2011, 2014, Oct and Dec 2020). Amphipod G. aequicauda was found only at site P2 during second low salinity period. In October 2020, after 2 months of salinity > 2 PSU and similar higher salinity in 2019 (Fig. 2), densities of E. stocki, G. aequicauda and L. hookeri increased, especially at V2, V4 and P1, where their total densities were 7–140 times higher than in previous second low salinity period. At V1 and V3, densities of these three species in Oct 2020 increased from 0 to 8 and 0 to 67 ind. m−2, respectively. Species E. stocki and L. hookeri survived in low abundance in the lake and in the Prosika Canal in the second low salinity period, when higher densities of these two species were recorded at sites V4 and P1.
In contrast, the density of the isopod P. coxalis (Fig. 3d) decreased with increasing salinity in 2011 in Kotarka and at three lake sites (V2-V4). In 2014 densities of P. coxalis reached highest values in Kotarka, but at lake sites densities remained low. In the second low salinity period P. coxalis was the most abundant macro-crustacean species at all lake sites, but, surprisingly, had low density in the Kotarka canal. After its appearance in 2016, the densities of the predatory shrimp P. antennarius on the stony substrate and the total abundance (in both quantitative and qualitative samples) were mostly the highest in Kotarka. The most pronounced peaks of abundance and densities of these species were observed in March 2019 (Fig. 3e, f), following the second-highest water level and low salinity in 2018.
Spatiotemporal Spearman correlations (all sites combined) between average density of the five dominant species and salinity measured during sampling were statistically significant (p < 0.05, n = 84) (Table 1) for amphipods E. stocki (rs = 0.31) and G. aequicauda (rs = 0.23). The same correlations were negative and marginally significant (p < 0.10) for density of isopod P. coxalis (rs = − 0.20) and abundance of decapod P. antennarius (rs = − 0.19). Spearman correlations of densities or abundance with all other environmental parameters measured during sampling are presented in Table 1 in Appendix.
Spearman correlations between average monthly densities of five dominant species and average salinity 0, 6, 12, and 24 months prior to sampling at four sites (K, V1, V2, V3) combined are shown in Fig. 4. As the time-lag increased positive correlations between E. stocki, G. aequicauda, L. hookeri and salinity increased. In contrast, P. coxalis and P. antennarius showed increased negative correlations with salinity as the time-lag increased. For all five species correlations for time-lags of 12 and 24 months were statistically significant (p < 0.0025 with Bonferroni correction), and only G. aequicauda showed a significant positive correlation with salinity for a time-lag of 6 months.
Spearman correlations (rs) between densities of five dominant macro-crustacean species and salinities in the month of sampling (0) or average salinities 6, 12 and 24 months before sampling at four sites combined (K, V1-V3). Triangles mark statistically significant correlations with Bonferroni correction (p < 0.0025) due to multiple testing (see caption of Table 1 for species abbreviations)
Spearman correlations of six tested macro-crustacean metrics with salinity measured during sampling were positive and statistically significant for three macro-crustacean metrics (EsGaLh-a, EsGaLh-d, %EsGaLh: rs = 0.33–0.45, p < 0.05, n = 84) and significantly negative for two metrics (PcPa-a, PcPa-d: rs = -0.24 to -0.27, p < 0.05, n = 84), while only %PcPa metric was not significantly correlated with salinity (rs = 0.09, p = 0.44). Spearman correlations of six tested metrics with all environmental parameters measured during sampling are in Table 1.
