Abstract
The northern San Francisco Estuary (nSFE) is an urban estuary supplied with anthropogenic nutrient inputs, yet spring blooms are uncommon and phytoplankton biomass is low. The low levels of chlorophyll (<5 µg L−1) have likely contributed to declines in several native fishes, and there is a need to evaluate the conditions that could allow for increased phytoplankton. Increased ammonium (NH4) loads have been hypothesized to modulate the magnitude of blooms in nSFE (the “NH4 hypothesis”) as a result of inhibition of phytoplankton NO3 uptake that limits access to the greater nitrogen (N) pool of nitrate (NO3). This hypothesis, tested in enclosures, but not in the field until now, is that lack of access to NO3 limits primary production and consequently the accumulation of chlorophyll. Here, we test this in the field with the following aims: (1) to observe the uptake response of phytoplankton in different flow and N loading conditions, (2) determine whether the sequence of uptake rates suggested by the “NH4 hypothesis” occurs and (3) obtain depth-integrated nutrient uptake rates to better constrain published criteria for bloom formation. Weekly measurements of NH4 and NO3 uptake, and primary production rates were made during spring 2011–2012, along with nutrient and chlorophyll concentrations during two contrasting hydrological conditions of high vs low freshwater flow. In conditions with high freshwater flow (maximum of 2405 m3 s−1), there were lower nutrient concentrations than with low/normal flows (e.g., NO3 of 10 µmol L−1 compared to 30 µmol L−1), with low N uptake and primary production rates. With low flow (maximum of 1304 m3 s−1), there was elevated chlorophyll and blooms occurred, especially in shallow well-lit shoals where chlorophyll reached 60 µg L−1. The higher levels of chlorophyll and primary productivity resulted from uptake of ambient NO3 by phytoplankton, and f-ratios >0.5. This was enabled by phytoplankton uptake of NH4 to below inhibitory levels, as proposed by the “NH4 hypothesis.” The depth-integrated uptake rate data were used to refine a model that yields flow and nutrient concentration criteria necessary for bloom formation and confirmed that washout flows were the most useful predictor of blooms. Understanding the interaction of phytoplankton biomass with nutrient variability requires evaluating changes in C and N uptake rates and river flow. These dynamic changes are central to understanding why some urban estuaries have lower productivity than expected, and would be difficult to evaluate using biomass data alone. This study points to the importance of treating inorganic N separately as NH4 and NO3 rather than lumping together as DIN and to use rate process data as a mechanistic way to understand, predict and minimize cultural eutrophication impacts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nutrient enrichment and cultural eutrophication in estuaries
Nutrient pollution is long recognized as a stressor for urbanized estuaries and coasts. With increased population growth, there are increased anthropogenic nitrogen (N) loads resulting from wastewater treatment and agricultural practices. These can have negative consequences for the timing of phytoplankton bloom initiation, magnitude of primary production, composition of the phytoplankton community composition and the organization of pelagic food webs, for example, shifting the phytoplankton toward harmful algal species. Estuaries are particularly susceptible to nutrient enrichment (e.g., Painting et al. 2007; Cloern 2001) and cultural eutrophication, an increase in organic matter production resulting from anthropogenic activity such as fertilizer runoff or sewage discharge (e.g., Fisher et al. 2006).
Over the past two decades, effort has been directed toward the development of decision-making tools such as nutrient threshold criteria (Glibert et al. 2010) to assess and predict the impacts of nutrient enrichment and cultural eutrophication on ecosystem function (e.g., OECD 1982; Painting et al. 2005; Bricker 1999). Typically, empirical relationship between nutrients and the product of phytoplankton growth, chlorophyll, are employed. For example, Gowen et al. (1992) showed that chlorophyll yield could be predicted from the available dissolved inorganic N (DIN) and phosphate (DIP). Edwards et al. (2003) proposed a steady-state yield of 0.95 µg chl (µmol N)−1 was the most appropriate value for assessing eutrophication potential resulting from continuous nutrient enrichment. More recently, it has been suggested that dynamic relationships between nutrients and microbial consortia may be more appropriate for developing nutrient criteria and should be developed alongside those using standing stock approaches. Consideration of nutrient transformation rates including nutrient assimilation offers a mechanistic way to understand, predict and reduce cultural eutrophication. This has become particularly important in San Francisco Estuary (SFE) where DIN and DIP concentrations greatly exceed those in other estuaries where water quality has been impaired by nutrient pollution (Cloern and Jassby 2012).
San Francisco Estuary and the pelagic organism decline (POD)
SFE is the largest estuary on the Pacific coast of the USA. It is a critical ecosystem that links freshwater and marine environments. The estuary is essential habitat for commercially important salmon and provides drinking water to over 22 million urban users, along with irrigation water for agriculture in the highly productive Central Valley of California. Long-term studies have documented the changes in the SFE as a result of water diversions, nutrient inputs, invasive species and other anthropogenic changes (reviewed by Cloern and Jassby 2012). The northern SFE (nSFE), specifically the low-salinity zone (LSZ, 0–5 salinity, Kimmerer et al. 2012), has experienced a decline in several pelagic fish species including the endangered delta smelt (Hypomesus transpacificus) and threatened longfin smelt (Spirinchus thaleichthys). This is termed the pelagic organism decline (POD) (Sommer et al. 2007). Similar situations could occur in other urban estuaries. The POD was first observed in 2002, and food availability, specifically low phytoplankton and zooplankton biomass, is considered an important causal factor (e.g., Kimmerer et al. 2012; Winder and Jassby 2010), in addition to decreasing amounts of suitable fish habitat (e.g., less turbid water conditions, Sommer et al. 2007).
The nSFE has historically had much lower rates of primary production compared to many other estuaries worldwide (Boynton et al. 1982). In the last decades, this has been overlaid by a long-term declining trend in phytoplankton biomass and primary productivity to very low levels (Jassby 2008; Jassby et al. 2002; Wilkerson et al. 2006; Kimmerer et al. 2012) that may be a principle cause for the fish declines. For example, Suisun Bay (Fig. 1) typically has chlorophyll levels that are <5 µg L−1 (Kimmerer et al. 2012) and rarely above the 10 µg L−1 food limitation threshold for zooplankton described by Müeller-Solger et al. (2002). The zooplankton consumer trophic level is now food-limited (Kimmerer et al. 2005).
