Abstract
Sea cucumbers have an important economic value and high demand. Despite that, there is a lack of knowledge about their biology, ecology and habitat distribution patterns, which is very important for sea cucumber stock management, establishment and sizing of no-take zones, restocking actions and selection of grow-out areas. This work aimed to determine the density, abundance and habitat associations of Holothuria arguinensis for a better understanding of its distribution along the coastal lagoon Ria Formosa and to select suitable areas for grow-out. In the duration of a year, monthly visual censuses were performed in two locations at Ria Formosa along the intertidal zone. The number and length of H. arguinensis´ individuals found were registered, and the coverage of algae and seagrass was estimated. It was found that H. arguinensis was distributed along the lower intertidal zone, linked to Zostera noltii meadows on muddy and sandy bottoms. These areas showed the densest population and the largest sizes of H. arguinensis. However, during the warmer months, H. arguinensis seemed to migrate to deeper waters in the channels, to avoid exposition to high temperatures and solar/UV irradiance during low tides. Areas located in the lower intertidal zone with Z. noltii meadows on sand-muddy bottoms should be selected for H. arguinensis grow-out sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sea cucumbers are a diverse group of marine invertebrates that play an important ecological role in benthic communities as deposit feeders for creating bioturbation, nutrient recycling and habitat structuring (Bruckner et al. 2003; Preston 1993; Uthicke and Karez 1999; Uthicke 2001a, b). Through their feeding activity, sea cucumbers process large volumes of benthic sediments, assimilating bacterial, fungal and detrital organic matter (Michio et al. 2003; Yokoyama 2013). Additionally, sea cucumbers have an important economic value since they are commercially exploited and provide an income to millions of fishers worldwide (Purcell et al. 2013), who extract 200 million animals each year (Purcell et al. 2016) and sell around 10,000 tons of dried sea cucumber (Purcell et al. 2013). The high market demand has driven uncontrolled over-exploitation of many sea cucumber stocks around the world (Conand 2004).
In the 1980 s, the sea cucumber aquaculture started to be developed in China and Japan focused on Apostichopus japonicus (Chen 2004); however, nowadays another six species are cultured and important efforts have been done to other many species (Eriksson et al. 2012; Purcell et al. 2012a; Domínguez-Godino et al. 2015; Domínguez-Godino and González-Wangüemert 2017, 2018a, b, c). In spite of their ecological and economic importance, there is a lack of knowledge about the ecology, biology and behaviour of most sea cucumber species from the Mediterranean Sea and NE Atlantic (Anderson et al. 2011; Bruckner et al. 2003; Conand 1990, 2004; Graham and Battaglene 2004; Navarro et al. 2014; Purcell et al. 2012a, b; Uthicke 2001b).
Most of the deposit-feeder sea cucumbers show a distribution linked with the sediment features in natural habitats; however, their density and movement patterns have been described to be dependent mainly on sediment type, habitat, substrate type, food abundance, migrations, aggregation behaviour, light intensity, depth and salinity (Sewell 1990; Brava 2005; Uthicke and Karez 1999; Sloan and von Bodungen 1980; Li et al. 1994; Slater et al. 2009; Dong et al. 2011; Hamel et al. 2001; Mercier et al. 2000a; Navarro et al. 2013, 2014; Shiell and Knott 2008). Characterisation of sea cucumber habitats is necessary to understand the main variables linked directly and/or indirectly to their patchy distribution and their environmental preferences. In addition, the different life stages should be considered in these studies, since for some species there is a habitat segregation associated with the age of individuals (Mercier et al. 2000a; Morgan 2011; Yamana et al. 2006). Juveniles of some sea cucumber species, such Holothuria scabra and/or Australostichopus mollis, are generally related to complex structures where juveniles are hidden from predators and attach themselves to macroalgae, seagrass leaves, rocks and/or shells (Mercier et al. 2000a; Morgan 2011). When they reach a determined size, juveniles migrate to soft sediment substrate (Conand 1993; Mercier et al. 2000a). However, adults are usually linked to more exposed areas (Yamana et al. 2006). Despite the reduced number of studies on sea cucumbers’ habitat preferences and movement patterns, this information is vital and very useful for sea cucumber stock management, establishment and sizing of no-take zones, restocking actions and selection of nursery and grow-out areas (Purcell and Kirby 2006; Shiell and Knott 2008; Ceccarelli et al. 2018).
The temperate sea cucumber Holothuria arguinensis (Koehler and Vaney 1906) is distributed along the North-Eastern Atlantic, from the Berlengas Island (Portugal) to Morocco and Mauritania, including the Canary Islands (Thandar 1988; Costello 2001; Rodrigues 2012; Rodrigues et al. 2015). However, this species has recently colonised the Mediterranean Sea (El Mojón, SE Spain and Bay of Algiers, Algeria) (González-Wangüemert and Borrero-Pérez 2012; Mezali and Thandar 2014). H. arguinensis has achieved high economic value, being intensively and illegally harvested in South Portugal and South Spain (González-Wangüemert et al. 2018). In the Natural Park Ria Formosa coastal lagoon, the management program and legislation (Decreto-Lei nº 373//87, de 9 de Dezembro; Resolução do Conselho de Ministros nº 78/2009, de 2 de Setembro) recognise the capture of 2 kg/person/day of marine invertebrates (no cephalopods) on the permitted areas. Despite the regulation, illegal captures were registered by the Maritime Police along the lagoon. Additionally, the aquaculture techniques for H. arguinensis have begun to be developed in the last years and important improvements have been achieved (Domínguez-Godino et al. 2015; Domínguez-Godino and González-Wangüemert 2017, 2018a, b, c).
Holothuria arguinensis has been described to be associated with sand, muddy, seagrass meadows and macroalgae habitats from 0 to 50 m (González-Wangüemert and Borrero-Pérez 2012; González-Wangüemert et al. 2013; Navarro et al. 2014; Siegenthaler et al. 2015), where it shows different densities depending on habitats (González-Wangüemert et al. 2013; Navarro et al. 2014; Siegenthaler et al. 2015). In the Ria Formosa Natural Park (S Portugal), H. arguinensis is the most abundant sea cucumber species distributed across the intertidal areas, showing different patterns of distribution and densities (González-Wangüemert et al. 2013; Siegenthaler et al. 2015). H. arguinensis is found to be a sea cucumber species that actively moves through its habitats for 24 h (Siegenthaler et al. 2015). H. arguinensis has a seasonal reproduction period from June to October (Domínguez-Godino et al. 2015, Marquet et al. 2017). However, no study has been done until now to establish the drivers that explain its patchy distribution at Ria Formosa. The aim of this work was to study the habitat association and spatial distribution pattern of H. arguinensis in Ria Formosa Natural Park (S. Portugal) along representative habitats. To achieve this objective, H. arguinensis abundance and sizes were recorded by visual censuses throughout 1 year in two sampling sites. Additionally, substratum coverage was registered, and sediment and faeces samples were collected to assess the habitat preference of this target species. This study will increase the ecological knowledge of H. arguinensis at Ria Formosa where a large and stable population can be found. Such information will be very useful for the selection of sea ranching and sea pens areas for the grow-out culture of this species in this lagoon where large areas are available. In addition, this ecological information will be also interesting for establishing further regulations of its fishery at Ria Formosa Natural Park and restocking programs since a many incidents of illegal harvesting have occurred in the last years (González-Wangüemert et al. 2018).
