Abstract
In the last years, increasing interest has been dedicated to the quality assessment of brackish-water systems. Traditionally, fish community is an important biological element used to assess the quality status of transitional water bodies. In this study, we analysed the effect of anthropogenic pressures on the population of a small teleost, the sand smelt Atherina boyeri, in a Mediterranean lagoon by means of body condition. Fish were sampled once a year during the period 2010–2012, in 32 sampling sites, and for each specimen individual body condition factor was estimated. A negative significant correlation was found between condition factor and pressures related to alteration of the hydrographic regime, while a significant positive correlation was found with trophic status indicators and fishery activities. Therefore, morphological and hydrological alteration of coastal lagoons, modifying the quality and the availability of resources, seems to influence the health of resident populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The contribution of biological elements to the assessment of ecological status in transitional waters is a relevant issue (Cabral et al. 2012). In the last decade, many papers dealt with this problem by many points of view. In particular, a great effort has been devoted to develop good methods able to define and assess the ecological status of these aquatic ecosystems, especially in Europe for the implementation of the Water Framework Directive (WFD). For the northern Atlantic region, official methodologies using fish as BQE (Biological Quality Elements) have been developed and intercalibrated (Lepage et al. 2016), but for the Mediterranean transitional water systems an official methodology is still lacking, even if different tools have been proposed (e.g. Franco et al. 2009; Drouineau et al. 2012).
Estuaries and coastal lagoons are widely known to be elective habitats for abundant and diversified fish populations (Blaber 1997; Franco et al. 2008; Able and Fahay 2010; Potter et al. 2015). The high productivity, coupled with physicochemical and morphological habitat features, allows these environments to play several ecological functions, such as feeding grounds or spawning and nursery areas for many fish species (Elliott and Hemingway 2002; McLusky and Elliott 2004; Franco et al. 2006; Rountree and Able 2007; Perez-Ruzafa et al. 2013), some of which are of conservation or economic value. On the other hand, fish fauna is a key component of aquatic ecosystems, mainly for its important ecological role in aquatic food webs, the significant lifespan, which integrates the effects of environmental pressures on a relative long timescale, and for its economical values as food resource (Whitfield and Elliott 2002; Van Der Oost et al. 2003; Harrison and Whitfield 2004; Cabral et al. 2012).
The increasing attention paid to the quality of lagoons and estuaries (Gilliers et al. 2004), which are subjected to high anthropogenic pressures (Kennish 2002; Airoldi and Beck 2007), raised the issue of evaluating and quantifying environmental quality using the health status of fish populations. One of the first attempts to deal with this issue involved the introduction of body condition factors at the beginning of the twentieth century (Froese 2006). These indices, firstly developed for fish, are simple and concise indicators that, integrating several aspects of fish biology, can be good predictors of individual fitness, thus giving a comprehensive picture of the status of natural populations (Vila-Gispert and Moreno-Amich 2001). Indeed, different factors contribute to the body condition of an individual, such as general conditions of the environment, food quality, quantity and availability. For these reasons, fish condition can be considered an integrative measure of environmental quality, summarising the health and physiological state of a population (Vila-Gispert and Moreno-Amich 2001; Jakob et al. 1996). Indeed, it integrates the effects of both biotic and abiotic environmental influences on fish (La Peyre et al. 2007; Searcy et al. 2007; Zapfe and Rakocinski 2008), thus allowing the comparison of habitat quality in different environments (Amara et al. 2007; Haas et al. 2009; Vasconcelos et al. 2009).
Individual health, quantified by means of condition indices, gives a snapshot of the physiological state of an animal (Jakob et al. 1996), hence providing information about the entire population (Stevenson and Woods 2006). Recently, Perez-Ruzafa et al. (2018) stressed the importance of analysing the physiological mechanisms involved in the allocation of energy resources by individuals to obtain information at the population level. This would help to evaluate the response of organisms and populations to human-induced alteration of ecosystems.
Among morphometric indices, one of the most commonly used is Fulton’s condition factor. However, it presents several issues, in particular when the assumption of isometric growth is not met (Jones et al. 1999). This drawback may be overcome by using the Kn index (Blackwell et al. 2000), calculated as the ratio between the measured weight and the theoretical weight predicted according to the length-weight regression. Kn values are not influenced by body shape, and the index can be used for the comparison of fishes having different lengths. Moreover, it showed to be influenced by environmental factors (Blackwell et al. 2000).
In the fields of fishery management and environmental monitoring policy, the link existing between the health of fish populations, and the effect of human activities on such populations, is of particular interest (Leamon et al. 2000; Ciotti et al. 2013). Many works used small-sized fish in the context of environmental monitoring activities, since these species are relatively abundant, can be easily captured and show a certain degree of site fidelity (Leamon et al. 2000; Galloway and Munkittrick 2006). Furthermore, in some cases, the use of the body condition indices allowed to detect the impacts of anthropogenic pressures on fish populations (Efitre et al. 2009; Piazza and La Peyre 2010).