Chlorophyll and nutrient concentrations in the northern SFE
However, phytoplankton blooms of >30 µg L−1 chlorophyll were common in the 1970s (Ball and Arthur 1979), in spite of elevated nutrients. Ball and Arthur (1979) reported chlorophyll concentrations of 30–40 µg L−1 in spring and 40–100 µg L−1 in summer from 1969 to 1979 with diatoms the dominant phytoplankton. The chlorophyll decline began in the early 1980s (see Jassby et al. 2002) with blooms rare after 1987, coincident with the introduction of the invasive clam, Potamocorbula amurensis which has been considered the major cause (Alpine and Cloern 1992).
Prior to the clam invasion (Glibert et al. 2011) and continuing to recent years, ammonium (NH4) concentrations have been increasing in the nSFE, reflecting increased wastewater discharge and the associated N loads as human population increases (Jassby 2008). The largest wastewater treatment facility/plant (WTP) currently discharges 15 t NH4-N day−1 to the SFE’s inland Delta and Suisun Bay (Jassby 2008), a threefold increase since 1987. Elevated NH4 from wastewater effluent has been implicated in depressed primary production along the California coast (MacIsaac et al. 1979), the Delaware Estuary (Yoshiyama and Sharp 2006), Wascana Creek, Canada (Waiser et al. 2010), and in the Sacramento River (Parker et al. 2012c). The association of wastewater NH4 and declining primary production appears related to decreased nitrate (NO3) uptake by the primary producers (e.g., Hong Kong waters, Xu et al. 2012) and low algal biomass (Scheldt Estuary, Cox et al. 2009). As noted by Cox et al. (2009), this is “contrary to expectations from the classical eutrophication response of estuarine and coastal systems.” Nixon (1990) observed a similar response of low productivity to elevated nutrients in Narragansett Bay and termed it oligotrophication.
The uncoupling of phytoplankton N and C uptake from the availability of nutrients appears to occur in the nSFE, where phytoplankton uptake and growth do not match the availability of N in the system. However, uptake rate data that reflect phytoplankton nutrient physiology have been rarely measured in this system. Instead, ecosystem management agencies rely on nutrient and biomass standing stock relationships to build simple models for decision making. One aim of this paper is to provide these uptake rate data for the nSFE.
The “NH4 hypothesis”
A common feature of the few spring blooms (>30 µg L−1 chlorophyll) observed in the nSFE since 1999 (i.e., in 2000, see Wilkerson et al. 2006; Kimmerer et al. 2012; Parker et al. 2012b, in 2010, see Dugdale et al. 2012 and in 2014, see Glibert et al. 2014) is a decline in the concentration of NH4 to ~1 µmol N L−1 (Wilkerson et al. 2006; Dugdale et al. 2012), indicating a possible role for this nutrient in modulating the timing and magnitude of blooms. Termed the “NH4 hypothesis” (Dugdale et al. 2007), the observations are suggested to occur as a result of inhibition of phytoplankton NO3 uptake (e.g., Pennock 1987) that limits access to the greater pool of available N (NO3). This hypothesis was tested in experimental enclosures, but not in the field, and led to the conclusion that lack of access to NO3 limits primary production (Parker et al. 2012a, c; Dugdale et al. 2013) and consequently the accumulation of chlorophyll. The association of lower chlorophyll with high NH4 and lack of NO3 uptake was also described as the “NH4 paradox” (Dugdale et al. 2012). Additionally, long-term increases in NH4 loadings to the nSFE may have resulted in changes in the food web structure via shifts in phytoplankton communities from large diatoms to “NH4-tolerant” phytoplankton taxa such as small-sized cryptomonads and flagellates and corresponding shifts from large zooplankton to smaller species (Glibert et al. 2011).
Based upon the results of 96-h enclosure experiments, the “NH4 hypothesis” holds that there is a predictable sequence of events leading to a phytoplankton bloom using the available NO3 (Dugdale et al. 2007; Parker et al. 2012a). First, phytoplankton N demand is satisfied by NH4 but with low phytoplankton growth; NO3 uptake is low or near zero due to NH4 inhibition. Once the NH4 has been drawn down to below an inhibitory concentration (<4 µmol N L−1), NO3 uptake is enabled. This is followed by continued phytoplankton growth, and NH4 concentration is further reduced to ≤1 µmol N L−1. This allows rapid uptake of NO3 coupled with high C uptake and a rapidly developing bloom in the enclosure. Eventually, the phytoplankton become N-limited, relying primarily on recycled NH4 (Parker et al. 2012a). Because of the ephemeral nature of phytoplankton bloom occurrences in the nSFE, there are few measured uptake rates of NH4, NO3 or carbon at high enough temporal or spatial resolution to capture a bloom event and test whether the sequence observed in enclosures occurs in the field. This study aimed to make such measurements at the appropriate time and space scales in nSFE.
Modeling bloom development in the SFE: the role of ammonium and flow
In the field, the suggested sequence described above for enclosure results will be modulated by river flow conditions and residence time as well as continual inputs of NH4 from the WTPs (Dugdale et al. 2012, 2013). Painting et al. (2007) showed the importance of residence time (and flushing rate) and light on the susceptibility of a water body to the impacts of nutrient enrichment through primary producers and incorporated this into a model to predict trophic status from nutrient input to apply to estuaries in the UK. In order to evaluate the effect of flow in the nSFE, a numerical model was developed by Dugdale et al. (2012, 2013) that combined flow, phytoplankton and nutrient data collected in the field during a spring bloom in the nSFE with enclosure data. This analysis indicated an optimal window where balanced river flow and NH4 conditions could support rapid phytoplankton growth on NO3 and result in a phytoplankton bloom. The model was used to develop three criteria for bloom initiation (Dugdale et al. 2012, 2013).