Benthic macro-crustacean assemblages and salinity
Non-parametric multidimensional scaling (NMDS) analysis of crustacean assemblages at all seven sites together showed a good separation of three periods (2011, 2014, October and December 2020) characterized by higher abundance of E. stocki, G. aequicauda and L. hookeri, from the second low salinity period (March 2016—March 2020), when P. coxalis and P. antennarius were more abundant (Fig. 5a). When all sites were analysed together, there were significant differences between sites (PERMANOVA, pseudo-F = 5.38, df = 6, p = 0.0001) and periods (pseudo-F = 10.73, df = 3, p = 0.0001), as well as significant site × period interaction (pseudo-F = 2.31, df = 15, p = 0.0001). Similar results were obtained for each site analysed separately, i.e. statistically significant differences between four periods were obtained for five sites (K, V1-V4, PERMANOVA, pseudo-F = 2.92–7.02, df = 3, p = 0.0001–0.005), even when Bonferroni correction was applied due to multiple testing (p = 0.007). At sites P1 and P2, only differences between the second low salinity period and the second period with increasing salinity (2020_2: Oct and Dec 2020) could be tested, and these differences were not significant (PERMANOVA, pseudo-F = 0.81–4.54, df = 1, p > 0.05).
a Non-parametric multidimensional scaling (NMDS) based on macro-crustacean abundance matrix (average abundance per site-month, square root transformation, Bray–Curtis similarity). Sites are shown as different symbols, four salinity periods in different colours: 2011—first increasing salinity period (sampled in five months Mar-Dec), 2014—first low salinity period (May and Oct), 2016–2020—second low salinity period (sampled once per year from Sep 2016-Mar 2019 and in Mar 2020), 2020_2—second period of increasing salinity (Oct and Dec in 2020); b Indicator value (IndVal) index for five dominant macro-crustacean species in for salinity periods calculated for the five sites combined (K, V1-V4), that were sampled in all four periods
To explain differences in macro-crustacean assemblages between salinity periods, IndVal values of the five dominant species were examined, as this index combines relative abundance and occurrence of a species. Since similar pattern of IndVal changes across species and periods were obtained for each site separately, the data from five sites (which were sampled in all four periods) were combined in Fig. 5b. The highest IndVal values for species E. stocki, G. aequicauda, L. hookeri were observed in 2014, while the lowest (barely visible in Fig. 5b) were in the second low salinity period (2016–2020). Permutation tests revealed that the lowest IndVal for those three species in 2016–2020 period were significantly lower than random (p < 0.005, p-value corrected for multiple testing using the Sidak method). In the second low salinity period species P. coxalis and P. antennarius had much higher values of the IndVal index, indicating their dominance among the macro-crustacean species.
Discussion
Our results demonstrate that increasing salinity variations in the Vrana Lake ecosystem have resulted in shifts in crustacean species assemblages over a period of 10 years. Comparison of hydrology data in this study with the previous 74 years indicates an increase in the intensity and duration of droughts and consequent salinization in the Vrana Lake ecosystem over the study period (2011–2020). Changes in the salinity of a lake ecosystem should result in changes in macroinvertebrate assemblages. Previously, relationships between salinity and macroinvertebrates have been studied in both freshwater (Piscart et al. 2005, 2006; Kefford et al. 2011; Szöcs et al. 2014; Le et al. 2021; Vidal et al. 2021) and brackish water ecosystems (Attrill et al. 1996; Boix et al. 2007; Obolewski et al. 2018). However, most studies used space-to-time substitution to examine the effects of salinity on macroinvertebrates, whereas studies that examined long-term changes in macroinvertebrate communities caused by changing salinity are rare (Attrill and Power 2000; Poizat et al. 2004; Boets et al. 2011). Thus, this study fills a gap in the understanding of how changes in salinity drive long-term changes in macro-crustacean assemblages in the Mediterranean region.