Estuarine residents are the elective guild of transitional water habitats, and they can be used to assess the quality of these ecosystems. Their entire life cycle is indeed spent within transitional waters, and thus, they integrate all the stimuli coming from such environment within their biological cycle (Bortone et al. 2005; Fiorin et al. 2007; Zucchetta et al. 2012). Despite that, the difficulties in the evaluation of anthropogenic pressures in transitional water ecosystems are exacerbated by the so-called Estuarine Quality Paradox (Elliott and Quintino 2007). Organisms inhabiting such environments, and resident species in particular, are well adapted to naturally stressing environmental conditions, and at the same time, these areas are often subjected to many different pressures deriving from human activities (Franco et al. 2009; Cavraro et al. 2017). Thus, determining and quantifying the health status of a population in relation to man-induced pressures it is not always an easy task in transitional water ecosystems. In this context, a particular key point is disentangling the relative contribution of natural variability and anthropogenic pressures in shaping both biological and life-history traits of a species (Cavraro et al. 2013, 2014).
In this study, we used the body condition of a small estuarine resident teleost, the sand smelt Atherina boyeri Risso, 1810, to evaluate the effects of anthropogenic pressures in a Mediterranean coastal lagoon. In particular, the aims of this study were to: (1) analyse the spatiotemporal variability of the body condition index, taking into account the natural heterogeneity that characterises coastal lagoons, and (2) verify whether this indicator might be sensitive to environmental alterations deriving from anthropogenic pressures and thus its contribution to the assessment of ecological status.
Materials and methods
Study area
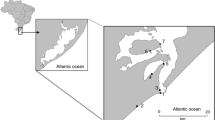
The Marano and Grado Lagoon belongs to the extended transitional system network of the northern Adriatic Sea basin (Fig. 1). It is located between the Tagliamento and Isonzo River deltas, and it extends over approximately 160 km2. The Lagoon is one of the best conserved wetlands of the whole Mediterranean area. This system is a coastal microtidal lagoon of large dimensions protected by the Ramsar Convention since 1971, and it is a Site of Community Importance at European level.
Based on geomorphological and hydraulic differences, two main basins are identifiable: Marano and Grado (De Vittor et al. 2012). The first one (western sector) shows few areas above the mean sea level and several channels linking the plain spring rivers flowing into the lagoon basin with the sea. The second one (eastern sector) is shallower (< 1 m, on average) with a complex hydrographical network including tidal flats, tidal channels and sub-tidal zones. Besides its natural significance, the lagoon and the neighbouring mainland host several socio-economic activities (e.g. industrial sites, commercial harbours, marinas, fishing, fish and clam farming, and tourism; Ramieri et al. 2011), which pose various environmental concerns (e.g. in terms of priority pollutants).
Field samplings
Preliminary studies conducted in the lagoon highlighted the presence of three water body types (mesohaline, polyhaline and euhaline) and 13 river mouths. Considering some specific descriptors such as geographical location, geomorphology, tides and surface salinity, 16 water bodies (WBs) were identified by local administration, three of them being heavily modified. The latter represent areas subjected to substantial changes due to anthropogenic activities (e.g. significant hydraulic regime changes and ex fish farms).
A three-year fish monitoring programme (2010–2012) was carried out on 32 stations (Fig. 1), where samples were collected by means of fyke nets. Four traps were used in each station. This gear is commonly used by traditional fishery throughout the northern Adriatic lagoons (Zucchetta et al. 2016). The fyke net consists of a barrier (about 60 m in length and 1.3 m in height) with a 0.7-cm mesh size, leading the nektonic organisms towards four cone-shaped, unbaited traps, of the same mesh size. Sampling was performed twice a year during spring and autumn with the aid of local fishermen, and all samples were collected after 24 h from the setting of the fyke nets. A. boyeri samples were preserved at − 20°C.
Data collection and analysis
Fish were measured to the nearest 0.1 mm (total length, TL) and weighed to the nearest 0.1 g (total fresh weight, TW). In the samples exceeding 100 individuals, 100 specimens were randomly selected for the measurements. A preliminary check of the population structure, using the length-frequency histograms of the sampled fish (“Appendix A”), was performed, on both spring and autumn samples, with the Bhattacharya method. Results of this analysis (not reported in the present study) showed that, in autumn, most of the individuals sampled belonged to the same cohort (0+). Since the species spawns during spring and summer, after September, females’ ovaries are empty (Boscolo 1970). Therefore, only fish sampled in autumn were considered in this analysis, to avoid the influence of gonad development on total weight. Individual condition was calculated as the relative condition factor, according to the equation Kn = W/W′, where W is the total weight of an individual fish and W′ is the length-specific weight predicted by the linear regression calculated for the relationship logTL − logTW (Blackwell et al. 2000). For each sampling station, mean values of TL, TW and Kn were then calculated, as well as mean salinity. Anthropogenic pressures were estimated for each water body (N = 16) as in Zucchetta et al. (2016), adapting the approach proposed by Aubry and Elliott (2006) (Table 1, “Appendix B”). An analysis of variance (ANOVA) was performed to test for differences in biological, environmental and pressure variables between the two sub-basins of Marano and Grado, using water bodies as replicates. The same data set was used to calculate Spearman correlations among Kn, salinity and the pressures. To test for the effect of salinity and pressures on Kn, a multiple regression approach was adopted, considering the interaction of each variable with the sub-basin. Collinear predictor variables were identified and removed from the model when the variance inflation factor resulted higher than 5 (Montgomery and Peck 1992).