The criteria were determined, assuming suitable irradiance conditions and a present-day discharge of 15 t NH4-N day−1 at the upstream WTP. They were as follows: (1) the NH4 loading criterion of 1.58 mmol NH4 m−2day−1 which must not be exceeded in order that phytoplankton uptake can assimilate all incoming NH4, enabling access to NO3. To change the balance in favor of bloom formation, either NH4 loading would need to decrease or the phytoplankton NH4 uptake rate would need to increase; (2) the NH4 concentration must be <4 µmol N L−1 to enable phytoplankton NO3 uptake, which requires a river flow of >800 m3 s−1 at the WTP for sufficient dilution of the effluent; and (3) the dilution rate of phytoplankton biomass set by river flow and volume of the bay must not exceed the phytoplankton growth rate to avoid “washout” and requires river flow at Suisun Bay <1100 m3 s−1. For example, if water residence time was too low (when there is high river flow) to allow phytoplankton to assimilate the inflowing NH4, or if there was elevated NH4 loading, phytoplankton growth would be based solely on NH4 (with lower C production and low biomass accumulation) and NO3 would go unused and be exported to the Pacific Ocean. Dugdale et al. (2012) calculated the criteria values using a number of broad assumptions since depth-integrated nutrient uptake rates for the nSFE were unavailable for comparison with loading values.
Aims of the study
There is a need to directly measure phytoplankton nutrient uptake and C productivity rates in the field for the nSFE, both to evaluate the criteria of Dugdale et al. (2012) and also to determine the physiological response of phytoplankton in nSFE to elevated nutrients and in different flow conditions. This study provides the first directly measured depth-integrated NO3 and NH4 uptake rates for nSFE phytoplankton. Rates were obtained during spring in the nSFE at intensive spatial and temporal scales, along with nutrient and chlorophyll concentrations during two contrasting hydrological conditions of high versus low freshwater flow. Our aims were to (1) observe the uptake response of phytoplankton in different flow and N loading conditions, (2) determine whether the sequence of uptake rates suggested by the “NH4 hypothesis” and observed in experimental enclosures occurs in the field and (3) obtain depth-integrated nutrient uptake data to better constrain the criteria for bloom formation in the nSFE to enable effective stochastic forecast modeling.
Materials and methods
Site description and sample collection
Weekly surveys were conducted from March to June during three years from 2010 to 2012 in the nSFE. Some data from 2010 were reported in Dugdale et al. (2012). No uptake rates were measured in 2010, but we have included the available hydrographic nutrient and chlorophyll data for 2010 here for comparison with 2011 and 2012. The SFE has a Mediterranean climate with highest freshwater runoff in winter to spring and little precipitation between June and October. The nSFE has freshwater inputs from the Sacramento and San Joaquin Rivers but also large water diversion facilities that can remove freshwater.
Seven to nine stations were occupied weekly for ten weeks. Most of the stations were located within the navigation channel and had water depths (at mean low water) ranging from 9.8 to 14.3 m (http://sfbay.wr.usgs.gov/access/wqdata). A shallow station 1.22 m at MLW (DWR-D7) was also sampled (Fig. 1) and is representative of the shoals of the northern Suisun Bay subembayment known as Grizzly Bay. Drifter studies have indicated that residence time in these shoals may be in days rather than hours as occurs in the adjacent deeper channel water that experiences stronger tidal forcing (Cuetara and Bureau 2007). Estimates of Sacramento River flow at Freeport, CA (SAC) and Delta Outflow (OUT) were obtained from the California Department of Water Resources Dayflow algorithm (http://www.water.ca.gov/dayflow/).
Surface measurements of temperature and salinity (expressed using the Practical Salinity Scale) were taken with a YSI 6920 sonde during 2010. During 2011 and 2012, vertical profiles of temperature and salinity measurements were made using a Seabird SBE-19 CTD mounted on a sampling rosette. At each station, water transparency was assessed with a Secchi disk. Surface water was collected using either a clean bucket in 2010 or 10-L Niskin bottle on a rosette for analysis of nutrients and chlorophyll concentrations as well as enumeration of phytoplankton species. Water samples were kept in a cooler with ice, transported to the laboratory (within 3 h of collection) and then analyzed immediately for nutrient and chlorophyll concentrations. In 2011 and 2012, surface water was also used for bottle incubations to measure phytoplankton nutrient uptake and primary productivity using 15N uptake of NO3 and NH4 and 13C uptake in stable isotope tracer experiments. Clam abundance data for P. amurensis were collected by the Environmental Monitoring Program (EMP) at the California Department of Water Resources and provided by Karen Taberski, San Francisco Bay Regional Water Quality Control Board.
Analytical protocols
Twenty-milliliter water samples for nutrient analysis were filtered through pre-combusted (450 °C, 4 h) 25-mm Whatman GF/F filters prior to analysis (Wilkerson et al. 2006). Nutrient analysis was performed on a Bran and Luebbe AutoAnalyzer II with MT-19 manifold chemistry module. NO3 and NO2 were assayed according to Whitledge et al. (1981) and Bran and Luebbe Method G-172-96 (Bran Luebbe Inc 1999c). Phosphate (PO4) was analyzed according to Bran and Luebbe Method G-175-96 (Bran Luebbe Inc 1999b) and silicate (Si(OH)4) by Bran and Luebbe Method G-177-96 (Bran Luebbe Inc 1999a). Separate 25 mL GF/F filtered samples were analyzed for NH4 according to Solorzano (1969) using a 10-cm pathlength cell. All nutrient concentrations are reported as µmol L−1.
Dissolved inorganic carbon (DIC) concentrations, required for calculating the primary productivity rates, were measured in 20-mL samples using a Monterey Bay Research Institute clone DIC analyzer with acid-sparging and non-dispersive infrared analysis (Friederich et al. 2002; Parker et al. 2006) following preservation with 200 μL of 5 % w/v HgCl2. Preserved samples were held for up to a month before DIC analysis.
Samples for chlorophyll a (termed chlorophyll throughout) were prepared by filtering 50–100 mL of sample water onto 25 mm GF/F filters (Wilkerson et al. 2006). Chlorophyll on the filters was determined by in vitro fluorometry after extraction in 90 % acetone using a Turner 10AU fluorometer (Arar and Collins 1992) calibrated with commercially available chlorophyll a (Turner Designs) and corrected for phaeophytin (Holm-Hansen et al. 1965) and is reported as µg L−1.
Laboratory quality assurance/quality control protocols were followed based on the California State Water Resources Control Board Surface Water Ambient Monitoring Program (SWAMP) (http://www.waterboards.ca.gov/water_issues/programs/swamp/qamp.html). This included implementation of standard laboratory procedures including replicates, field blanks, matrix spikes, certified reference materials, setting of control limits, criteria for rejection, and data validation methods. All nutrient and chlorophyll analyses were carried out on fresh samples within 24 h of collection.