Salinization of the Vrana Lake ecosystem stems from climate change, rising sea levels, and agricultural water and land use (Katalinić et al. 2012; Rubinić and Katalinić 2014). Climate change in the Vrana Lake basin is marked by higher air temperatures, reduced precipitation (Rubinić and Katalinić 2014), and intensified droughts and floods over the past two decades. Hydrologic records indicate both the lowest and highest water levels occurred in the past decade (2011–2020). Salinity increases during droughts, notably in 2007/2008 and 2011/2012. Rising Adriatic Sea levels (Vilibić et al. 2017), coupled with more frequent droughts, result in increased saltwater intrusions into Vrana Lake. Oligohaline conditions, after mesohaline peaks in 2008 and 2012, show delayed return in the lake. Agricultural land use contributes to water abstraction, leading to salinization of the Kotarka Canal. High discharge after heavy rains causes rapid salinity fluctuations in the canal, outpacing those in Vrana Lake. The interplay of climate change, sea level rise, and agricultural water use triggers more frequent transitions from normal (low oligohaline, < 1–2 PSU) to higher (high oligohaline-mesohaline, > 2 PSU) salinities in extremely dry years, impacting macro-crustacean assemblages in the lake and the Kotarka canal.
The macro-crustacean fauna of Vrana Lake and connecting canals (Table 1) was characterized by a combination of five dominant species during the study period: three brackish/marine species (Echinogammarus stocki, Gammarus aequicauda, and Lekanesphaera hookeri) and two freshwater/oligohaline species (Proasellus coxalis and Palaemon antennarius). The first three brackish/marine species are widely distributed along the Croatian Adriatic coast, inhabiting estuaries of larger rivers and smaller streams (Žganec unpublished). Together with other brackish/marine macro-crustaceans, they inhabit the mouth of the Prosika Canal and colonized the study sites in the lake before the start of this study in March 2011. This likely occurred during some prior salinization events as in 2008. Before more significant salinization, the macro-crustacean fauna of the lake and its tributaries was probably dominated by the species P. coxalis and P. antennarius, as was the case in the second low salinity period (2016–2020).
Increased salinity during the study period led to an increase in the abundance and distribution of three brackish/marine species, typical for Mediterranean brackish waters. Two of these species, G. aequicauda and L. hookeri, are widespread in Mediterranean coastal systems, such as lagoons and estuaries, and have been extensively studied (Casagranda et al. 2006; Castaneda and Drake 2008; Kevrekidis et al. 2009). These two eurihaline species occur within a wide range of temperatures and salinities in Mediterranean waters (Kouwenberg and Pinkster 1985; Charmantier and Charmantier Daures 1994; Casagranda et al. 2006; Delgado et al. 2009; Kevrekidis et al. 2009). In the Vrana Lake basin, G. aequicauda was found in a wide range of salinity (Table 1), but preferred higher salinity, as suggested by the significantly positive correlation with salinities both during sampling and 6, 12, and 24 months prior to sampling. Similar results were obtained for the isopod L. hookeri. This species is also widespread in the most sheltered habitats of European estuaries and coastal lagoons (Kouwenberg and Pinkster 1985; Casagranda et al. 2006; Castaneda and Drake 2008), where it prefers lower salinity (< 15‰, Frier 1976). The amphipod E. stocki, on the other hand, is much less studied. It is a widespread Mediterranean species found in a relatively wide range of salinity (0.1–18.9‰) (Pinkster 1993). Populations of this species in Vrana Lake were found within a similar range of salinity as G. aequicauda and L. hookeri (Table 1), but its median and average salinity values are between the upper oligohaline and lower mesohaline salinity class.