Materials and methods
Study area and sampling
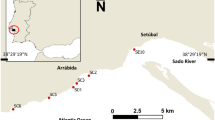
The Ria Formosa Natural Park is a coastal lagoon sheltered by a sand barrier island system. It extends for about 55 km with a width of up to 6 km along the south coast of Portugal (Fig. 1) (Barbosa 2010). The lagoon has a surface of 170 ha, and it is connected to the ocean by 6 outlets (Pacheco et al. 2010; Pilkey et al. 1989). The Ria Formosa has a semi-diurnal tide with amplitudes ranging from 0.7 m for neap tides to 3.5 m for spring tides, favouring a water exchange of 50 to 75% each tide depending on the tide’s amplitude (Águas 1986). The lagoon, which includes barrier islands, barrier platforms, back barrier lagoons and mainland, is composed of a highly branched system of creeks and channels. The intertidal area covers 1/3 of the total area, 14% of which is composed by permanent submersed subtidal channels (Newton and Mudge 2003; Ribeiro et al. 2008). The average depth is 3–4 m with a maximum depth up to 20 m in the channels (Malaquias and Sprung 2005; Sprung 2001). Water temperature ranges from 12 to 13 °C in winter to 27–28 °C in summer (Ribeiro et al. 2008) and the salinity from 35.5 to 36.9 psu (Ribeiro et al. 2008); however, during heavy rainfalls, salinity can drop to 25 psu in some places (Pilkey et al. 1989). The main habitats composing the Ria Formosa lagoon are sand, mud and seagrass meadows, which show Zostera noltii in the intertidal areas, Zostera marina and Cymodocea nodosa in the subtidal areas, and Spartina maritima in the higher intertidal (Malaquias and Sprung 2005). During winter, thick mats of the macroalgae Ulva spp. and Enteromorpha spp. can be found (Sprung 2001).
Bi-monthly samplings were done at two sites at Ria Formosa, Praia de Faro (PF) and Ramalhete (RM) from August 2014 to August 2015 (Fig. 1) to cover the possible changes of habitat association and spatial distribution pattern of H. arguinensis along the year seasonality on Ria Formosa. The two sites were composed of the main subtidal habitats from Ria Formosa and where large densities of H. arguinensis could be found. Visual censuses were conducted at each site during the lowest tide of the month at daytime (http://www.hidrografico.pt/previsao-mares.php). At each sampling site, two transects of 100 × 2 m were established at the minimum level (Transect 1: Low; 2 m deep) and maximum level (Transect 2: High; 1 m deep) of low tide, with a distance of 20 m between them along the shore (González-Wangüemert et al. 2013). For each transect, three replicates were carried out; therefore, 600 m2 of the surface was sampled by transect and sampling site. Along each transect, the number of H. arguinensis individuals, their total relaxed length and position were recorded (metre on transect and right/left position). The weight of each individual was estimated using the following formula: weight = 9.016 length = 3.6 (González-Wangüemert et al. 2016). For the total area covered in each transect and site, the density and biomass of H. arguinensis were calculated. Since habitat substratum composition is an important factor for sea cucumber distribution and density, the habitat along the transects was characterised; every 5 m, the substratum coverage was estimated by the same sampler estimating the coverage percentage of sand, mud, rock, seagrass Z. noltii and/or algae on 1 m2 quadrant (González-Wangüemert et al. 2013). Sediment samples (n = 3) close to the mouth and faeces samples close to the anus of the sea cucumbers (n = 3) were collected at the beginning, middle and end of each replicate transect using a spun. The sediment and faeces samples were oven-dried at 60 °C for 48 h, and the organic matter content was estimated by loss of ignition (LOI). Three sub-samples of 1 g were placed in a furnace for 5 h at 525 °C and then re-weighed, the total organic matter (TOM) being the difference of weight. The absorption efficiency (AE) (Conover 1966) of organic matter was estimated for each sampled individual using its set of sediment and faeces samples, calculated as follows:
Since H. arguinensis has been described to be associated with seagrass meadows at Ria Formosa (González-Wangüemert et al. 2013; Siegenthaler et al. 2015), two parameters related to this were considered. First, when the individuals were found in the seagrass meadows of Z. noltii (only seagrass species were found along the transect), a core sample was collected (8 cm of diameter) to estimate Z. noltii shoots density. After the sampling, the core samples were washed at the laboratory, to eliminate the mud and the Z. noltii shoots were counted. Secondly, the mean distance from each individual of H. arguinensis to a seagrass quadrat with minimum a 20% of coverage was estimated.
Statistical analysis
The statistical analyses were performed in R statistic software (Core Team 2013). Mean values of the obtained and estimated variables previously described were compared among transects and sites for the sampling period using one-way ANOVA, posterior assessment of normality using Shapiro–Wilk’s test (Shapiro and Wilk 1995) and homogeneity of variance with Bartlett test (Bartlett 1937). Tukey’s test (Tukey 1949) was used as post hoc analyses. If data do not accomplish normality and homogeneity, comparisons of mean values of variables were done using Kruskal–Wallis test (Kruskal and Wallis 1952) or Mann–Whitney–Wilcoxon test (Mann and Whitney 1947). Differences were considered significant if P < 0.05.
Linear correlation between the H. arguinensis variables (length, weight, density and biomass) and the environmental parameters (substratum coverage, number of shoots, TOM of sediment samples) and TOM of the faeces was tested using Spearman correlation, which was considered significant when R > 0.70 (P < 0.05). A redundancy analysis (RDA) was used to examine the relationships between H. arguinensis’ variables (length, density and biomass) and environmental parameters, which could play an important role in the sea cucumber habitat association and spatial distribution pattern.
Results
Holothuria arguinensis’ population variables
A total of 354 individuals of H. arguinensis were found and measured along the sampling period, corresponding to 85 individuals at Praia de Faro and 269 at Ramalhete sites (Table 1). The mean length and weight of H. arguinensis individuals at the two High transects were similar among them (Table 1, Kruskal–Wallis, P > 0.05) and significantly smaller than the values obtained for the two Low transects (Table 1, Kruskal–Wallis, P < 0.05). The individuals found at the Low transect at Praia de Faro were slightly larger and heavier (24.04 ± 5.62 cm and 213.08 ± 51.23 g) than the ones registered at the Low transect at Ramalhete (21.87 ± 4.84 cm and 193.54 ± 43.59 g); however, they were not significantly different (Table 1, Kruskal–Wallis, P > 0.05). The smallest and lightest individual was found at Ramalhete Low transect with 14 cm length and 122.624 g weight, and the largest and heaviest individual at Praia de Faro Low transect with 41 cm length and 366.056 g weight. H. arguinensis individuals from Praia de Faro ranged in length from 12 to 41 cm, showing multimodal size–frequency distribution, with the highest frequency for the size class of 23 cm (Fig. 2a). At Ramalhete site, the individuals ranged in length from 10 to 35 cm, showing similar multimodal size–frequency distribution; however, the highest frequencies were found for the size classes of 22 and 25 cm (Fig. 2b). The weight of individuals from Praia de Faro and Ramalhete sites showed a multimodal distribution with the most frequent weight between 221 and 240 g (Fig. 3).