Dual-labeled 13C/15N stable isotope tracer incubations were carried out to estimate C and N uptake rates (Slawyk et al. 1977; Parker 2005). Trace additions of NaH13CO3 and either K15NO3 or 15NH4Cl (99 at%) were added to samples to approximately 10 % of the ambient concentration. Samples were incubated in 180-mL polycarbonate bottles for 24 h, held in baywater-cooled incubator tables screened to four irradiances representing 50, 25, 10 and 5 % of surface photosynthetically active radiation (PAR). Incubations were terminated by gentle vacuum filtration onto pre-combusted (450 °C for 4 h) 25-mm Whatman GF/F filters. Filters were frozen and stored at −20 °C for up to a year, until analysis for 13C and 15N enrichment and particulate organic carbon and nitrogen concentration with a Europa 20/20 isotope-ratio mass spectrometer system. Near-surface (50 % of surface PAR) carbon and nitrogen uptake rates (ρ, µmol C or N L−1 day−1) and biomass specific uptake rates (V, day−1) were calculated according to Dugdale and Wilkerson (1986) and Legendre and Gosselin (1996). No correction for NH4 regeneration and isotope dilution was made to NH4 uptake rates. However, the potential impact of NH4 regeneration on calculated NH4 uptake rates is likely low because of relatively low NH4 uptake and high NH4 concentrations. Further discussion of this is given in Parker et al. (2012a).
Depth-integrated C and N uptake rates were calculated for three stations during 2011 and 2012 by trapezoidal integration, based on an empirical conversion factor of light attenuation from Secchi depth determined previously for the LSZ in the nSFE (Kimmerer et al. 2012). For calculating depth-integrated production, a few missing productivity values were estimated using either the mean of the productivity values found at the irradiance above and below the missing value or in cases where the missing value was either at the highest irradiance (50 % PAR) or at the lowest irradiance (5 %), and the productivity value from the closest irradiance was used. Using depth-integrated N uptake, f-ratios (Eppley and Peterson 1979) were calculated as the ratio of NO3 uptake to total DIN uptake and used as an index of the relative importance of NO3 uptake for phytoplankton N demand, with f-ratios of zero indicating no NO3 uptake, while f-ratios of one would indicate that NO3 was the sole N source for phytoplankton (Dugdale et al. 2007).
NH4 loadings to the nSFE in spring
In order to re-evaluate the model criteria of Dugdale et al. (2012) that used 2010 flow values, NH4 loading to the nSFE was calculated using freshwater flow and NH4 concentrations from spring 2011 and 2012. Bay-wide loading for Suisun Bay was calculated as well as depth-weighted (water column) loading at a shoal and channel station (DWR-D7, USGS5) for comparison with measured depth-integrated NH4 uptake rates. This comparison assumes a static water column and so is an oversimplification. However, with residence time over the shoals in Suisun Bay being in days (Lucas et al. 1999; Cuetara and Bureau 2007) this approach is not unreasonable. These loadings are calculated according to:
where NH4 inp is input of NH4 to Suisun Bay in mmol N day−1, NH4 DWR-D4 is the concentration of NH4 at station DWR-D4 (µmol N L−1) and OUT is delta outflow (m3 day−1).
Then
where \(L_{{{\text{NH}}_{4} {\text{Sui}}}}\) (mmol NH4–N m−2 day−1) is bay-wide loading to Suisun Bay and A is surface area of Suisun Bay (in m2).
where δNH4 is the daily increase in NH4 concentration (mmol m−3 day−1) in Suisun Bay due to NH4 input and V is volume of Suisun Bay (m3). This is then used to calculate the depth-weighted (water column) loading (L in mmol NH4–N m−2day−1) at DWR-D7 (LDWR-D7) and USGS5 (LUSGS5) using the mean depth at those locations (Zm in m).
Results
The uptake response of phytoplankton in different flow and nutrient conditions
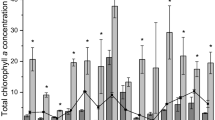
Conditions were different during the three years that nSFE was sampled, enabling our first aim to be addressed. Physical factors, nutrients and chlorophyll concentrations measured were also different spatially along the nSFE transect that was sampled. These temporal and spatial patterns will be described for conditions in 2010–2012, followed by the phytoplankton uptake response measured in 2011 and 2012. We describe mean data for each nSFE station over the entire spring study period, including the shoal station DWR-D7 (Figs. 2, 3).
Mean values (± standard error of mean) for all cruises plotted versus station location in northern San Francisco Estuary for spring 2010, 2011, 2012, a temperature, b salinity, c Secchi depth, concentrations of d chlorophyll, e NH4, f NO3, g PO4, h Si(OH)4, i ρNH4, j ρNO3, k ρC. (Color figure online)
Flow
Contrasting flow conditions were experienced with differences each year in peak flows in early spring (March) and minimum flows in April and May (Fig. 2). In March 2012, flow was as low as 250 m3 s−1, whereas in 2011 flow reached 2405 m3 s−1 (March 26, 2011) declining to ~1100 m3 s−1 in May, 2011. In the April–May period, flows (Fig. 2) were lowest in 2010 (max of 918 m3s−1), highest in 2011 (max of 2405 m3s−1) and intermediate in 2012 (max of 1304 m3s−1). Arrows on this diagram point to peak chlorophyll accumulation observed at the shoal station DWR-D7.
Temperature, salinity, Secchi depth, chlorophyll
Along the transect and at the shallow shoal station, mean temperatures were similar along the transects although there was variability during the spring. Warmest temperatures occurred during 2012, followed by 2011 and 2010 (Fig. 3a). Salinity was almost zero throughout the study area in the high flow spring, 2011. In contrast, in 2010 and 2012, salinity decreased from 6.5 going upstream, to almost zero (0.14) with ~2 at the shoal station DWR-D7 (Fig. 3b). Water transparency (Secchi depths) (Fig. 3c) was greatest in the high flow spring 2011 and showed increasing trend going upstream, but with low transparency at the shallow station. Transparency in spring 2010 was similar to 2012, with a slight increase going upstream (Fig. 3c). Again the lowest Secchi depths (i.e., transparency) were at the shoal station DWR-D7. However, due to the shallow depth (1.6 m) at DWR-D7, PAR nearly always reached the bottom of the water column (Fig. 4a), and mean Zeu/Zm was 1.03 in 2010, 1.18 in 2011 but 0.66 in 2012. The irradiance profile for a deeper channel station USGS5 shows that this situation is unlikely to occur and that only the upper water column will be available for photosynthesis (Fig. 4b). Mean chlorophyll (Fig. 3d) in the channel stations exhibited no clear spatial patterns in 2011 and 2012 with 3–4 µg L−1 throughout the Suisun Bay, whereas a bloom was apparent in 2010 bloom in the mid-section of the transect with 14 µg L−1 at USGS5. In all years, the shoal station showed elevated average chlorophyll concentrations compared to the channel stations (9 µg L−1 in 2011, 10 µg L−1 in 2012).