Observed spatiotemporal variations in macro-crustacean density and abundance were the direct consequence of both spatial gradients and temporal changes in salinity. Brackish/marine species (E. stocki, G. aequicauda, and L. hookeri) were more abundant at sites with higher salinity, during periods of increased salinity (second half of 2011 and 2020) and in the first low salinity period in 2014. During the oligohaline phase in the second low salinity period (Sep 2016–Mar 2020), their populations declined precipitously. Their spatiotemporal variations were significantly positively correlated with salinity 12 and 24 months before sampling (Fig. 4). Slower salinity changes in the lake and euryhaline ecophysiology of these three species are responsible for observed time-delays in their population changes with respect to salinity. The inertia of the large volume of water in Vrana Lake delayed salinity decreases and enabled a longer period with more favourable higher salinity for these species, relative to the Kotarka Canal. After the strongest salinization of the lake in 2011 and 2012, and with recovery to lower salinities in 2013 and 2014, their population sizes remained high in 2014, due to higher salinities one and two years before. However, during the prolonged second low salinity period (September 2016–March 2020), the populations of these species strongly declined. Thus, two years of low salinity (1–2 PSU) were needed for a strong population decline (E. stocki and L. hookeri) or the disappearance of G. aequicauda. Previous research indicated that prolonged low salinities are limiting factors for the survival of embryos and juveniles of the amphipod G. aequicauda (Delgado et al. 2011). Thus, prolonged low salinity (< 2 PSU) seems to be the main limiting factor for those three brackish/marine species, due to the low survival of juveniles in such conditions. Density increases of E. stocki, G. aequicauda, and L. hookeri in October 2020, followed salinity increases above 2 PSU for two and four months in the lake and in the Kotarka Canal, respectively. Also, salinity was above 2 PSU in 2019. Thus, the sudden population increase in October 2020 of these three species was due to increased salinity (> 2 PSU) in both 2019 and 2020. The time lag in population increases of 1 year for these species also contributed to the strong positive correlations with salinity one and two years prior to sampling (Fig. 4).
While unfavourable for the previous three species, the prolonged oligohaline phase provided time for population increase of the isopod P. coxalis and the shrimp P. antennarius, making them the dominant species in the lake after the population declines of the three more halotolerant species. The isopod P. coxalis is frequently found in Mediterranean fresh and brackish waters (Koprnická 2013; Kemp et al. 2020), and it can tolerate higher salinity (range: 0.05–5.60 ppt in Boets et al. 2011). Here, we showed that it can survive very high salinity (31.8 PSU at site V4), but it prefers lower salinity, with the optimum probably in oligohaline conditions. Higher salinity 12 and 24 months prior to sampling were associated with decreased abundance of this species in the lake. The decapod shrimp P. antennarius is also a freshwater/oligohaline species, widely distributed in rivers, lakes, and coastal systems of the Apennine and Balkan peninsulas (Tzomos and Koukouras 2015). Its optimal salinity is between 3—11‰, and 20‰ is indicated as the physiological limit for this species (Dalla Via 1987a; 1987b). In Croatia, it inhabits freshwater/oligohaline waters in the Neretva River Delta at salinities between 0–5‰ (Gottstein Matočec and Kerovec 2002; Gottstein Matočec et al. 2006). In the Vrana Lake basin, it was found once at site P2 at euhaline salinity (Table 1), but its median and average values align well with its optimal conditions in oligohaline to mesohaline salinity from the literature mentioned above. Spatiotemporal correlations with salinity were negative for this species, and higher salinities 12 and 24 months prior to sampling were associated with its lower abundance.
Three tested macro-crustacean metrics were significantly positively correlated with salinity, and two metrics had a significant negative correlation with salinity (Table 1). Although individual species had the strongest significant positive or negative correlations with average salinity one and two years prior to sampling, the tested metrics, which combined the abundance of three or two species, could be used as indicators of past and present salinity on benthic macroinvertebrates and aquatic biota. Furthermore, since the five dominant macro-crustacean species can be easily distinguished in the field, their use is highly recommended for future ecological monitoring of salinization in the lake and connecting canals.
Salinization is a major stressor in the Vrana Lake basin, and the combination of sea-level rise, increased frequency of drought, and agricultural withdrawals of water will increase the frequency and duration of high salinity events. Thus, measures for its active control are urgently needed. Plans for the adaptive management of the lake salinization by controlling the water level with a movable gate at the Prosika Canal have been postponed for too long. If saltwater intrusion increases in the future, and salinity rises to upper oligohaline and lower mesohaline conditions, the macro-crustacean fauna could permanently shift to halotolerant species. As a result, E. stocki, G. aequicauda, and L. hookeri, as well as other halotolerant species like Melita palmata, could become the dominant species in the littoral macroinvertebrate assemblages of Vrana Lake. In contrast, P. coxalis and P. antennarius are expected to decline as salinity increases, with refuges in the northeastern parts of the lake and in the lake tributaries. This could have positive impact on detritus processing by the macroinvertebrate community in the littoral zone of the lake. Overall, the increase in salinity and shift to the dominance of halotolerant species will have strong negative impacts on the species richness and diversity of benthic macroinvertebrates in the Vrana Lake basin.