The density range was smaller at the two High transects compared to the Low transects: Praia de Faro from 0 to 50 ind/Ha and Ramalhete from 0 to 200 ind/Ha. Larger density was found at the two Low transects, which oscillated from 66 to 316 ind/Ha in Praia de Faro and from 316 to 933 ind/Ha in Ramalhete. The lowest mean density was obtained in the High transect at Praia de Faro with 19.05 ± 29.48 ind/Ha (Table 1, Fig. 4a, Kruskal–Wallis, P > 0.05). Significantly higher density was found at the Low transect at Ramalhete with a mean value of 595.25 ± 412.58 ind/Ha (Table 1, Fig. 4a, Kruskal–Wallis, P < 0.05). Significant lower mean biomasses were also found at both High transects in Praia de Faro and Ramalhete sites in comparison with the Low transects at both sites (Table 1, Fig. 4b, Kruskal–Wallis, P < 0.05); however, significant differences were not found among High transects (Table 1, Fig. 4b, Kruskal–Wallis, P > 0.05). The highest biomass was obtained at each Low transect with a total of 38.05 ± 42.7 kg/Ha and 115.21 ± 79.15 kg/Ha for Praia de Faro and Ramalhete, respectively; however, the differences found among them were not significant (Table 1, Fig. 4b, Kruskal–Wallis, P > 0.05).
The highest mean absorption efficiency (AE) was obtained for H. arguinensis individuals found at the High transect at Praia de Faro (Table 1). The Low transect at Praia de Faro and the High transect at Ramalhete had similar values of AE, which were approximately half of the value obtained for the High transect at Praia de Faro, without showing significant differences (Table 1, Kruskal–Wallis, P > 0.05). A negative value of AE was obtained for the Low transect at Ramalhete which was significantly different from the AE value obtained for the High transect at Praia de Faro (Table 1, Kruskal–Wallis, P < 0.05).
Holothuria arguinensis’ seasonality patterns density, weight and biomass
Holothuria arguinensis showed different patterns of density, mean weight and total biomass along the seasons for the different transects. The High transect at Praia de Faro showed the highest densities, mean weight and biomasses at autumn and winter, the density and biomass being higher in autumn than in winter (Table 2, Fig. 5a–c). During summer in the Hight transect at Praia de Faro, no sea cucumbers were found and low density was registered during spring with small and light individuals; therefore, low biomass was also recorded to be significantly different compared to the values obtained for the winter (Table 2, Fig. 5a–c; Kruskal–Wallis, P < 0.05). At the Low transect at Praia de Faro, the highest density and biomass were found for autumn and winter, with the lowest values found during the summer season (Table 2, Fig. 5b, d, f); however, heavier individuals were found in spring and summer than for autumn and winter (Table 2, Fig. 5f). For the case of the High transect at Ramalhete, the largest density, weight and biomass were registered in winter (Table 2, Fig. 6a–c), with significantly heavier sea cucumbers than the ones found in summer (Kruskal–Wallis, P < 0.05). Along this transect, no sea cucumbers were found during spring (Table 2, Fig. 6a). The Low transect at Ramalhete showed high density and biomass along the different seasons, summer being the season with the largest density and biomass (Table 2, Fig. 6d, e). At this transect, the lowest density of sea cucumbers was found in spring, with the lightest individuals registered during winter (Table 2, Fig. 6d–f).
Mean density (ind/Ha), mean biomass (kg/Ha) and mean estimated weight (g) for H. arguinensis in the High transect (a, c, e, respectively), and in the Low transect (b, d, f, respectively), at Praia de Faro (PF) site in the different seasons. Values are given as mean ± SD. Different letters indicate significant differences (Nemenyi post hoc, P < 0.05)
Mean density (ind/Ha), mean biomass (kg/Ha) and mean estimated weight (g) for H. arguinensis in the High transect (a, c, e, respectively), and in the Low transect (b, d, f, respectively), at Ramalhete (RM) site in the different seasons. Values are given as mean ± SD. Different letters indicate significant differences (Nemenyi post hoc, P < 0.05)
Habitat description
Three different major habitats were identified in our sampling sites. The High transect at Praia de Faro was characterised by a coverage of 89.63% of sand: a percentage significantly higher than the sand coverage registered in the others transects (Table 3, Kruskal–Wallis, P < 0.05). Additionally, along this transect was found a 10.37% of Ulva spp. covering the sand. The Lower transect at Praia de Faro was significantly dominated by a dense seagrass meadow of Zostera noltii accounting to a 93.09% coverage (Table 3, Kruskal–Wallis, P < 0.05). The seagrass was crossed by several sandy and muddy channels covering 2.89% and 2.9% of the transect, respectively. The two transects at Ramalhete were characterised by a heterogeneous habitat composed of medium level of Z. noltii coverage (48.64% and 44.02% for the High and Low transect, respectively; no significant differences were found among them; Table 3, Kruskal–Wallis, P > 0.05), sand (22.51% for the High and 39.24% for the Low transect; showing significant differences; Table 3, Kruskal–Wallis, P < 0.05) and mud (16.15% for the High and 9.36% for the Low transect, showing significant differences among them; Table 3, Kruskal–Wallis, P < 0.05). Along these two transects at Ramalhete site, a similar mean coverage of Ulva spp. was registered (~ 5%), with a 7.24% and 1.58% of Enteromorpha spp. for the High and Low transects, respectively. The Ramalhete site recorded the lowest and highest estimation of Z. noltii’s shoots for the High and Low transects, respectively; however, no significant differences were obtained (Table 2, Kruskal–Wallis, P > 0.05). No significant differences were registered between transects among sampling sites (Kruskal–Wallis, P > 0.05). The Praia de Faro site showed the highest and lowest percentage of total organic matter (TOM): 6.72% for the Low transect and 1.18% for the High transect. The two transects at Ramalhete showed medium values, the records for the Low transect being slightly higher (3.31% TOM) than the ones obtained for the High transect (2.38% TOM). More significant differences of the percentage of TOM were found among the High transects in comparison with the Low transects (Kruskal–Wallis, P < 0.05 (Table 3). Similar significant differences were found in the percentage of TOM in H. arguinensis’ faeces (Table 3) (Kruskal–Wallis, P < 0.05). No significant lineal correlation was found between H. arguinensis’ variables (length, weight, density and biomass) and the environmental parameters (substratum coverage, number of shoots, TOM of sediment samples) and TOM of faeces (Spearman correlation: R > 0.70, P > 0.05). The transect at Ramalhete could be defined as a complex mosaic habitat, where seagrass meadows with different shoot density and coverage were mixed with sandy and muddy patches; Praia de Faro, however, was found to be a homogeneous muddy substratum of dense seagrass meadow (Table 3).
Redundancy analysis (RDA)
The first two components explained 99.99% of the total variance. The first component has a strong positive correlation with density, while the second component has a negative correlation with the biomass, weight and length (Fig. 7). The environmental factors that are significantly important to the variability explained by the two components are the number of shoots (Nshoots), the percentage of coverage (Seagrass) and the percentage of TOM in the sediment (pOMsed), which were significantly and positively correlated with the first component (P < 0.05). The distance of the individuals to a metre of the transect with the seagrass coverage larger than 20% (dist20) was significantly negatively correlated with the second component (Fig. 7, P < 0.05). The RDA analyses revealed a separation of the different transects, where the Low transect of Ramalhete (RMLow) showed a significant positive correlation to the first component (P < 0.05), with the density as the most important variable contributing to this separation (Fig. 7). However, the Low transect of Praia de Faro (PFLow) showed a significant negative correlation to the second component (P < 0.05); therefore, length, weight and biomass were the most influential variables (Fig. 5). The two High transects (RMHigh and PFHigh) showed a close similar significant negative correlation to the first component (Fig. 7, P < 0.05).