Results
Overall, total anthropogenic pressure level of intensity, calculated as the average of the three pressure categories, did not differ between the two sub-basins (Fig. 2, Table 2). The Marano sub-basin was subjected to significantly higher pressure related to the use of lagoon resources and to environmental quality, while morphological alteration significantly affected the Grado sub-basin more strongly, mainly due to the alteration of the hydrographic regime (Fig. 2, Table 2). Within the pressure category related to the use of the lagoon, fishery effort and the extent of ports in particular were significantly higher in Marano compared to Grado. Regarding the pressure category related to environmental quality, despite the significantly lower values of the biotic index for the macrozoobenthic community and the dissolved oxygen concentration in the Marano sub-basin, Grado showed an overall lower intensity of pressure, probably as the result of one indicator, the low DIN values recorded (Fig. 2).
Significant differences (Table 2) in average salinity were found between the two sub-basins (Fig. 3). In the Grado sub-basin, salinity was on average 10 points higher than in Marano. Furthermore, a substantial spatial homogeneity was observed in the former sub-basin, while Marano showed a clear sea-lagoon gradient (Fig. 4b).
Significant differences were found also in average length and weight of A. boyeri (Fig. 3), with fish in Marano being longer and heavier compared to those sampled in Grado. On the contrary, in terms of condition factor (Fig. 3) no significant difference was found between the two basins (Table 2). Finally, no significant relationship was found between size and condition factor.
Significant correlations were found among A. boyeri condition, salinity and the indicators/pressures considered in the two parts of the lagoon (Table 3). In the Grado sub-basin, no significant correlation of salinity was found with the condition factor or any indicator/pressure. In turn, a significant negative correlation was found between condition factor and morphological pressures (− 0.34) and, in particular, between condition factor and pressure deriving from the alteration of the hydrographic regime (− 0.42). On the contrary, a positive correlation between condition factor and DIN was found (0.45).
Conversely, the condition factor in the Marano basin showed a significant negative correlation with salinity (− 0.60). Indeed, moving from the sea inlets to the inner parts of the lagoon, higher values of Kn were observed (Fig. 4). Also, in the Marano sub-basin some indicators/pressures resulted to be correlated with salinity. When an indicator/pressure showed a positive correlation with salinity (thus decreasing along the sea-lagoon gradient), the possible effect of this indicator/pressure on fish cannot be distinguished from the natural gradient’s effect. Therefore, the attention was focused only to the indicators/pressures showing no significant correlation, or a negative correlation, with salinity. In this context, in both sub-basins, a negative correlation was found between condition factor and the alteration of the hydrographic regime (− 0.41), and a positive correlation was found not only with dissolved nitrogen concentration (0.72) but also with reactive phosphorous concentration (0.46). Furthermore, a significant positive correlation was found between fishery and condition factor (0.43).
At first, multiple regression analysis considered as predictors all the pressure variables and salinity in the two sub-basins. Once collinear variables were excluded, the model included only the sub-basin, the alteration of the hydrographic regime and concentrations of dissolved oxygen and reactive phosphorous. Among predictors, only alteration of the hydrographic regime resulted to be significant (Table 4), with a negative relationship with condition factor in both sub-basins (Fig. 4).
Looking at spatial variability in condition factor (Fig. 5), it is clear that this indicator showed higher values in the more confined water bodies of Marano sub-basin, following the salinity gradient (Fig. 5). This gradient was absent in Grado, while the condition factor showed higher values near the sea inlets and lower values in proximity of the mainland. Higher values of morphological and, in particular, hydrological pressures were found in Grado (Fig. 2), particularly in the eastern part of the sub-basin (Fig. 5c), while Marano showed a higher intensity of pressure fishery activities (Table 2; Fig. 5d). Considering the water quality indicators, an east–west gradient of DIN was observed (Fig. 5e), with significant higher values in Marano; no statistical difference was found for RP (Table 2).
Discussion
Estuarine resident fish species are known to be well adapted to stressful environments such as coastal lagoons (Franco et al. 2008). This guild is the most abundant in lagoon shallow-water habitats, accounting for about 90% of the total fish abundance (Franzoi et al. 2010). A. boyeri is one of the most abundant resident fish in Mediterranean coastal lagoons (Franzoi et al. 1989; Malavasi et al. 2004; Manzo 2010; Perez-Ruzafa et al. 2007a; Maci and Basset 2010; Verdiell-Cubedo et al. 2012), with a key role in estuarine food web being one of the main links between primary benthic and planktonic consumers and the higher trophic levels (Bartulovic et al. 2004). Although A. boyeri is not a sedentary benthic species, it is characterised by a certain degree of site fidelity, in particular to spawning grounds, resulting in semi-isolated populations (Koutrakis et al. 2004). Nevertheless, the plasticity of this species allows the colonisation of nearly all any shallow-water habitats (Leonardos 2001; Koutrakis et al. 2004), coping with the often strong and rapid variations in temperature, salinity and dissolved oxygen (Perez-Ruzafa et al. 2007b). In transitional water systems, salinity is one of the main drivers of biological communities (Attrill 2002; McLusky and Elliott 2004), influencing species abundance and distribution. In the Adriatic lagoon of Acquatina, for example, Maci and Basset (2010) analysed the populations of A. boyeri along a decreasing salinity gradient, observing higher densities and a better body condition moving from the sea to the inner portion of the lagoon. A similar pattern was found also in the present study, but with a distinction between the two sub-basins investigated. Indeed, a clear salinity gradient was present only in Marano, while it was absent in Grado (Ferrarin et al. 2010). Overall, the mean size of A. boyeri was higher in Marano, probably due to the higher trophic status of this basin. The significant river discharge that characterises this part of the lagoon not only determines the freshwater input that generates the observed salinity gradient, but also causes a relevant input of nutrients, i.e. nitrogen, deriving from agricultural activities and untreated domestic sewage (Acquavita et al. 2015). Thus, A. boyeri can take advantage of the high productivity of lagoon waters: despite the stressful environmental conditions often found in transitional water ecosystems, lagoon fish species were found to adopt life-history strategies that allow them to cope with natural stress (Perez-Ruzafa et al. 2013). The differences in size and weight found between the two sub-basins should not be ascribed to differences in age, as reported in Materials and Methods. This would be in accordance with the biology of A. boyeri: other authors (Fernandez-Delgado et al. 1988; Bartulovic et al. 2004) found a short life cycle, with only a small number of individuals older than 1+ age class, with most of the spawners dying after the reproductive season. Anyway, further studies are needed to highlight if some of the differences found between the two sub-basins could depend on differences in life-history traits related to growth and reproduction.