Data availability
The dataset is available from the corresponding author on reasonable request.
References
Argano R (1979) Isopodi (Crustacea Isopoda). In: Ruffo S (ed) Guide per il riconoscimento delle specie animali delle acque interne Italiane, AQ/1/43. Consiglio Nazionale delle Richerche, Verona, Italy, pp 1–63
Attrill MJ, Power M (2000) Effects on invertebrate populations of drought-induced changes in estuarine water quality. Mar Ecol Prog Ser 203:133–143
Attrill MJ, Rundle SD, Thomas MR (1996) The influence of drought-induced low freshwater flow on an upper-estuarine macroinvertebrate community. Water Res 30:261–268
Bellan Santini D, Karaman G, Krapp-Schickel G, et al (1982) The Amphipoda of the Mediterranean. Part 1. Gammaridea (Acanthonotozomatidae to Gammaridae). In: Ruffo S (ed) The Amphipoda of the Mediterranean. Part 1. Mémoires de l’Institut océanograophique 13, Monaco, pp 1–364
Bellan Santini D, Karaman G, Krapp-Schickel G, et al (1993) The Amphipoda of the Mediterranean. Part 3. Gammaridea (Melphidippidae to Talitridae), Ingolfiellidea, Caprellidea. In: Ruffo S (ed) The Amphipoda of the Mediterranean. Part 3. Mémoires de l’Institut océanograophique 13, Monaco, pp 577–814
Boets P, Lock K, Goethals PLM (2011) Shifts in the gammarid (Amphipoda) fauna of brackish polder waters in Flanders (Belgium). J Crustac Biol 31:270–277. https://doi.org/10.1651/10-3357.1
Boix D, Sala J, Gascón S et al (2007) Comparative biodiversity of crustaceans and aquatic insects from various water body types in coastal Mediterranean wetlands. Hydrobiologia 584:347–359. https://doi.org/10.1007/s10750-007-0579-8
Cañedo Argüelles IM (2020) A review of recent advances and future challenges in freshwater salinization. Limnetica 39:185–211. https://doi.org/10.23818/limn.39.13
Castaneda E, Drake P (2008) Spatiotemporal distribution of Lekanesphaera species in relation to estuarine gradients within a temperate European estuary (SW Spain) with regulated freshwater inflow. Ciencias Mar 34:125–141
Casagranda C, Dridi MS, Boudouresque CF (2006) Abundance, population structure and production of macro-invertebrate shredders in a Mediterranean brackish lagoon, Lake Ichkeul, Tunisia. Estuar Coast Shelf Sci 66:437–446. https://doi.org/10.1016/j.ecss.2005.10.005
Caspers H (1959) Vorschläge einer Brackwassernomenklatur (‘“The venice system”’). Int Rev Ges Hydrobiol 44:312–315
Charmantier G, Charmantier Daures M (1994) Ontogeny of osmoregulation and salinity tolerance in the isopod crustacean Sphaeroma serratum. Mar Ecol Prog Ser 114:93–102. https://doi.org/10.3354/meps114093
Cunillera-Montcusí D, Beklioğlu M, Cañedo-Argüelles M et al (2022) Freshwater salinisation: a research agenda for a saltier world. Trends Ecol Evol 37:440–453. https://doi.org/10.1016/j.tree.2021.12.005
Dalla Via G-J (1987a) Salinity response in brackish water populations of the freshwater shrimp Palaemonetes antennarius - I. Oxygen Consumption Comp Biochem Physiol 87A:471–478
Dalla Via G-J (1987b) Effects of salinity and temperature on oxygen consumption in a freshwater population of Palaemonetes antennarius (Crustacea, Decapoda). Comp Biochem Physiol 88A:299–305
De Cáceres M, Legendre P (2009) Associations between species and groups of sites: indices and statistical inference. Ecology 90:3566–3574. https://doi.org/10.1890/08-1823.1
Delgado L, Guerao G, Ribera C (2009) The Gammaridea (Amphipoda) fauna in a Mediterranean coastal lagoon: Considerations on population structure and reproductive biology. Crustaceana 82:191–218. https://doi.org/10.1163/156854008X397194