Redundancy analysis (RDA) plots of H. arguinensis measured variables: density, length and total biomass (Biomass Ha); and the obtained significant environmental variables: coverages of Z. noltii seagrass (Seagrass), number of Z. noltii´s shoots (Nshoots), percentage of TOM in the sediment (pOMsed) and distance to a Z. noltii seagrass coverage superior of 20% (dist20)
Discussion
Holothuria arguinensis showed different distribution patterns across the study area with densities ranging from 0 to 933 ind/Ha depending on the transects with a mean value of 212 ± 328 ind/Ha. The maximum density recorded in this study was higher than the described one for this species at Ria Formosa previously, using visual censuses (140–480 ind/Ha, González-Wangüemert et al. 2013); however, slightly lower mean density was obtained in our sampling (267 ± 152 ind/Ha, González-Wangüemert et al. 2013). In addition, our mean density was also smaller than the capture–recapture estimated density for this area (563 ind/Ha, Siegenthaler et al. 2015). The density of this species at the Canary Islands on seagrass meadows and macroalgal bed was also higher than in our sampling, with 250 ind/Ha and 460 ind/Ha, respectively (Navarro et al. 2014). Therefore, H. arguinensis shows different mean densities across habitats, location and time, what might also be influenced by the different sampling methods used in the different studies.
Holothuria arguinensis in our study showed a clear preference for the lower part of the intertidal zone, since significant lower density, length, weight and biomass were found at the two High transects. The area covered by the High transects, due to their position at the upper part of the intertidal, had longer emersion times along the year in comparison with the Low transects (Praia de Faro High transect 261.1 h and Low transect 10.93 h), which could be considered the main factor explaining the low presence of sea cucumbers at these transects. This is reinforced by the seasonal change of density and biomass registered in both High transects, showing the lowest density/biomass during the spring and summer periods. During the summer, the mean air temperature at Ria Formosa is 22–23 °C; however, the mean number of days with air temperature higher than 25 °C is 79 days (http://portaldoclima.pt). In addition, during these months, the highest global radiation is attained with a mean value of 250 W/m2 (http://portaldoclima.pt), together with the highest level of UV radiation. Therefore, the upper part of the intertidal became an unsuitable habitat for sea cucumbers during summer due to the long periods of desiccation under high temperatures and UV irradiance, which can produce skin ulceration and some diseases (González-Wangüemert et al. 2013, Cánovas et al. 2019). This fact can be also accentuated in the case of the High transect at Praia de Faro by the non-existence of seagrass meadow that could provide some covering protection to H. arguinensis against sun irradiance, which has been already described for this species at Ria Formosa (González-Wangüemert et al. 2013; Siegenthaler et al. 2015). Tidal level has already been described as a factor influencing sea cucumbers’ density, as is the case of Holothuria grisea in the Brazilian coast, where higher densities were found in the subtidal zone (Tommasi 1969, Mendes et al. 2006). H. grisea showed a reduction in densities during summer, which could be driven by the high summer temperatures. In addition, high aggregations of H. grisea were found in tidal pools as emersion response during the low tide periods (Mendes et al. 2006). Studies focused on Holothuria theeli also suggested that its habitat preferences and occupation might be linked to the tidal level (Sonnenholzner 2003). Additionally, H. arguinensis has been described to have day/night movement pattern, showing displacement directed to the low part of the intertidal during the day and to the high part of the intertidal during the night: movements that were independent of habitat and mainly driven by avoidance of sunlight (Siegenthaler et al. 2015).
Holothuria arguinensis’ length, weight, density and/or biomass were not linearly correlated with any of the environmental parameters assessed. However, some of them, such as the number of seagrass shoots, seagrass coverage, the distance to a seagrass coverage of 20% and the percentage of organic matter in the sediment, were significantly related to the variability of H. arguinensis’ density, length and biomass according to the RDA analysis. H. arguinensis’ density seems to be influenced by the shoot’s density and the organic matter content in the sediment, and their length and biomass by the seagrass density and the distance to seagrass meadows. Ramalhete site showed higher density of smaller and lighter H. arguinensis individuals, and this sampling site is characterised by sand-muddy bottom, medium coverage of seagrass meadows with large shoots of Z. noltii and low organic matter content. In contrast, Praia de Faro showed lower densities of larger and heavier individuals; this site showed muddy bottom rich in organic matter and highly covered with thin Z. noltii shoots. In addition, H. arguinensis significantly prefers the low intertidal of our study area, although differences in the studied parameters on H. arguinensis among the two Low transects from the different sampling places were found. The Low transect at Ramalhete site has significantly less dense seagrass meadow than Praia de Faro, showing slightly smaller and lighter sea cucumbers than the Low transect at Praia de Faro. These differences could be due to the habitat features linked to both transects. The transect at Ramalhete could be defined as complex mosaic habitat, where seagrass meadows with different shoot density and coverage were mixed with sandy and muddy areas. This complex and heterogeneous habitat could provide many diverse food sources for sea cucumbers, in contrast to a more homogeneous muddy substratum and dense seagrass meadow found at Praia de Faro. Therefore, it is likely that H. arguinensis prefers a more complex habitat which has different microhabitats across small areas, which is able to support importantly larger population density with smaller and lighter individuals. Diversity of mycrophytobenthos (MPB: microalgae, cyanobacteria and other photosynthetic bacteria) and their spatial–temporal biomass variability are partially controlled by tidal exposure and sediment features (Brotas et al. 1995). The sediment type is the parameter that explains most of the diversity (Paterson and Hagerthey 2001; Perkins et al. 2003), determining the presence of certain MPB groups: muddy sediments are dominated by diatoms and sandy sediments by more diverse groups, such as cyanobacterias and euglenids (Underwood and Barnett 2006). So, probably differences in MPB could be also found at the different sampling sites and influence the individual distribution. H. arguinensis can process large volumes of sand, showing a mean feeding rate of 27.88 ± 3.63 g ind-1 d-1 for individuals with a mean weight of 272.89 ± 32.04 g (Domínguez-Godino and González-Wangüemert 2018b). However, it could be expected that in muddy bottoms with dense seagrass meadow, this feeding rate could be smaller due to sediment size and compaction. This together with the poorest food source (lower diversity of MPB) of this habitat might influence the lower density and larger sizes of H. arguinensis at the Low transect in Praia de Faro. In spite of this hypothesis on habitat preference, it is important to highlight that H. arguinensis at Praia de Faro could have also suffered heavier illegal harvesting pressure than Ramalhete site, what could explain the obtained results. At Ria Formosa, since 2014, there have been an important incidence of illegal sea cucumber fishing mainly focused on H. arguinensis, whose density has reduced in areas such as Ramalhete Channel, Culatra Island and Armona Island, with a decrease in the largest sizes (González-Wangüemert et al. 2018). The same two transects at Praia de Faro were sampled from November 2012 to February 2013 using the same methodology (González-Wangüemert et al. 2013); however, higher densities were found more during that period (150 ind/Ha and 480 ind/Ha for the High and Low transects, respectively), than the obtained ones during our study (19 ind/Ha and 193 ind/Ha for the High and Low transects, respectively). In fact, the registered densities in the sampling of 2012–2013 at Praia de Faro are comparable with the ones obtained at the Ramalhete site in the Low transect during our study (595 ind/Ha). Similar important H. arguinensis’ abundance reduction has been found along Ria Formosa in other locations due to illegal harvesting, such as in Armona Island where densities reduced from 118 ind/Ha in 2014 to 30 ind/Ha in 2016 (González-Wangüemert et al. 2018). In addition, H. arguinensis’ size distribution changed from a mean size class between 26–30 and 21–25 cm in this location (González-Wangüemert et al. 2018). The sampling site at Praia de Faro is an accessible area of the Ria Formosa, which is the main beach of Faro city, residential area and fishermen urban area (Zacarias et al. 2011; Almeida et al. 2011). Along the lagoon, many species with commercial interest are harvested during low tides (e.g. cockles, ensis, clam, spiny dye-murex) by shellfish catchers; therefore, H. arguinensis could have been harvested illegally during the time period between the two samplings (González-Wangüemert et al. 2018; Pires 2016a, b). The Ramalhete site is located in the north of the main channel of Praia de Faro. Being less accessible from mainland, a ship was required to reach this area. Additionally, in the adjacent areas of the transects in Ramalhete, some clam and oyster farms are present, which are frequented by the farmers and not by fishermen harvesting other commercial species that could also restrict the incidence of illegal fishing.