In coastal lagoons, a high nutrient enrichment may be detrimental to the ecological status, since it can be the cause of dystrophic crisis (Solidoro et al. 2010). Usually, these events occur during the summer period, when the high temperatures cause mass mortality of the accumulated biomass of macroalgae (Sfriso et al. 2003). In the studied lagoon, such events are usually rare and occur on a small spatial scale, since the system is phosphorous limited, and the water renewal through the sea inlets guarantees a dilution of nutrients (Acquavita et al. 2015). This would explain the positive correlation found between the condition factor and water concentration of nitrogen and phosphorous, although no significant differences in Kn were found between the two sub-basins. The higher trophic status stimulates the productivity of the basin, maintaining a trophic network that allows secondary consumers like A. boyeri to find more food, thus reaching a larger size.
Also the intensity of fishing activity was positively linked to the body condition of A. boyeri. In the lagoon, the main fishing activity is represented by artisanal fishery carried out by means of fyke nets (Bettoso et al. 2013), with a mean fishing effort higher in Marano than in Grado (25 fishermen in Marano, 5 in Grado). Usually, fishing results in a reduction in the mean individual size in natural populations (Berkeley et al. 2004), but in the present study A. boyeri in Marano were significantly larger than in Grado. Artisanal fishery, such as that carried out in North Adriatic lagoons, is thought to be a sustainable activity. For example, in a similar ecosystem (the Venice lagoon), minor effects of artisanal fishery were observed on the fish community analysed by Zucchetta et al. (2016) and only the 6% of the catches, in terms of biomass, is discarded (Pranovi et al. 2013). Furthermore, fishing effort is usually concentrated in the areas that guarantee the maximum yield. According to the findings of Zucchetta et al. (2016) for the Venice lagoon, also in the present study the positive correlation observed between fishery pressure and body condition of A. boyeri might be explained by a sustainable fishing effort focused on the most productive areas of the lagoon, which therefore host fish populations in better conditions.
While pressures deriving from nutrient concentrations and fishing effort did not cause significant negative impacts on the lagoon ecosystem, a different pattern emerged considering the morphological alterations. In the last 60 years, severe morphological changes deeply altered the lagoon landscape (Fontolan et al. 2012). Drowning (the combined effect of eustatism, subsidence and autocompaction) and erosion by vessel-generated waves are two of the main causes of the reduction in salt marsh extension (144 ha, − 16%). Also submerged habitats, such as tidal and sub-tidal flats, are affected by erosion processes. The loss of fine sediments is determining a general deepening of the lagoon and a progressive shift into a marine embayment (Fontolan et al. 2012; Ferrarin et al. 2016). In the present study, a negative correlation was found between the intensity of morphological pressures and the body condition of A. boyeri. This picture is quite clear in the Grado basin, where no salinity gradient was observed and the intensity of morphological pressures was higher. On the other hand, in the Marano basin the positive correlation between salinity and some of the indicators of pressure (i.e. intertidal, marina, DO and port) does not allow to assess whether the decreasing values of body condition observed moving from the inner lagoon towards the sea inlets are due to a reduction in the pressure intensity or whether it is a natural decreasing linked to the salinity gradient. Considering the single-pressure indicators, alteration of the hydrological regime gives a clearer picture. In both basins, no significant correlation with salinity was found, while body condition showed a significant negative correlation with the pressure indicator. As an example of alteration to the hydrographic regime, the eastern part of the lagoon is physically divided into two sections by the bridge connecting the main land to the city of Grado (Ferrarin et al. 2010). Considering the water bodies, such bridge separates TEU1 and TPO1 from FM2 and FM3. The latter were indeed classified as heavily modified water bodies in which the sediment particles entering the lagoon via the easternmost inlet remain trapped due to the presence of the bridge (Ferrarin et al. 2016).
A clearly negative response of fish community to morphological alterations was also found by Zucchetta et al. (2016) in the Venice lagoon. In that study, effects of pressures on the lagoon morphology were stronger for resident species. Thus, in coastal lagoons it seems that morphological pressures, and the alteration of the hydrodynamic conditions in particular, might play a key role, affecting those species which spend all the life cycle within transitional waters.