Delgado L, Guerao G, Ribera C (2011) Effects of different salinities on juvenile growth of Gammarus aequicauda (malacostraca: Amphipoda). Int J Zool 2011:1–6. https://doi.org/10.1155/2011/248790
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Dugan HA, Bartlett SL, Burke SM et al (2017) Salting our freshwater lakes. Proc Natl Acad Sci USA 114:4453–4458. https://doi.org/10.6073/pasta/455d73d4cb43514e503826211eba4e99
Frier JO (1976) Oxygen consumption and osmoregulation in the isopods Sphaeroma hookeri leach and S. rugicauda leach. Ophelia 15:193–203. https://doi.org/10.1080/00785326.1976.10425458
Fritz F (1984) Postanak i starost Vranskog jezera kod Biograda na moru [Origin and age of Vrana Lake near Biograd na moru]. Geološki Vijesnik 37:231–243 (in Croatian)
Froglia C (1978) Decapodi (Crustacea Decapoda). In: Ruffo S (ed) Guide per il riconoscimento delle specie animali delle acque interne Italiane, AQ/1/9. Consiglio Nazionale delle Richerche, Verona, Italy, pp 1–39
Giorgi F (2006) Climate change hot-spots. Geophys Res Lett 33:1–4. https://doi.org/10.1029/2006GL025734
Giorgi F, Lionello P (2008) Climate change projections for the Mediterranean region. Glob Planet Change 63:90–104. https://doi.org/10.1016/j.gloplacha.2007.09.005
Gottstein Matočec S, Kerovec M (2002) Atyaephyra desmaresti and Palaemonetes antennarius (Crustacea: Decapoda, Caridea) in the delta of the Neretva river (Croatia). Biol - Sect Zool 57:181–189
Gottstein Matočec S, Kuzman A, Kerovec M (2006) Life history traits of the grass shrimp Palaemonetes antennarius (Decapoda, Palaemonidae) in the delta of the Neretva River, Croatia. Limnologica 36:42–53. https://doi.org/10.1016/j.limno.2005.10.002
Harrell JF (2023) Hmisc: Harrell Miscellaneous. R package version 5.1–1
Henry J-P, Magniez G (1983) Introduction pratique à la systématique des organismes des eaux continentales françaises - 4. Crustacés Isopodes (principalement Asellotes). Bull Mens La Société Linnéenne Lyon 52:319–357. https://doi.org/10.3406/linly.1983.10613
Herbert ER, Boon P, Burgin AJ et al (2015) A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6:1–43. https://doi.org/10.1890/ES14-00534.1
IPCC (2014) Climate change 2014: synthesis report. contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. IPCC, Geneva, Switzerland
Jacobs BJM (1987) A taxonomic revision of the European, Mediterranean and NW. African species generally placed in Sphaeroma Bosc, 1802 (Isopoda: Flabellifera: Sphaeromatidae). Zool Verh Leiden 238:1–71
Jeppesen E, Brucet S, Naselli-Flores L et al (2015) Ecological impacts of global warming and water abstraction on lakes and reservoirs due to changes in water level and related changes in salinity. Hydrobiologia 750:201–227. https://doi.org/10.1007/s10750-014-2169-x
Jiménez Cisneros B, Oki T, Arnell N, et al (2014) Freshwater resources. In: Field CB, Barros VR, Dokken DJ, et al. (eds) Climate change 2014: impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. contribution of working group ii to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp 229–269
Katalinić A, Denona MĆ, Rubinić J, Morell M (2012) Vrana Lake in Dalmatia—Water, surroundings and protection. In: proc. of international scientific conference on water, climate and environment "Balwois 2012"
Kefford BJ, Marchant R, Schäfer RB et al (2011) The definition of species richness used by species sensitivity distributions approximates observed effects of salinity on stream macroinvertebrates. Environ Pollut 159:302–310. https://doi.org/10.1016/j.envpol.2010.08.025