Holothuria arguinensis showed two different patterns of density distribution in the Low transects along the year. At Praia de Faro, the same pattern of density’ decrease during the warmer months was observed in the High and Low transects; therefore, the individuals might move to deeper areas of the channel during this season. Although the data were not statistically significant, it is interesting to note that the Low transect at Ramalhete showed the opposite pattern, summer being the period with the highest density. As other sea cucumbers (e.g. Holothuria tubulosa, Parastichopus californicus), H. arguinensis has density-dependent reproduction and aggregation behaviour during reproductive season (Brava 2005; Bruckner et al. 2003; Cousteau and Dugan 1963; McEuen 1988), which is on summer months (Domínguez-Godino et al. 2015; Marquet et al. 2017). Therefore, it could be possible that Ramalhete site is an aggregation area of breeders during the period of summer, which explains the high density registered on Low Transects during summer.
Holothuria arguinensis showed different absorption efficiency along the different transects. Sea cucumbers can modulate their absorption efficiency as compensatory response to sediment quality and quantity (Roberts et al. 2003; Yuan et al. 2006; Zamora and Jeffs 2011). H. arguinensis showed the highest absorption efficiency at the High transect of Praia de Faro, which was characterised by sandy bottom with the lowest content of organic matter. However, in the Low transect at Praia de Faro and High transect at Ramalhete, similarly low values of absorption efficiency were obtained: in these places, the transects showed lower coverage of sand and high content of organic matter, due probably to the high coverage of mud and seagrass. Therefore, H. arguinensis needs to be more efficient in obtaining nutrients in poorer substratum such as sandy areas which are less efficient in rich muddy and seagrass meadows areas. It is important to highlight that the absorption efficiency obtained for the Low transect at Ramalhete was negative, meaning that most of the faeces collected had higher total organic content than the sediment samples. This result is typically associated with selective feeder sea cucumbers, which select organic-rich sediment from the surrounding areas. However, H. arguinensis has been categorised as non-selective feeder (Navarro et al. 2014), which is also supported by our results of absorption efficiency obtained from the other three transects. The negative values of absorption efficiency might be explained by the continuous feeding and movement of H. arguinensis along this heterogeneous transect (in terms of coverage and organic matter content). In this way, the sediment collected underneath the sea cucumbers could not correspond to the faeces features (TOM), because the individual displaces along a very heterogeneous habitat during the time period from ingestion to defecation. This fact was observed during the collection of the mentioned samples, e.g. the individual was on sandy bottom; however, the faeces was composed of muddy sediment. Therefore, possible mismatching of sediment and faeces samples can be recorded due to sea cucumber displacement along its habitat. This should be considered when the absorption efficiency is applied on field surveys since they might induce some bias.
Sea cucumbers are unevenly distributed due to several factors such as food availability, bottom sediment features, substrate type, depth, tidal regime, predator avoidance. (Sloan and Von Bodungen 1980; Young and Chia 1982; Hammond 1983; Massin and Doumen 1986; Bulteel et al. 1992; Uthicke and Karez 1999; Mercier et al. 2000a, b; Uthicke et al. 2004; Abdl Razek et al. 2006; Eckert 2007; Entrambasaguas et al. 2008; Shiell and Knott 2010). Additionally, their habitat preferences have been described to be species-specific and within the same species with differences across life stages (Conand 1990; Mercier et al. 2000b; Purcell 2004; Shiel 2004; James 2005; Yamana et al. 2006; Conand 2008; Purcell et al. 2009). Therefore, the above mentioned parameters could have a certain influence on H. arguinensis distribution, density and size. Specially for our target species, the tidal regime together with the depth, which determine the number of emersions’ hours for different areas of the lagoon, could be the most important factors. In addition, the bottom sediment features and substrate type which determine the food availability for H. arguinensis seem to also be decisive.
The results obtained in this study have important applications for H. arguinensis’ aquaculture, target species in Europe whose biotechnology for its culture was being developed in the last years (Domínguez-Godino et al. 2015; Domínguez-Godino and González-Wangüemert 2017). However, there is a need for a better understanding of natural habitat selection of juveniles and adults to apply this information on grow-out selection sites. Sea cucumber could be cultured on sea pens and sea ranching in natural areas (Han et al. 2016). The Ria Formosa has an important potential to develop these culture systems, such as using them for other commercial species, such cockles, clam and oysters along it (Newton et al. 2003). According to the obtained results, grow-out areas for large juveniles and adults of H. arguinensis should be allocated in medium and dense seagrass meadows at the lower intertidal areas along the Ria Formosa.
Conclusions
Holothuria arguinensis showed different distribution patterns across the study areas, recording lower densities than the previously ones found for this species at Ria Formosa (S Portugal) and/or the Canary Islands (Spain). H. arguinensis was significantly more abundant in the lower areas of the intertidal, which could likely be driven by the tidal level at the lagoon Ria Formosa. Additionally, more dense populations with smaller and lighter individuals were found in sandy-muddy with medium coverage of Z. noltii seagrass meadows, due likely to the increase in food sources by the habitat complexity. However, the differences among sites could also have been driven by the illegal sea cucumber fishing affecting the Ria Formosa. These areas where large populations of H. arguinensis could be found should be protected and regulated as non-taking zones to ensure stable population which favour the persistence of the target species and production of offspring. Finally, H. arguinensis showed a modulation of absorption efficiency according to the associated substratum type. However, the presented methodology for field surveys might induce some bias which must be considered in ecology research on sea cucumbers. In addition, these results have an application for H. arguinensis’ aquaculture in terms of selection of grow-out areas for large juveniles and adults; which should be allocated in the lower intertidal on sandy-muddy bottoms with medium dense Z. noltii seagrass meadows.