Conclusions
An official method based on fish fauna for the assessment of ecological status in Mediterranean coastal lagoons is still lacking, although a multimetric index has been proposed by Franco et al. (2009). Multimetric indices are usually focused on the whole fish assemblage, considering metrics related to species/guilds abundance and biomass, but also information about disturbance-sensitive species might be used as supporting information to assess the ecological status of aquatic environment. An approach considering the health status of individuals of key species might provide complementary information on environmental conditions, although reliable predictions and assessments of cause–effect relationships should rely on a good knowledge about the links between biological and physiological parameters with the health status of individuals (Perez-Ruzafa et al. 2018). In some cases, the combined effect of natural variability and environmental parameters could have prevented to directly identify the impacts on fish caused by anthropogenic pressures. Despite this, results of the present study showed how even a simple index like the body condition factor could be a good indicator of the health status of A. boyeri populations affected by anthropogenic pressures, in particular for those pressures deriving from the morphological (hydrological) alteration of the environment. The extension of this approach to other lagoons, possibly using different species or indicators [i.e. the Wr proposed by Blackwell et al. (2000) or other physiological indices], could support the fish-based assessment of ecological status on a broader geographical scale.
References
Able KW, Fahay MP (2010) Ecology of estuarine fishes: temperate waters of the western North Atlantic. Johns Hopkins University Press, Baltimore
Acquavita A, Aleffi IF, Bencia C, Bettoso N, Crevatina E, Milania L, Tamberlicha F, Toniatti L, Barbieri P, Licen S, Mattassi G (2015) Annual characterization of the nutrients and trophic state in a Mediterranean coastal lagoon: the Marano and Grado Lagoon (northern Adriatic Sea). Reg Stud Mar Sci 2:132–144
Airoldi L, Beck MW (2007) Loss, status and trends for coastal marine habitats of Europe. Oceanogr Mar Biol: Ann Rev 45:345–405
Amara R, Maziane T, Gilliers C, Hrmel G, Laffargue P (2007) Growth and condition indices in juvenile Solea solea measured to assess the quality of essential fish habitat. Mar Ecol Prog Ser 351:201–208
Attrill MJ (2002) A testable linear model for diversity trends in estuaries. J Anim Ecol 71:262–269
Aubry A, Elliott M (2006) The use of environmental integrative indicators to assess seabed disturbance in estuaries and coasts: application to the Humber Estuary, UK. Mar Poll Bull 53:175–185
Bartulovic V, Glamuzina B, Conides A, Ducic J, Lucic D, Njire J, Kozul V (2004) Age, growth, mortality and sex ratio of sand smelt, Atherina boyeri Risso, 1810 (Pisces: Atherinidae) in the estuary of the Mala Neretva River (middle-eastern Adriatic, Croatia). J Appl Ichthyol 20:427–430
Berkeley SA, Hixon MA, Larson RJ, Love MS (2004) Fisheries sustainability via protection of age structure and spatial distribution of fish populations. Fisheries 29:23–32
Bettoso N, Acquavita A, D’Aietti A, Matassi G (2013) The Marano and Grado lagoon: a brief synopsis on the aquatic fauna and fisheries resources. Ann Ser Hist Nat 23:135–142
Blaber SJM (1997) Fish and fisheries of tropical estuaries (fish and fisheries series 22). Chapman and Hall, London
Blackwell BG, Brown ML, Willis DW (2000) Relative weight (Wr) status and current use in fisheries assessment and management. Rev Fish Sci 8:1–44
Bortone SA, Dunson WA, Greenawalt JM (2005) Fishes as estuarine indicators. In: Bortone SA (ed) Estuarine indicators. CRC Press, Boca Raton
Boscolo L (1970) Osservazioni sulla biologia e sulla pesca dell’Atherina boyeri Risso 1810 (Osteichthyes, Atherinidae) vivente nelle acque dell’Alto Adriatico. Boll pes piscicol idrobiol 25:61–79
Cabral HN, Fonseca VF, Gamito R, Gonçalves CI, Costa JL, Erzini K, Gonçalves J, Martins J, Leite L, Andrade JP, Ramos S, Bordalo A, Amorim E, Neto JM, Marques JC, Rebelo JE, Silva C, Castro N, Almeida PR, Domingos I, Gordo LS, Costa MJ (2012) Ecological quality assessment of transitional waters based on fish assemblages in Portuguese estuaries: the Estuarine Fish Assessment Index (EFAI). Ecol Indic 19:144–153
Cavraro F, Torricelli P, Franzoi P, Malavasi S (2013) Productivity in natural and artificial habitats in brackish water systems: an example from Aphanius fasciatus populations. Trans Wat Bull 7:23–31
Cavraro F, Daouti I, Leonardos I, Torricelli P, Malavasi S (2014) Linking habitat structure to life history strategy: insights from a Mediterranean killifish. J Sea Res 85:205–213
Cavraro F, Zucchetta M, Malavasi S, Franzoi P (2017) Small creeks in a big lagoon: the importance of marginal habitats for fish populations. Ecol Eng 99:228–237
Ciotti BJ, Targett TE, Nash RDM, Burrows MT (2013) Small-scale spatial and temporal heterogeneity in growth and condition of juvenile fish on sandy beaches. J Exp Mar Biol Ecol 448:346–359
De Vittor C, Faganeli J, Emili A, Covelli S, Predonzani S, Acquavita A (2012) Benthic fluxes of oxygen, carbon and nutrients in the Marano and Grado Lagoon (northern Adriatic Sea, Italy). Est Coast Shelf Sci 113:57–70