Depth versus estimates of mean spring photosynthetically active radiation (PAR, expressed as % surface irradiance) for a shoal station DWR-D7 and b channel station USGS5 during 2010 (blue), 2011 (red) and 2012 (green). Estimates of PAR were derived from an empirical relationship between PAR and Secchi for the low-salinity zone of the nSFE (Kimmerer et al. 2012) and weekly Secchi depth measurements. Arrows indicate the 50, 25, 10 and 5 % PAR values, equivalent to the light intensity at which C and N uptake rates were estimated in stable isotope tracer incubations. (Color figure online)
Nutrient concentrations
All nutrient concentrations were lower in 2011 than other years, consistent with dilution by the higher flow. Along the transect, NH4 concentrations (Fig. 3e) were lower in the middle reaches of Suisun Bay (at stations USGS3, USGS5, USGS6) and high at the river inflow station, DWR-D4, and at the outflow area of Suisun Bay, USGS7. The shoal station DWR-D7 had lower mean NH4 (<4 µmol N L−1) than in the channel. Mean NO3 and PO4 patterns and concentrations (Fig. 3f, g) were virtually identical for 2010 and 2012, decreasing upstream. In 2011, concentrations were reduced by 50 % (NO3) and 40 % (PO4) or more compared to 2010 and 2012. The shoal station had slightly higher nutrient concentrations than the channel. Silicate was high throughout the Bay (>225 µmol Si L−1) with lowest concentrations in the high flow spring, 2011 (Fig. 3h).
Nitrogen and carbon uptake rates
N and C uptake rates were not measured in 2010, the high chlorophyll spring. In 2011 and 2012, in the channel transect, NH4 uptake (ρNH4) was higher (Fig. 3i) than ρNO3 (Fig. 3j). The converse was observed at the shoal station. Lower uptake rates were observed in the higher flow conditions of 2011. Interestingly, there were no clear spatial trends compared with the nutrient distributions, except slightly higher ρNO3 rates toward the seaward end of the transect (Fig. 3j), where higher NO3 was observed (Fig. 3b). The shoal station DWR-D7 showed much higher mean rates than the channel, seven and ten times higher in 2011 and 2012, respectively. Carbon uptake (ρC, Fig. 3k) showed high spatial variability with a minimum in mid-Suisun Bay and similar rates (ρC ~ 10 µmol C L−1day−1) in both years. As observed for NO3 uptake, mean near-surface rates at the shoal station DWR-D7 were higher (twofold) than those at the channel stations in both years (~20 µmol C L−1 day−1).
The sequence of N and C uptake rates observed
Since there was little spatial variability between stations for many parameters, especially the nutrient uptake, we selected three stations to study in more detail—particularly how rates and parameters (nutrient and chlorophyll concentrations) changed as the spring progressed, in order to address our second aim to observe whether the “NH4 hypothesis” sequence occurred in the field. The three locations selected represent the inflowing conditions to Suisun Bay in the channel at DWR-D4 where elevated NH4 was observed (Fig. 3e), a channel location in mid-Suisun Bay (USGS5) where NH4 was lower, and a phytoplankton bloom had been observed in 2010 (Fig. 3d), and the shallow shoal (DWR-D7) that showed elevated mean chlorophyll and primary productivity (Fig. 3d, k). The 2010 data set was included for comparison even though rates were unavailable, since the nutrient depletion rates could be used to look for the sequence.
Time series and sequence of changes in 2010 and 2011
NO3 concentrations exceeded NH4 at all stations in all years (Fig. 5, 6, 7). As the spring proceeded in 2010, the nutrient and chlorophyll patterns were similar for all three stations (Fig. 5), with declining nutrient concentrations and two phytoplankton blooms (peak chlorophyll in mid-April and late May reaching 34 µg L−1at DWR-D7) observed when NH4 concentrations were low.
Time series from March to June 2011 at a, d DWR-D4, b, e USGS5 and c, f DWR-D7 of surface concentrations (a–c) of NO3 (circles), NH4 (triangles) and chlorophyll (squares); and rates of (d–f) nitrate uptake, ρNO3 (circles), ammonium uptake, ρNH4 (triangles) and carbon uptake, ρC (squares). (Color figure online)
Time series from March to June 2012 at a, d DWR-D4; b, e USGS5 and c, f DWR-D7 of surface concentrations a–c of NO3 (circles), NH4 (triangles) and chlorophyll (squares); and rates of (d–f) nitrate uptake, ρNO3 (circles), ammonium uptake, ρNH4 (triangles) and carbon uptake, ρC (squares). (Color figure online)
These patterns were not observed in 2011 (Fig. 6) except at DWR-D7 (Fig. 6c), where declining NO3, and low NH4 co-occurred with chlorophyll peaks later in spring (compared to 2010) reaching 17.2 µg L−1 on 17 May and 13.0 µg L−1 on 31 May. As observed throughout the Bay (Fig. 3e, f), N concentrations were lower in this high flow condition than in 2010, with little variability throughout the spring. Interestingly, the uptake rates at DWR-D4 and USGS5 in 2011 (Fig. 6d, e, h, i) showed some peaks, with a small peak in ρC (21 and 16 µmol C L−1 day−1, respectively) on 3 May accompanying by a peak in ρNH4 (1.58 and 1.16 µmol N L−1 day−1, respectively). Throughout the spring, ρNH4 exceeded ρNO3 at the channel stations. In contrast, at DWR-D7, in 2011 for most of the spring ρNO3 was greater than ρNH4 and the peaks in ρC were accompanied by peaks in ρNO3 (Fig. 6f). Note that the scale for the y-axes for DWR-D7 (Fig. 6f) is greater than for the other two stations. The higher ρC peak was on 3 May and reached 55 µmol C L−1 day−1; the peak ρNO3 was 1.89 µmol N L−1 day−1 (Fig. 6f).