References
Abdl Razek FA, Ael-Shimy NA, Abdel Rahman SH, Omar HA (2006) Ecological observations on the abundance, distribution of holothuroids (Echinodermata–Holothuroidea) in the Red Sea coast, Egypt. Egypt J Aquat Res 32:346–362
Águas M (1986) Simulação da circulação hidrodinâmica na Ria Formosa. Os sistemas lagunares do Algarve. University of Algarve, Faro, pp 78–90
Almeida LP, Ferreira Ó, Taborda R (2011) Geoprocessing tool to model beach erosion due to storms: application to Faro beach (Portugal). J Coast Res 64:1830. https://doi.org/10.1016/j.geomorph.2011.04.047
Anderson SC, Flemming JM, Watson R, Lotze HK (2011) Serial exploitation of global sea cucumber fisheries. Fish Res 12(3):317–339. https://doi.org/10.1111/j.1467-2979.2010.00397.x
Barbosa AB (2010) Seasonal and interannual variability of planktonic microbes in a mesotidal coastal lagoon (Ria Formosa, SE Portugal): impact of climatic changes and local human influences. In: Kennish MJ, Paerl HW (eds) Coastal Lagoons: critical habitats of environmental change. CRC Press, Boca Raton, pp 335–366
Bartlett M (1937) Properties of sufficiency and statistical tests. Proc Roy Soc Lond Ser A 160:268–282
Brava C (2005) Spawning of Holothuria tubulosa (Holothurioidea, Echinodermata) in the Alboran Sea (Mediterranean Sea). Zool Baetica 16:147–150
Brotas V, Cabrita T, Portugal A, Serôdio J, Catarino F (1995) Spatio-temporal distribution of the microphytobenthic biomass in intertidal flats of Tagus Estuary (Portugal). In: Balvay G (ed) Space partition within aquatic ecosystems. Developments in hydrobiology, vol 104. Springer, Dordrecht, pp 93–104
Bruckner AW, Johnson KA, Field JD (2003) Conservation strategies for sea cucumbers: can a CITES Appendix II listing promote sustainable international trade. SPC Bêche-de-mer Inf Bull 18:24–33
Bulteel P, Jangoux M, Coulon P (1992) Biometry, bathymetric distribution, and reproductive cycle of the holothuroid Holothuria tubulosa (Echinodermata) from Mediterranean seagrass beds. Mar Ecol 13:53–62. https://doi.org/10.1111/j.1439-0485.1992.tb00339.x
Cánovas F, Domínguez-Godino JA, González-Wangüemert M (2019) Short term approach to wildlife epidemiology of skin ulceration disease in the new target species of sea cucumber Holothuria arguinensis. Dis Aquat Organ. https://doi.org/10.3354/dao03373
Ceccarelli DM, Logan M, Purcell SW (2018) Analysis of optimal habitat for captive release of the sea cucumber Holothuria scabra. Mar Ecol Prog Ser 588:85–100. https://doi.org/10.3354/meps12444
Chen J (2004) Present status and prospects of sea cucumber industry in China. In: Lovatelli A, Conand C, Purcell SW, Uthicke S, Hamel JF, Mercier A (eds) Advances in sea cucumber aquaculture and management. FAO Fisheries Technical Paper 463. FAO, Rome, pp 25–38
Conand C (1990) The fishery resources of Pacific Island countries. Part 2. Holothurians, FAO Fisheries technical paper, No. 272. FAO, Rome, p 143
Conand C (1993) Ecology and reproductive biology of Stichopus variegatus an Indo-Pacific coral reef sea cucumber (Echinodermata: Holothuroidea). Bull Mar Sci 52(3):970–981
Conand C (2004) Present status of world sea cucumber resources and utilization: an international overview. Adv Sea Cucumber Aquac Manag (463):13–23
Conand C (2008) Population status, fisheries and trade of sea cucumbers in Africa and the Indian Ocean. In: Toral-Granda V, Lovatelli A, Vasconcellos M (eds) Sea cucumbers—a global review of fisheries and trade. FAO fisheries and aquaculture technical paper, No. 516. FAO, Rome, pp 143–193
Conover R (1966) Assimilation of organic matter by zooplankton. Limnol Oceanogr 11:338–345
Core Team R (2013) R: a language and environment for statistical computing, Vienna, Austria. http://www.R-project.org/. Accessed 16 Dec 2018
Costello M (2001) European register of marine species: a check-list of the marine species in Europe and a bibliography of guides to their identification. Muséum national d’histoire naturelle, Paris
Cousteau JY, Dugan J (1963) The living sea. Hamish Hamilton, London
Domínguez-Godino JA, González-Wangüemert M (2017) Establishing the baseline of sea cucumber aquaculture in Europe. In: World Aquaculture Society Congress, Cape Town, South Africa, June 2017, pp 26–30
Domínguez-Godino JA, González-Wangüemert M (2018a) Breeding and larval development of Holothuria mammata, a new target species for aquaculture. Aquac Res 49(4):1430–1440. https://doi.org/10.1111/are.13597
Domínguez-Godino JA, González-Wangüemert M (2018b) Assessment of Holothuria arguinensis feeding rate, growth and absorption efficiency under aquaculture conditions. New Zeal J Mar Fresh 53(1):60–76. https://doi.org/10.1080/00288330.2018.1480499
Domínguez-Godino JA, González-Wangüemert M (2018c) Does space matter? Optimizing stocking density of Holothuria arguinensis and Holothuria mammata. Aquac Res 49(9):3107–3115. https://doi.org/10.1111/are.13773
Domínguez-Godino JA, Slater MJ, Hannon C, González-Wangüemert M (2015) A new species for sea cucumber ranching and aquaculture: breeding and rearing of Holothuria arguinensis. Aquaculture 438:122–128. https://doi.org/10.1016/j.aquaculture.2015.01.004
Dong G, Dong S, Tian X, Wang F (2011) Effects of photoperiod on daily activity rhythm of juvenile sea cucumber, Apostichopus japonicus (Selenka). Chin J Oceanol Limnol 29(5):1015
Eckert GL (2007) Spatial patchiness in the sea cucumber Pachythyone rubra in the California Channel Islands. J Exp Mar Biol Ecol 348:121–132. https://doi.org/10.1016/j.jembe.2007.04.004
Entrambasaguas L, Pérez-Ruzafa A, García-Charton JA, Stobart B, Bacallado JJ (2008) Abundance, spatial distribution and habitat relationships of echinoderms in the Cabo Verde Archipelago (eastern Atlantic). Mar Fresh Res 59:477–488. https://doi.org/10.1071/MF07109
Eriksson H, Robinson G, Slater MJ, Troell M (2012) Sea cucumber aquaculture in the Western Indian Ocean: challenges for sustainable livelihood and stock improvement. Ambio 41(2):109–121. https://doi.org/10.1007/s13280-011-0195-8
González-Wangüemert M, Borrero-Pérez G (2012) A new record of Holothuria arguinensis colonizing the Mediterranean Sea. Mar Biodiver Rec. https://doi.org/10.1017/S1755267212000887
González-Wangüemert M, Braga T, Silva M, Valente S, Rodrigues F, Serrao E (2013) Volunteer programme assesses the Holothuria arguinensis populations in Ria Formosa (southern Portugal). SPC Bêche-de-mer Inf Bull 33:44–48
González-Wangüemert M, Valente S, Henriques F, Domínguez-Godino JA, Serrão EA (2016) Setting preliminary biometric baselines for new target sea cucumbers species of the NE Atlantic and Mediterranean fisheries. Fish Res 179:57–66. https://doi.org/10.1016/j.fishres.2016.02.008
González-Wangüemert M, Domínguez-Godino JA, Cánovas F (2018) The fast development of sea cucumber fisheries in the Mediterranean and NE Atlantic waters: from a new marine resource to its over-exploitation. Ocean Coast Manag 151:165–177. https://doi.org/10.1016/j.ocecoaman.2017.10.002
Graham JC, Battaglene SC (2004) Periodic movement and sheltering behaviour of Actinopyga mauritiana (Holothuroidea: Aspidochirotidae) in Solomon Islands. SPC Beche-de-mer Inf Bull 19:23–31