Kemp JL, Ballot A, Nilssen JP et al (2020) Distribution, identification and range expansion of the common Asellidae in Northern Europe, featuring the first record of Proasellus meridianus in the Nordic countries. Fauna nor 40:93–108. https://doi.org/10.5324/fn.v40i0.3353
Kevrekidis T, Kourakos G, Boubonari T (2009) Life history, reproduction, growth, population dynamics and production of Gammarus aequicauda (Crustacea: Amphipoda) at extremely low salinities in a Mediterranean lagoon. Int Rev Hydrobiol 94:308–325. https://doi.org/10.1002/iroh.200811097
Koprnická M (2013) Phylogeography and dispersal routes of the Proasellus coxalis s.l. (Crustacea: Isopoda). Dissertation, Univerzita Karlova v Praze
Kouwenberg J, Pinkster S (1985) Population dynamics of three brackish water Isopods species (Crustacea) in the lagoon system of Bages-Sigean (France). II. Life cycles, sexual activity and fecundity. Vie Milieu 35:79–92
Le TDH, Schreiner VC, Kattwinkel M, Schäfer RB (2021) Invertebrate turnover along gradients of anthropogenic salinisation in rivers of two German regions. Sci Total Environ 753:1–10. https://doi.org/10.1016/j.scitotenv.2020.141986
Legendre P, Legendre L (2012) Numerical ecology. Elsevier, Amsterdam
Little S, Wood PJ, Elliott M (2017) Quantifying salinity-induced changes on estuarine benthic fauna: the potential implications of climate change. Estuar Coast Shelf Sci 198:610–625. https://doi.org/10.1016/j.ecss.2016.07.020
Martínez-Megías C, Rico A (2022) Biodiversity impacts by multiple anthropogenic stressors in Mediterranean coastal wetlands. Sci Total Environ 818:1–13. https://doi.org/10.1016/j.scitotenv.2021.151712
Mastrocicco M (2021) Studies on water resources salinization along the Italian coast: 30 years of work. Acque Sotter - Ital J Groundw 10:7–13. https://doi.org/10.7343/as-2021-537
Obolewski K, Glińska-Lewczuk K, Szymańska M et al (2018) Patterns of salinity regime in coastal lakes based on structure of benthic invertebrates. PLoS ONE 13:1–19. https://doi.org/10.1371/journal.pone.0207825
Pinkster S (1993) A revision of the genus Echinogammarus Stebbing, 1899 with some notes on related genera (Crustacea, Amphipoda). Mem Del Mus Di Stor Nat 10:1–185
Piscart C, Moreteau JC, Beisel JN (2005) Biodiversity and structure of macroinvertebrate communities along a small permanent salinity gradient (Meurthe River, France). Hydrobiologia 551:227–236. https://doi.org/10.1007/s10531-004-4783-9
Piscart C, Usseglio-Polatera P, Moreteau JC, Beisel JN (2006) The role of salinity in the selection of biological traits of freshwater invertebrates. Arch Fur Hydrobiol 166:185–198. https://doi.org/10.1127/0003-9136/2006/0166-0185
Poizat G, Rosecchi E, Chauvelon P et al (2004) Long-term fish and macro-crustacean community variation in a Mediterranean lagoon. Estuar Coast Shelf Sci 59:615–624. https://doi.org/10.1016/j.ecss.2003.11.007
Poore GCB (2001) Families and genera of Isopoda Anthuridea. In: Kensley B, Brusca RC (eds) Isopod systematics and evolution. Balkema, Rotterdam, pp 63–173
R Core Team, 2023. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Roberts DW (2023) labdsv: Ordination and Multivariate Analysis for Ecology. R package version 2.1–0, https://CRAN.R-project.org/package=labdsv
Rubinić, J., 2014. Vodni režim Vranskog jezera u Dalmaciji i klimatski utjecaji [Water regime of the Vransko Lake in Dalmatia and climate impacts]. Dissertation, University of Rijeka (in Croatian).