Time series and sequence of changes in 2012
In 2012 (Fig. 7a–c), nutrients were similar in value to 2010 (Fig. 5a–c) and showed similar decline as the spring progressed, with some increase in chlorophyll although not reaching the dense bloom levels of 2010, except at the shoal station (Fig. 7c). At USGS5, chlorophyll reached 11 µg L−1 on May 8, 2012, when NH4 was ~2 µmol N L−1 (Fig. 7b). At DWR-D7, very high chlorophyll (60 µg L−1) occurred on May 8, 2012, coinciding with very low NH4 (0.3 µmol N L−1) (Fig. 7c). The peak of the blooms in all years at DWR-D7 occurred in May during the latter part of the spring season in the lower flow periods in all years (Fig. 2). In 2012, at the channel stations DWR-D4 (Fig. 7d) and USGS5 (Fig. 7e), ρNH4 was greater than ρNO3 except at USGS5 during the small bloom on May 8 when there was a peak in ρNO3 (2.48 µmol N L−1 day−1) and high ρC (34 µmol C L−1 day−1) and low NH4 concentration (1.8 µmol N L−1). At DWR-D7, the maximum uptake rates of ρC and ρNO3 (Fig. 7f) were much greater (about 5 times higher) than at DWR-D4 or USGS5 or at DWR-D7 in 2011. As in 2011, ρNO3 was mostly greater than ρNH4 although on 24 April a small increase in ρC (18 µmol C L−1 day−1) was matched by a peak in ρNH4 (2.65 µmol N L−1 day−1). The high chlorophyll condition on May 8, 2012, at DWR-D7 (Fig. 7c) was when the highest ρNO3 (12.66 µmol N L−1 day−1 and ρC (153 µmol C L−1 day−1) rates were measured, accompanied by low ρNH4 (0.24 µmol N L−1 day−1) (Fig. 7f) and NH4 concentration (0.31 µmol N L−1) (Fig. 7c).
The trends seen in experimental enclosures of NH4 drawdown followed by NO3 uptake when levels are <4 µmol L−1 (Parker et al. 2012a) were observed at DWR-D7 and at USGS5. The high primary production leading to the high chlorophyll concentrations was typically accompanied by high NO3 uptake, as was also described in enclosures (Dugdale et al. 2007; Parker et al. 2012a).
Depth-integrated C and N uptake rates
Depth-integrated rates in 2011
To address the third aim of the study, depth-integrated NH4, NO3 and C phytoplankton uptake values along with the photic zone depths for the three stations (DWR-D4, USGS5 and DWR-D7) were measured in 2011 (Table 1) and 2012 (Table 2). The rates in 2011 show similar trends and the same seasonal peaks of C, NH4 and NO3 production in early May as in the surface values (Fig. 6d–f). C production was nearly the same at all three stations. Mean integrated NH4 uptake was substantially lower at DWR-D7 than at DWR-D4 or USGS5, while mean NO3 uptake was an order of magnitude higher at DWR-D7 than at the two channel stations (Table 1). The low NO3 uptake rates compared to NH4 uptake rates at the two channel stations resulted in low mean f-ratios of 0.1. Elevated f-ratios were only observed at shoal station, DWR-D7 (mean f-ratio = 0.7). Depth-integrated NH4 uptake ranged from 0.04 to 2.94 mmol N m−2 day−1. Integrated C uptake ranged widely from 9.34 to 41.1 mmol C m−2 day−1. C/N uptake ratios were 6.6–22.0 with similar mean values for the three stations (~12), higher than the Redfield ratio of 6.6.
Depth-integrated rates in 2012
In 2012, ρC ranged from 1.61 to 100 mmol C m−2day−1 (Table 2). Except for the higher values during the bloom in early May 2012, C uptake was reduced by about half compared to 2011 at the two channel stations, but much less so at DWR-D7. Integrated NH4 uptake also was less in 2012, NO3 uptake was low at the two channel stations and similar to 2011 values except at DWR-D7 that was lowest except on the bloom date in early May 2012. Depth-integrated NH4 uptake ranged from 0.08 to 1.56 mmol N m−2 day−1. The C/N uptake ratios were slightly lower than 2011 ranging from 4.4 to 17.6 with similar mean values at DWR-D4 and USGS5 of 9.5, whereas the mean value at DWR-D7 of 11.7 was close to that of 2011 (12.2). Mean f-ratios remained low in 2012 including at DWR-D7, except for the bloom period when the f-ratio reached 0.6–1.0, as productivity was fueled almost entirely by NO3.
Comparison of depth-integrated NH4 uptake and calculated NH4 loads in the nSFE
In order for re-analysis of the criteria for bloom formation developed in Dugdale et al. (2012), especially the loading criterion, the measured depth-integrated uptake values (“Depth-integrated C and N uptake rates” section) were compared with NH4 loading in nSFE. Also, this comparison could be made separately for channel and shoal situations. In 2011, bay-wide loading of NH4 to Suisun Bay declined from 4.16 mmol N m−2 day−1 from 5 April to a low of 2.03 mmol N m−2 day−1 on 10 May (Table 3). The mean April loading 3.42 mmol N m−2day−1 was about twice that observed in April 2009 and 2010, 1.63 and 1.51 mmol N m−2 day−1 (Dugdale et al. 2012). This exceeded both the loading criterion in Dugdale et al. (2012) of 1.58 mmol N m−2 day−1 and the mean integrated uptake rates measured here (Table 1), and no large scale phytoplankton bloom was observed, as would be expected from Dugdale et al. (2012).
When loading within the water column was separated out to specific locations over the channel (USGS5) or shoal (DWR-D7) and compared to the integrated NH4 uptake rates (Table 3), loading always exceeded uptake in 2011. The exceptions were at DWR-D7 on April 12, 2011, when both rates are almost equal, and June 7, 2011, when uptake was three times greater than loading. Since the loading criterion was not met most of the spring, blooms would not be expected in 2011, and none were observed except for a small increase in chlorophyll at DWR-D7 of 17 µg L−1 on May 17, 2011 (Fig. 6c).