Hamel JF, Conand C, Pawson DL, Mercier A (2001) The sea cucumber Holothuria scabra (Holothuroidea: Echinodermata): its biology and exploitation as beche-de-mer. Adv Mar Biol 41:129–223
Hammond LS (1983) Nutrition of deposit-feeding holothuroids and echinoids (Echinodermata) from a shallow reef lagoon, Discovery Bay, Jamaica. Mar Ecol Prog Ser 10:297–305
Han Q, Keesing JK, Liu D (2016) A review of sea cucumber aquaculture, ranching, and stock enhancement in China. Rev Fish Sci Aquac 24(4):326–341. https://doi.org/10.1080/23308249.2016.1193472
James DB (2005) Information on juvenile holothurians. SPC Beche-de-Mer Inf Bull 21:26–27
Koehler R, Vaney C (1906) Mission des Pêcheries de la Côte occidentale d’Afrique. II. Echinodermes. Actes de la Société Linnéenne de Bordeaux 60:58–66
Kruskal W, Wallis W (1952) Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47(260):583–621
Li YS, Wang YL, Wang P (1994) Studies on the living environment and the selection of sea area for Stichopus japonicus. Trans Oceanol Limnol China 4:42–47
Malaquias MAE, Sprung MJ (2005) Population biology of the cephalaspidean mollusc Haminoea orbygniana in a temperate coastal lagoon (Ria Formosa, Portugal). Estuar Coast Shelf Sci 63:177–185. https://doi.org/10.1016/j.ecss.2004.11.005
Mann H, Whitney D (1947) On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat 18:50–60
Marquet N, Conand C, Power DM, Canário AV, González-Wangüemert M (2017) Sea cucumbers, Holothuria arguinensis and H. mammata, from the southern Iberian Peninsula: variation in reproductive activity between populations from different habitats. Fish Res 191:120–130
Massin C, Doumen C (1986) Distribution and feeding of epibenthic holothuroids on the reef flat of Laing Island (Papua New Guinea). Mar Ecol Prog Ser 31:185–195
McEuen FS (1988) Spawning behaviours of northeast Pacific sea cucumbers (Holothuroidea: Echinodermata). Mar Biol 98(4):565–585
Mendes FM, Marenzi AW, Di Domenico M (2006) Population patterns and seasonal observations on density and distribution of Holothuria grisea (Holothuroidea: Aspidochirotida) on the Santa Catarina Coast, Brazil. SPC Beche-de-mer Inf Bull 23:5–10
Mercier A, Battaglene SC, Hamel JF (2000a) Periodic movement, recruitment and size-related distribution of the sea cucumber Holothuria scabra in Solomon Islands. In: Jones MB, Azevedo JMN, Neto AI, Costa AC, Martins AMF (eds) Island, ocean and deep-sea biology. Springer, Dordrecht, pp 81–100
Mercier A, Battaglene SC, Hamel JF (2000b) Settlement preferences and early migration of the tropical sea cucumber Holothuria scabra. J Exp Mar Biol Ecol 249:89–110. https://doi.org/10.1016/S0022-0981(00)00187-8
Mezali K, Thandar AS (2014) First record of Holothuria (Roweothuria) arguinensis (Echinodermata: Holothuroidea: Aspidochirotida: Holothuriidae) from the Algerian coastal waters. Mar Biodivers Rec. https://doi.org/10.1017/S1755267214000438
Michio K, Kengo K, Yasunori K, Hitoshi M, Takayuki Y, Hideaki Y, Hiroshi S (2003) Effects of deposit feeder Stichopus japonicus on algal bloom and organic matter contents of bottom sediments of the enclosed sea. Mar Pollut Bull 47(1–6):118–125. https://doi.org/10.1016/S0025-326X(02)00411-3
Morgan AD (2011) Patterns of distribution and abundance of the temperate sea cucumber Australostichopus mollis on a rocky subtidal reef. New Zeal J Zool 38(3):195–206
Navarro PG, García-Sanz S, Barrio JM, Tuya F (2013) Feeding and movement patterns of the sea cucumber Holothuria sanctori. Mar Biol 160(11):2957–2966. https://doi.org/10.1007/s00227-013-2286-5
Navarro PG, García-Sanz S, Tuya F (2014) Contrasting displacement of the sea cucumber Holothuria arguinensis between adjacent nearshore habitats. J Exp Mar Biol Ecol 453:123–130. https://doi.org/10.1016/j.jembe.2014.01.008
Newton A, Mudge SM (2003) Temperature and salinity regimes in a shallow, mesotidal lagoon, the Ria Formosa, Portugal. Estuar Coast Shelf Sci 57:73–85. https://doi.org/10.1016/S0272-7714(02)00332-3
Newton A, Icely JD, Falcão M, Nobre A, Nunes JP, Ferreira JG, Vale C (2003) Evaluation of eutrophication in the Ria Formosa coastal lagoon, Portugal. Cont Shelf Res 23(17–19):1945–1961. https://doi.org/10.1016/j.csr.2003.06.008
Pacheco A, Ferreira O, Williams J, Garel E, Vila-Concejo A, Dias JA (2010) Hydrodynamics and equilibrium of a multiple-inlet system. Mar Geol 274:32–42. https://doi.org/10.1016/j.margeo.2010.03.003
Paterson DM, Hagerthey SE (2001) Microphytobenthos in contrasting coastal ecosystems: biology and dynamics. In: Reise K (ed) Ecological comparisons of sedimentary shores. Springer, Berlin, pp 105–125
Perkins RG, Honeywill C, Consalvey M, Austin HA, Tolhurst TJ, Paterson DM (2003) Changes in microphytobenthic chlorophyll a and EPS resulting from sediment compaction due to de-watering: opposing patterns in concentration and content. Cont Shelf Res 23(6):575–586. https://doi.org/10.1016/S0278-4343(03)00006-2
Pilkey O, Neal W, Monteiro J, Dias J (1989) Algarve barrier islands: a noncoastal-plain system in Portugal. J Coast Res 5:239–261
Pires BF (2016a) Polícia Marítima apreende 120 quilos de Pepinos-do-Mar em Faro. http://barlavento.pt/destaque/policia-maritima-apreende-mais-de-120-quilos-de-pepinos-do-mar-emfaro. Accessed 16 Dec 2018
Pires BF (2016b) Pepinos-do-mar da Ria Formosa já estao em risco de extinçao. http://barlavento.pt/destaque/pepinos-do-mar-da-ria-formosa-ja-estao-em-risco-de-extincao. Accessed 16 Dec 2018
Preston GL (1993) Beche-de-mer. In: Nearshore marine resources of the Southern Pacific: information for fisheries development and management. Institute of Pacific Studies, Fiji; Forum Fisheries Agency, Solomon Islands; International Centre for Ocean Development, Canada, pp 371–407
Purcell SW (2004) Criteria for release strategies and evaluating the restocking of sea cucumbers. In: Lovatelli A, Conand C, Purcell SW, Uthicke S, Hamel JF, Mercier A (eds) Advances in sea cucumber aquaculture and management. FAO fisheries and aquaculture technical paper No. 463. FAO, Rome, pp 181–189
Purcell SW, Kirby DS (2006) Restocking the sea cucumber Holothuria scabra: Sizing no-take zones through individual-based movement modelling. Fish Res 80(1):53–61. https://doi.org/10.1016/j.fishres.2006.03.020
Purcell SW, Gossuin H, Agudo NS (2009) Status and management of the sea cucumber fishery of La Grande Terre, New Caledonia. World Fish Center studies and reviews No. 1901. The World Fish Centre, Penang
Purcell SW, Hair CA, Mills DJ (2012a) Sea cucumber culture, farming and sea ranching in the tropics: progress, problems and opportunities. Aquaculture 368:68–81. https://doi.org/10.1016/j.aquaculture.2012.08.053
Purcell SW, Samyn Y, Conand C (2012b) Commercially important sea cucumbers of the world. FAO Species Catalogue for Fishery Purposes No. 6, FAO, Rome. ISBN: 9789251067192
Purcell SW, Mercier A, Conand C, Hamel JF, Toral-Granda MV, Lovatelli A, Uthicke S (2013) Sea cucumber fisheries: global analysis of stocks, management measures and drivers of overfishing. Fish Res 14(1):34–59. https://doi.org/10.1111/j.1467-2979.2011.00443.x