Drouineau H, Lobry J, Delpech C, Bouchoucha M, Mahévas S, Courrat A, Pasquaud S, Lepage M (2012) A Bayesian framework to objectively combine metrics when developing stressor specific multimetric indicator. Ecol Indic 13:314–321
Efitre J, Chapman LJ, Murie DJ (2009) Fish condition in introduced tilapias of Ugandan crater lakes in relation to deforestation and fishing pressure. Environ Biol Fish 85:63–75
Elliott M, Hemingway KL (2002) Fishes in estuaries. Blackwell, Oxford
Elliott M, Quintino V (2007) The Estuarine Quality Paradox, environmental homeostasis and the difficulty of detecting anthropogenic stress in naturally stressed areas. Mar Poll Bull 54:640–645
Fernandez-Delgado C, Hernando JA, Herrera M, Bellido M (1988) Life-history patterns of the sand smelt Atherina boyeri Risso, 1810 in the estuary of the Guadalquivir river, Spain. Est Coast Shelf Sci 27:697–706
Ferrarin C, Umgiesser G, Bajo M, Bellafiore D, De Pascalis F, Ghezzo M, Mattassi G, Scroccaro I (2010) Hydraulic zonation of the lagoons of Marano and Grado, Italy. A modeling approach. Est Coast Shelf Sci 87:561–572
Ferrarin C, Umgiesser G, Roland A, Bajo M, De Pascalis F, Ghezzo M, Scroccaro I (2016) Sediment dynamics and budget in a microtidal lagoon: a numerical investigation. Mar Geol 381:163–174
Fiorin R, Malavasi S, Franco A, Franzoi P (2007) Comparative energy allocation in two sympatric, closely related gobies: the black goby Gobius niger and the grass goby Zosterisessor ophiocephalus. J Fish Biol 70:483–496
Fontolan G, Pillon S, Bezzi A, Villalta R, Lipizer M, Triches A, D’Aietti A (2012) Human impact and the historical transformation of saltmarshes in the Marano and Grado Lagoon, northern Adriatic Sea. Est Coast Shelf Sci 113:41–56
Franco A, Franzoi P, Malavasi S, Riccato F, Torricelli P, Mainardi D (2006) Use of shallow water habitats by fish assemblages in a Mediterranean coastal lagoon. Est Coast Shelf Sci 66:67–83
Franco A, Elliott M, Franzoi P, Torricelli P (2008) Life strategies of fishes in European estuaries: the functional guild approach. Mar Ecol Progr Ser 354:219–228
Franco A, Torricelli P, Franzoi P (2009) A habitat-specific fish-based approach to assess the ecological status of Mediterranean coastal lagoons. Mar Poll Bull 58:1704–1717
Franzoi P, Trisolini R, Carrieri A, Rossi R (1989) Caratteristiche ecologiche del popolamento ittico ripario della sacca di Scardovari (Delta de Po). Nova Thalassia 10:399–405
Franzoi P, Franco A, Torricelli P (2010) Fish assemblage diversity and dynamics in the Venice lagoon. Rend Lincei 21:269–281
Froese R (2006) Cube law, condition factor and weight-length relationships: history, meta-analysis and recommendations. J Appl Ichthyol 22:241–253
Galloway BJ, Munkittrick KR (2006) Influence of seasonal changes in relative liver size, condition, relative gonad size and variability in ovarian development in multiple spawning fish species used in environmental monitoring programmes. J Fish Biol 69:1788–1806
Gilliers C, Amara R, Bergeron J-P, Le Pape O (2004) Comparison of growth and condition indices of juvenile flatfish in different coastal nursery grounds. Environ Biol Fish 71:189–198
Haas HL, Freeman CJ, Logan JM, Deegan L, Gaines EF (2009) Examining mummichog growth and movement: are some individual making intra-season migrations to optimize growth? J Exp Mar Biol Ecol 369:8–16
Harrison TD, Whitfield AK (2004) A multi-metric fish index to assess the environmental condition of estuaries. J Fish Biol 65:683–710
Jakob EM, Marshall SD, Uetz GW (1996) Estimating fitness: a comparison of body condition indices. Oikos 77:61–67
Jones RE, Petrell RJ, Pauly D (1999) Using modified length–weight relationships to assess the condition of fish. Aquac Eng 20:261–276
Kennish MJ (2002) Environmental threats and environmental future of estuaries. Env Cons 29:78–107
Koutrakis ET, Kamidis NI, Leonardos ID (2004) Age, growth and mortality of a semi-isolated lagoon population of sand smelt, Atherina boyeri (Risso, 1810) (Pisces: Atherinidae) in an estuarine system of northern Greece. J Appl Ichthyol 20:382–388
La Peyre MK, Gossman B, Nyman JA (2007) Assessing functional equivalency of nekton habitat in enhanced habitats: comparison of terraced and unterraced marsh ponds. Est Coast 30:526–536
Leamon JH, Schultz ET, Crivello JF (2000) Variation among four health indices in natural populations of the estuarine fish, Fundulus heteroclitus (Pisces, Cyprinodontidae), from five geographically proximate estuaries. Environ Biol Fish 57:451–458
Leonardos I (2001) Ecology and exploitation pattern of a landlocked population of sand smelt, Atherina boyeri (Risso 1810), in Trichonis Lake (western Greece). J Appl Ichthyol 17:262–266
Lepage M, Harrison T, Breine J, Cabral H, Coates S, Galván C, García P, Jager Z, Kelly F, Mosch EC, Pasquaud S, Scholle J, Uriarte A, Borja A (2016) An approach to intercalibrate ecological classification tools using fish in transitional water of the North East Atlantic. Ecol Indic 67:318–327
Maci S, Basset A (2010) Spatio-temporal patterns of abundance, size structure and body condition of Atherina boyeri (Pisces: Atherinidae) in a small non-tidal Mediterranean lagoon. Est Coast Shelf Sci 87:125–134
Malavasi S, Fiorin R, Franco A, Franzoi P, Granzotto A, Riccato F, Mainardi D (2004) Fish assemblages of Venice Lagoon shallow waters: an analysis based on species, families and functional guilds. J Mar Syst 51:19–31
Manzo C (2010) Fish assemblages in three Mediterranean coastal lagoons: structure, functioning and spatio-temporal dynamics. PhD thesis