Rubinić J, Katalinić A (2014) Water regime of Vrana Lake in Dalmatia (Croatia): changes, risks and problems. Hydrol Sci J 59:1908–1924. https://doi.org/10.1080/02626667.2014.946417
Šiljeg A, Lozić S, Šiljeg S (2015) A comparison of interpolation methods on the basis of data obtained from a bathymetric survey of Lake Vrana, Croatia. Hydrol Earth Syst Sci 19:3653–3666. https://doi.org/10.5194/hess-19-3653-2015
Stroj, A., 2012. Vransko jezero—Hidrogeološka istraživanja [Vrana Lake—hydrogeological study]. Report (in Croatian).
Szöcs E, Coring E, Bäthe J, Schäfer RB (2014) Effects of anthropogenic salinization on biological traits and community composition of stream macroinvertebrates. Sci Total Environ 468–469:943–949. https://doi.org/10.1016/j.scitotenv.2013.08.058
Tzomos T, Koukouras A (2015) Redescription of Palaemon antennarius H. Milne Edwards, 1837 and Palaemon migratorius (Heller, 1862) (Crustacea, Decapoda, Palaemonidae) and description of two new species of the genus from the circum-Mediterranean area. Zootaxa 3905:27–51. https://doi.org/10.11646/zootaxa.3905.1.2
Vidal N, Yu J, Gutierrez MF et al (2021) Salinity shapes food webs of lakes in semiarid climate zones: a stable isotope approach. Inl Waters 11:476–491. https://doi.org/10.1080/20442041.2020.1859290
Vilibić I, Šepić J, Pasarić M, Orlić M (2017) The Adriatic Sea: a long-standing laboratory for sea level studies. Pure Appl Geophys 174:3765–3811. https://doi.org/10.1007/s00024-017-1625-8
Wagner RJ, Boulger Jr. RW, Oblinger CJ, Smith BA (2006) In: Guidelines and standard procedures for continuous water-quality monitors: station operation, record computation, and data reporting. U.S. Geological survey techniques and methods 1–D3, Washington, DC.
Williams WD (2001) Anthropogenic salinisation of inland waters. Hydrobiologia 466:329–337
Wittmann KJ, Ariani AP, Daneliya M (2016) The Mysidae (Crustacea: Peracarida: Mysida) in fresh and oligohaline waters of the Mediterranean. Taxonomy, biogeography, and bioinvasion. Zootaxa 4142:1–70
Acknowledgements
We would like to thank all those that helped during the field and laboratory work, or with the logistic: Maja Ćuže Denona, Norma Fressel, Antonio Karaga, Petar Crnčan, Nikolina Knežević, Tomislav Kralj, Andreja Lucić, Ivana Pusić, Antonio Svorenji, Ivana Zrinščak. This research was funded by the Natural Park “Vransko jezero” within four separate expert projects for mollusca fauna (lead by JL, author of this study), crustacean fauna (KŽ) and two projects for littoral macroinvertebrates assemblages (KŽ).
Funding
Nature Park Vransko jezero
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Krešimir Žganec and Jasna Lajtner, and data analysis by Krešimir Žganec and C. Brannon Andersen. The first draft of the manuscript was written by Krešimir Žganec and C. Brannon Andersen, while all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Handling Editor: S. S. S. Sarma.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Žganec, K., Andersen, C.B. & Lajtner, J. The impact of salinization on benthic macro-crustacean assemblages in a Mediterranean shallow lake. Aquat Ecol 58, 689–706 (2024). https://doi.org/10.1007/s10452-024-10099-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-024-10099-1