In 2012, bay-wide loading decreased from 4.18 mmol N m−2day−1 at the beginning of April to 0.33 mmol N m−2 day−1 at the end of May. The mean April loading 2.81 mmol N m−2day−1 was lower than in the high flow conditions of 2011, but higher than of 2009, and of 2010. From 15 May onward, the bay-wide loading (Table 3) was below both the loading criterion (1.58 mmol m2 day−1) of Dugdale et al. (2012) and the mean NH4 uptake for DWR-D4, USGS5 and DWR-D7 of 0.68 mmol m2 day−1 in this study (Table 2), indicating bloom potential. At USGS5 and DWR-D7, the measured depth-integrated NH4 uptake rates were equal to or exceeded the NH4 loading (italic font in Table 3) beginning on 24 April at DWR-D7 and on 29 May at USGS5, also supporting the prediction of a bloom after these times. This uptake would result in NH4 being drawn down to allow NO3 uptake and phytoplankton growth. In accordance, increased chlorophyll was observed at both locations (Fig. 7c, e) with a large bloom at DWR-D7 on May 8, 2012, when NH4 was almost zero (Fig. 7c).
Discussion
The response of phytoplankton in different flow and nutrient conditions
The nSFE showed large inter-annual differences in inorganic nutrients and phytoplankton dynamics related to the ambient delta outflow conditions with the high river flow conditions of spring 2011 resulting in large differences compared to the other years. Specifically, we observed lower salinity and nutrients (consistent with greater dilution) as well as lower chlorophyll and lower N and C uptake rates (consistent with shorter water residence time). In 2010 and 2012, we saw initial inorganic nitrogen and chlorophyll concentrations that were nearly threefold higher than in 2011. N and C uptake rates in 2012 (not measured in 2010) were also generally higher than in 2011. These inter-annual differences were observed both at channel stations and at our shoal stations and were also characterized by low NH4. Similar to previous observations (Wilkerson et al. 2006; Parker et al. 2012a), chlorophyll that exceeded 10 µg L−1 was associated with increased NO3 uptake and higher rates of carbon production. The shoal station, DWR-D7, showed the most regularity in supporting phytoplankton blooms in accordance with Lucas et al. (1999). This is likely the result of the favorable light conditions found in the shoals, with 1 % of surface PAR reaching the sediment surface (Fig. 4a), despite the shallowest Secchi depths of any stations surveyed (Fig. 4b).
Northern SFE is a High-Nutrient Low-Growth (HNLG) region
Mean depth-integrated primary production values in the channel were about twofold higher than those measured previously in the LSZ (50–100 mg C m−2 day−1) by Kimmerer et al. (2012) in spring and summer 2006 and 2007. In this study, the higher depth-integrated C production rates were supported almost entirely by NO3 and were achieved under photic zone depth conditions that were not different from the spring mean for DWR-D7 (Table 2). The mean C/N uptake ratios of 11.5 to 12.2 are more than the Redfield ratio of 6.6 (Redfield et al. 1963). The relative constancy of the C uptake and the larger excursions in N uptake in 2011 suggest that the phytoplankton in the nSFE were starved for N.
The availability of directly measured (with 14C or 13C) depth-integrated carbon uptake rates (primary productivity) in the LSZ for the years 2006, 2007 (25 and 31 gCm−2 year−1, Kimmerer et al. 2012), 2011 and 2012 (66 and 40 gCm−2 year−1, daily values multiplied by 270 days, Tables 1, 2) makes it possible to place nSFE productivity within the worldwide estuaries framework provided by Cloern et al. (2014).
Our estimated annual primary production values place Suisun Bay in the lower portion of the oligotrophic category of Nixon (1995), i.e., <100 gC m−2 year−1 and among the lowest primary production of estuarine-coastal ecosystems of the world, for example, the Gulf of Finland, Durnbell Bay, Colne (Fig. 4 in Cloern et al. 2014), and should be considered HNLG (high-nutrient low-growth) described by Cloern (2001) and Sharp (2001) and experiencing oligotrophication (Nixon 1990). Here, this condition was associated with elevated NH4 restricting access to the more abundant form of DIN, NO3: the “NH4 hypothesis” (Dugdale et al. 2007; 2012). The HNLG conditions exist in an area that is critical for a number of fish species (including the endangered delta smelt). According to the relation between primary productivity and fish yield provided by Nixon (1988), the fish yield from the nSFE would be 3 kg ha−1 year−1, a very low value.
The role of benthic grazing on nSFE phytoplankton
While phytoplankton growth in the SFE is generally thought to be regulated by both suspended sediment that decreases light availability (Cole and Cloern 1984; Alpine and Cloern 1992) and nutrient composition and availability (Dugdale et al. 2007; Parker et al. 2012a, c), chlorophyll accumulation is proposed to be controlled largely by grazing (e.g., Kimmerer 2004). Recent analysis (Kimmerer and Thompson 2014) suggests that grazing by the invasive clam Potamocorbula amurensis represents half to two-thirds of phytoplankton losses in the low-salinity zone. Given this high potential for regulation of chlorophyll accumulation, and that phytoplankton losses to grazing in shallow water should be much faster in shoals versus channels (Kimmerer and Thompson 2014), it would be expected that clam abundance would be low in the shoal during peak chlorophyll occurrences (at DWR-D7 in May 2010 and 2012). However, in spring 2010 and 2012, clam densities at the shoal station were much higher than the average, and in spring 2011, with the lowest clam density (Fig. 8), no phytoplankton bloom was observed. The relation between spring blooms observed here and clam grazing in this study is unclear.
Using numerical models, Kimmerer and Thompson (2014) estimated zero to slightly positive net growth of phytoplankton in shoal habitats of Grizzly Bay and concluded that improved irradiance conditions likely balanced or overcompensated for greater grazing potential in shallow water. Here, we suggest that improved light and optimal freshwater flow combined with faster N uptake on NO3 are important for chlorophyll accumulation.