McLusky DS, Elliott M (2004) The estuarine ecosystem: ecology, threats and management, 3rd edn. Oxford University Press, New York, p 214
Montgomery DC, Peck EA (1992) Introduction to Linear regression analysis, 2nd edn. Wiley, New York
Muxika I, Borja A, Bald J (2007) Using historical data, expert judgement and multivariate analysis in assessing reference conditions and benthic ecological status, according to the European Water Framework Directive. Mar Poll Bull 55:16–29
Perez-Ruzafa A, Marcos C, Perez-Ruzafa IM, Barcala E, Hegazi MI, Quispe J (2007a) Detecting changes resulting from human pressure in a naturally quick-changing and heterogeneous environment: spatial and temporal scales of variability in coastal lagoons. Est Coast Shelf Sci 75:175–188
Perez-Ruzafa A, Mompean MC, Marcos C (2007b) Hydrographic, geomorphologic and fish assemblage relationships in coastal lagoons. Hydrobiologia 577(107):125
Perez-Ruzafa A, Marcos C, Perez-Ruzafa IM, Perez-Marcos M (2013) Are coastal lagoons physically or biologically controlled ecosystems? Revisiting r versus K strategies in coastal lagoons and estuaries. Est Coast Shelf Sci 132:17–33
Perez-Ruzafa A, Perez-Marcos M, Marcos C (2018) From fish physiology to ecosystems management: keys for moving through biological levels of organization in detecting environmental changes and anticipate their consequences. Ecol Indic 90:334–345
Piazza BP, La Peyre MK (2010) Using Gambusia affinis growth and condition to assess estuarine habitat quality: a comparison of indices. Mar Ecol Prog Ser 412:231–245
Potter IC, Tweedley JR, Elliott M, Whitfield AK (2015) The ways in which fish use estuaries: a refinement and expansion of the guild approach. Fish Fish 16:230–239
Pranovi F, Caccin A, Franzoi P, Malavasi S, Zucchetta M, Torricelli P (2013) Vulnerability of artisanal fisheries to climate change in the Venice lagoon. J Fish Biol 83:847–863
Ramieri E, Barbanti A, Picone M, Menchini G, Bressan E, Dal Forno E (2011) Integrated plan for the sustainable management of the Lagoon of Marano and Grado. Littoral 2010:05008
Rountree RA, Able KW (2007) Spatial and temporal habitat use patterns for salt marsh nekton: implications for ecological functions. Aquat Ecol 41:25–45
Sarretta A, Pillon S, Molinaroli E, Guerzoni S, Fontolan G (2010) Sediment budget in the Lagoon of Venice, Italy. Cont Shelf Res 30:934–949
Searcy SP, Egglestone DB, Hare JA (2007) Is growth a reliable indicator of habitat quality and essential fish habitat for a juvenile estuarine fish? Can J Fish Aquat Sci 64:681–691
Sfriso A, Facca C, Ghetti PF (2003) Temporal and spatial changes of macroalgae and phytoplancton in a Mediterranean coastal area: the Venice lagoon as a case study. Mar Env Res 56:617–636
Solidoro C, Bandelj V, Aubry Bernardi F, Camatti E, Ciavatta S, Cossarini G, Facca C, Franzoi P, Libralato S, Melaku Canu D, Pastres R, Pranovi F, Raicevich S, Socal G, Sfriso A, Sigovini M, Tagliapietra D, Torricelli P (2010) Response of venice lagoon ecosystem to natural and anthropogenic pressures over the last 50 years. In: Paerl Hans, Kennish Mike (eds) Coastal lagoons: systems of natural and anthropogenic change. CRC Press, Taylor and Francis, pp 483–511
Stevenson RD, Woods WA (2006) Condition indices for conservation: new uses for evolving tools. Int Comp Biol 46:1169–1190
Van Der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers risk assessment: a review. Environ Tox Pharm 13:57–149
Vasconcelos RP, Reis-Santos P, Fonseca V, Ruano M, Tanner S, Costa MJ, Cabral HN (2009) Juvenile fish condition in estuarine nurseries along the Portuguese coast. Estuar Coast Shelf Sci 82:128–138
Verdiell-Cubedo D, Torralva M, Andreu-Soler A, Oliva-Paterna FJ (2012) Effects of shoreline urban modification on habitat structure and fish community in littoral areas of a Mediterranean coastal lagoon (Mar Menor, Spain). Wetlands 32:631–641
Vila-Gispert A, Moreno-Amich R (2001) Mass-length relationship of Mediterranean barbel as an indicator of environmental status in South-west European stream ecosystems. J Fish Biol 59:824–832
Whitfield AK, Elliott M (2002) Fishes as indicators of environmental and ecological changes within estuaries: a review of progress and some suggestions for the future. J Fish Biol 61:229–250
Zapfe GA, Rakocinski CF (2008) Coherent growth and diet patterns in juvenile spot (Leiostomus xanthurus Lacepede) reflect effects of hydrology on access to shoreline habitat. Fish Res 91:107–111
Zucchetta M, Cipolato G, Pranovi F, Antonetti P, Torricelli P, Franzoi P, Malavasi S (2012) The relationships between temperature changes and reproductive investment in a Mediterranean goby: insights for the assessment of climate change effects. Est Coast Shelf Sci 101:15–23
Zucchetta M, Scapin L, Cavraro F, Pranovi F, Franco A, Franzoi P (2016) Can the effects of anthropogenic pressures and environmental variability on nekton fauna be detected in fishery data? Insights from the monitoring of the artisanal fishery within the Venice Lagoon. Est Coasts 39:1164–1182
Acknowledgments
We would like to thank T. Ghenda, A. Longo and E. Lugnan for their precious help during the field activities, and G. Mattassi for his supervision. The collection of data used in the present work was funded by ARPA FVG, as part of the monitoring activities carried out for the implementation of the WFD.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Piet Spaak.