Purcell SW, Conand C, Uthicke S, Byrne M (2016) Ecological roles of exploited sea cucumbers. Oceanogr Mar Biol Annu Rev 54:367–386
Ribeiro J, Monteiro CC, Monteiro P, Bentes L, Coelho R, Gonçalves JMS, Lino PG, Erzini K (2008) Long-term changes in fish communities of the Ria Formosa coastal lagoon (southern Portugal) based on two studies made 20 years apart. Estuar Coast Shelf Sci 76:57–68. https://doi.org/10.1016/j.ecss.2007.06.001
Roberts D, Gebruk A, Levin V, Manship B (2003) Feeding and digestive strategies in deposit-feeding holothurians. Oceanogr Mar Biol Annu Rev 38:257
Rodrigues NV (2012) New geographic distribution records for Northeastern Atlantic species from Peniche and Berlengas Archipelago. ARQUIPÉLAGO Life Mar Sci (29):4
Rodrigues F, Valente S, González-Wanguemert M (2015) Genetic diversity across geographical scales in marine coastal ecosystems: Holothuria arguinensis a model species. J Exp Mar Biol Ecol. https://doi.org/10.1016/j.jembe.2014.12.006
Sewell MA (1990) Aspects of the ecology of Stichopus mollis (Echinodermata: Holothuroidea) in north-eastern New Zealand. New Zeal J Mar Fresh 24(1):97–103. https://doi.org/10.1080/00288330.1990.9516405
Shapiro S, Wilk M (1995) An analysis of variance test for normality (complete samples). Biometrika 52(3/4):591–611
Shiel G (2004) Field observations of juvenile sea cucumbers. SPC Beche-de-Mer Inf Bull 20:6–11
Shiell GR, Knott B (2008) Diurnal observations of sheltering behaviour in the coral reef sea cucumber Holothuria whitmaei. Fish Res 91(1):112–117. https://doi.org/10.1016/j.fishres.2007.12.010
Shiell GR, Knott B (2010) Aggregations and temporal changes in the activity and bioturbation contribution of the sea cucumber Holothuria whitmaei (Echinodermata: Holothuroidea). Mar Ecol Prog Ser 415:127–139
Siegenthaler A, Cánovas F, González-Wangüemert M (2015) Spatial distribution patterns and movements of Holothuria arguinensis in the Ria Formosa (Portugal). J Sea Res 102:33–40. https://doi.org/10.1016/j.seares.2015.04.003
Slater MJ, Jeffs AG, Carton AG (2009) The use of the waste from green-lipped mussels as a food source for juvenile sea cucumber, Australostichopus mollis. Aquaculture 292(3–4):219–224. https://doi.org/10.1016/j.aquaculture.2009.04.027
Sloan NA, von Bodungen B (1980) Distribution and feeding of the sea cucumber Isostichopus badionatus in relation to shelter and sediment criteria of the Bermuda Platform. Mar Ecol Prog Ser 2:14–28
Sonnenholzner J (2003) Seasonal variation in the food composition of Holothuria theeli (Holothuroidea: Aspidochirotida) with observations on density and distribution patterns at the central coast of Ecuador. Bull Mar Sci 73(3):527–543
Sprung M (2001) Larval abundance and recruitment of Carcinus maenas L. close to its southern geographic limit: a case of match and mismatch. Hydrobiologia 449:153–158
Thandar AS (1988) A new subgenus of Holothuria with a description of a new species from the south-east Atlantic Ocean. J Zool 215(1):47–54. https://doi.org/10.1111/j.1469-7998.1988.tb04884.x
Tommasi LR (1969) Lista dos Holothurioidea recentes do Brasil. Instituto oceanográfico, Universidade de São Paulo, São Paulo
Tukey JW (1949) Comparing individual means in the analysis of variance. Biometrics 5(2):99–114
Underwood GJC, Barnett M (2006) What determines species composition in microphytobenthic biofilms. In: Kromkamp J (ed) Functioning of microphytobenthos in estuaries. Microphytobenthos symposium, pp 121–138. Royal Netherlands Academy of Arts and Sciences, Amsterdam
Uthicke S (2001a) Interactions between sediment-feeders and microalgae on coral reefs: grazing losses versus production enhancement. Mar Ecol Prog Ser 210:125–138
Uthicke S (2001b) Nutrient regeneration by abundant coral reef holothurians. J Exp Mar Biol Ecol 265(2):153–170. https://doi.org/10.1016/S0022-0981(01)00329-X
Uthicke S, Karez R (1999) Sediment patch selectivity in tropical sea cucumbers (Holothurioidea: Aspidochirotida) analysed with multiple choice experiments. J Exp Mar Biol Ecol 236:69–87. https://doi.org/10.1016/S0022-0981(98)00190-7
Uthicke S, Welch D, Benzie JAH (2004) Slow growth and lack of recovery in overfished holothurians on the Great Barrier Reef: evidence from DNA fingerprints and repeated large-scale surveys. Conserv Biol 18:1395–1404. https://doi.org/10.1111/j.1523-1739.2004.00309.x
Yamana Y, Hamano T, Miki K (2006) Distribution of the Japanese sea cucumber Apostichopus japonicus in the intertidal zone of Hirao Bay, eastern Yamaguchi Prefecture, Japan—suitable environmental factors for juvenile habitats. J Nat Fish Univ 54:111–120
Yokoyama H (2013) Growth and food source of the sea cucumber Apostichopus japonicus cultured below fish cages—potential for integrated multi-trophic aquaculture. Aquaculture 372:28–38. https://doi.org/10.1016/j.aquaculture.2012.10.022
Young CM, Chia FS (1982) Factors controlling spatial distribution of the sea cucumber Psolus chitionoides settling and post-settling behaviour. Mar Biol 69:195–205
Yuan X, Yang H, Zhou Y, Mao Y, Zhang T, Liu Y (2006) The influence of diets containing dried bivalve feces and/or powdered algae on growth and energy distribution in sea cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea). Aquaculture 256:457–467. https://doi.org/10.1016/j.aquaculture.2006.01.029
Zacarias DA, Williams AT, Newton A (2011) Recreation carrying capacity estimations to support beach management at Praia de Faro, Portugal. Appl Geogr 31(3):1075–1081. https://doi.org/10.1016/j.apgeog.2011.01.020
Zamora LN, Jeffs AG (2011) Feeding, selection, digestion and absorption of the organic matter from mussel waste by juveniles of the deposit-feeding sea cucumber, Australostichopus mollis. Aquaculture 317(1):223–228. https://doi.org/10.1016/j.aquaculture.2011.04.011
Acknowledgements
This research was supported by the CUMFISH (PTDC/MAR/119363/2010; http://www.ccmar.ualg.pt/cumfish/) and CUMARSUR (PTDC/MAR-BIO/5948/2014) projects funded by Fundacão para Ciência e Tecnologia (FCT, Portugal). Dr. Mercedes González-Wangüemert was supported by Fundação para a Ciência e Tecnologia (FCT) postdoctoral Grant (SFRH/BPD/70689/2010) and later by FCT Investigator Programme-Career Development Contract (IF/00998/2014). Jorge Antonio Domínguez-Godino was supported by Piscifactorias Albadalejo S.L and later by research fellow (CCMAR/BI/0007/2015). Special thanks to the volunteers that collaborated along the samplings and to Nacho Pérez Blasco for his help during the sampling and laboratory work. In addition, special thanks to Fernando Cánovas for his help during data analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Télesphore Sime-Ngando
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Domínguez-Godino, J.A., González-Wangüemert, M. Habitat associations and seasonal abundance patterns of the sea cucumber Holothuria arguinensis at Ria Formosa coastal lagoon (South Portugal). Aquat Ecol 54, 337–354 (2020). https://doi.org/10.1007/s10452-020-09746-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-020-09746-0