Appendices
Appendix A

Appendix B
Pressure | Indicator | Measure | Degree of changes | Note | |||||
|---|---|---|---|---|---|---|---|---|---|
No change (0) | Very low (1) | Low (3) | Medium (5) | High (7) | Very high (9) | ||||
Coastline morphological change | Intertidal | % of intertidal area lost between 1954 and 2006 (Fontolan et al. 2012) | Increase | No change | < 1% lost since 1850 | ≥ 1% and < 5% lost | ≥ 5% é < 10% lost | ≥ 10% lost | Fixed in time |
Coastline | Relative change in bottom depth between 1927 and 2002 (Sarretta et al. 2010), taking a variation of ±0.75 m as minimum significant change | No development | < 5% of the coastline impacted by industrial or urban activities | ≥ 5% and < 30% | ≥ 30% and < 60% | ≥ 60% and < 90% | ≥ 90% | ||
Hydro | % of area affected by interferences with the hydrographic regime | No construction | < 5% of the intertidal and sub-tidal area affected | >=5% and < 10% | >=10% and < 20% | >=20% and < 40% | >=40% | ||
No resource use (0) | Very low (1) | Low (3) | Medium (5) | High (7) | Very high (9) | ||||
|---|---|---|---|---|---|---|---|---|---|
Resource use change | Aquaculture | % of area covered by clam farming | No fish farming | < 1% of the intertidal and sub-tidal area covered | ≥ 1% and < 10% | ≥ 10% and < 30% | ≥ 30% and < 50% | ≥ 50% | Fixed in time |
Fisheries | number of traps km−2 | No fishery activities | < 10% of the surface of WB affected by fishery | ≥ 10% and < 30% | ≥ 30% and < 60% | ≥ 60% and < 90% | ≥ 90% | ||
Marinas | number of berths km−2 | No marina | < 100 berths in marina | ≥ 100 and < 150 berths | ≥ 150 and < 300 berths | ≥ 300 and < 500 berths | ≥ 500 berths | ||
Intensity of port developments | Extent of ports | No harbour | < 500 m of quays | ≥ 500 and < 2 km | ≥ 2 and < 5 km | ≥ 5 and < 10 km | ≥ 10 km of quays |
Very low (1) | Low (3) | Medium (5) | High (7) | Very high (9) | |||||
|---|---|---|---|---|---|---|---|---|---|
Environmental quality and its perception | Sediment | Sediment chemical status based on decree 56/2009 | 100% of substances below the EQS (Environmental Quality Standard) | One not priority substance fails to comply with EQSs | More than one not priority substance fails to comply with EQSs | One priority substance fails to comply with EQSs | More than one priority substance fails to comply with EQSs | More than one priority substance and one or more than one not priority substance fail to comply with EQSs | Fixed in time |
Benthos | M_AMBI index level (Muxika et al. 2007) | Normal | Normal | Normal | Recovering or deteriorating | Modified | Severely modified | Fixed in time | |
DIN | µg L−1 of dissolved inorganic nitrogen | Pressure calculated as seasonal average of concentration on a monthly basis | |||||||
RP | µg L−1 of reactive phosphorous | Pressure calculated as seasonal average of concentration on a monthly basis | |||||||
Chl-a | µg L−1 of chlorophyll-a | Pressure calculated as seasonal average of concentration on a monthly basis | |||||||
DO | % saturation of dissolved oxygen | Pressure calculated as seasonal average of concentration on a monthly basis |
Rights and permissions
About this article
Cite this article
Cavraro, F., Bettoso, N., Zucchetta, M. et al. Body condition in fish as a tool to detect the effects of anthropogenic pressures in transitional waters. Aquat Ecol 53, 21–35 (2019). https://doi.org/10.1007/s10452-018-09670-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-018-09670-4