Sequence observed in the field matched enclosure observations
As we hypothesized, the seasonal progression of events leading to a phytoplankton bloom in the nSFE followed the sequence developed from enclosures filled with water from the low-salinity zone (Parker et al. 2012a; Dugdale et al. 2012). This is well illustrated by the stages leading to 60 µg L−1 of chlorophyll in the field at DWR-D7 in 2012 (Fig. 7c). As spring progressed, with improved solar irradiance conditions (Table 2), phytoplankton NH4 uptake increased (Fig. 7f) and NH4 was drawn down by the phytoplankton (Fig. 7c) until the concentration was low enough for NO3 uptake to start and then rapidly increased (Fig. 7f). Following the description by Parker et al. (2012a), C uptake tracked NO3 uptake and chlorophyll accumulated sufficiently rapidly to overcome grazing and led to a phytoplankton bloom.
The time series of NH4, NO3 and C uptake at station USGS5 in 2012 showed a similar sequence. First, NH4 uptake increased (Fig. 7e) and then NH4 concentration declined (Fig. 7b), followed by a rapid increase in NO3 uptake and C uptake rates (Fig. 7e); in this case, only a relatively small increase in chlorophyll resulted (Fig. 7b). At the upstream channel station, DWR-D4, NH4 (Fig. 7a) was never drawn down to levels low enough to enable NO3 uptake (Fig. 7d) and so only NH4 uptake contributed to the low levels of C productivity and low chlorophyll.
Comparison of depth-integrated NH4 uptake and calculated NH4 loads in the nSFE
The measured depth-integrated rates of NH4 uptake (Tables 1, 2) allowed for a more informed calculation of the bloom criteria previously defined by Dugdale et al. (2012), and the criteria can also be re-evaluated for the different delta outflow conditions experienced in 2011 and 2012. The loading criterion (Criterion 1; 1.58 mmol NH4 m−2 day−1) established by Dugdale et al. (2012) was developed using a mean surface phytoplankton NH4 uptake value from Wilkerson et al. (2006) and was used to calculate a water column integrated rate using the Secchi depth and assuming uniform uptake throughout the photic zone. Here, the directly measured depth-integrated NH4 uptake rates were similar to the original Criterion 1 in the channel at USGS5 (1.52 and 0.8 mmol NH4 m−2 day−1 in 2011 and 2012) but lower in the shoal (0.64 and 0.42 mmol m−2 day−1 for 2011 and 2012) (Tables 1, 2). Another way to re-valuate Criterion 1 is by calculating for 2011 and 2012, the bay-wide and depth-weighted loading at DWR-D7 and USGS5 for comparison with the measured uptake rates (Table 3). When the loading exceeded the uptake (and the published criterion), no phytoplankton blooms were observed, as in most of 2011. When uptake exceeded the loading as in 2012, increased chlorophyll would be predicted and occurred, with a large bloom at DWR-D7 (Fig. 7c).
Re-evaluating the flow and washout criteria for bloom formation in the nSFE
Besides the loading criterion, the other criteria proposed by Dugdale et al. (2012) can also be re-evaluated using data and conditions of 2011 and 2012 (Table 4). In 2011 (the high flow condition), NH4 concentrations at the upstream end of Suisun Bay (DWR-D4) were below the second criterion of 4 µmol N L−1 for most of April and May (Table 3) although the minimum flow at the WTP for dilution (800 m3 s−1) was met at all times. In 2012, flow was below the criterion in nearly all of April and May (Table 3), and NH4 concentrations were above the concentration criterion of 4 µmol N L−1 until mid-May 2012 when blooms occurred. In this case, NH4 reduction took place in the river below the WTP, likely due to pelagic nitrification and not due to dilution processes resulting from changes in flow.
The third criterion, the washout flow of <1100 m3 s−1 was always exceeded in spring 2011 (the high flow year), and consequently, there were no blooms. The washout flow criterion was met in 2012 (a low flow year) during April and May (Table 3), and as in 2010, blooms were observed at USGS5 and DWR-D7, when Criteria 1 and 2 were also met. Kimmerer et al. (2012) described a poor response of phytoplankton to river flow in Suisun Bay in 2006 and 2007; these observations may have been the result of exceeding the washout criteria during 2006 (a very wet year) and exceeding the concentration criteria during 2007 (a very dry year).
Among the three criteria, the most important are the loading and the upper limit for washout (the river flow). The flow in 2011 was greater than washout, the loading criterion was exceeded, and even though the concentration criterion was met, no bloom would be predicted and no blooms were observed (Table 4). In 2012, washout was not exceeded, the minimum flow for NH4 dilution was not met, but loading and NH4 concentrations at the entrance to Suisun Bay fell below criterion values and blooms were observed in both shoal and channel (Table 4).
The critical elements for chlorophyll accumulation to occur in the spring are the balance between NH4 loading and the ability of the phytoplankton to absorb the loading (both linked to flow), and so control and reduce the ambient NH4 concentration, enabling them to access the greater concentration of N that is available in the NO3 pool. The loading criterion is not fixed but is linked to the integrated NH4 uptake rate which we now know varies spatially (channel versus shoal) and inter-annually (Tables 1, 2). This parameter is not a typically monitored water quality parameter. So, for model prediction of potential blooms, the estimate in Dugdale et al. (2012) of 1.58 mmol m−2 day−1 could be applied, or alternatively the mean uptake values from this data set (Tables 1, 2) used: for high flow years, as in 2011, 1.52 mmol m−2 day−1 and 0.64 mmol m−2 day−1 for channel and shoal locations, respectively, and for low flow years, 0.80 mmol m−2 day−1 and 0.42 mmol m−2 day−1.
Decreased NH4 loading at DWR-D4 played a major role in the 2012 bloom events but no decline in discharge at the WTP occurred during April 2012 as happened in 2010 (Dugdale et al. 2012). This reduction in NH4 concentration may have been due to pelagic nitrification (Parker et al. 2012c; Hager and Schemel 1992) resulting from the warmer spring temperatures (Fig. 3a) and higher residence times compared to other years. Seasonal increases in nitrification rates would appear at DWR-D4 as decreased NH4 loading and may be an additional factor in the timing and enabling of spring blooms. Pelagic nitrification, resulting in decreased NH4 and increased NO3, was proposed by Glibert et al. (2014) to explain increased chlorophyll in the LSZ during the drought conditions of spring 2014. However, there are currently no published direct measurements of pelagic nitrification for the Sacramento River to evaluate this.
By monitoring some easily measured parameters (delta outflow and NH4 concentration) and applying a constant for NH4 uptake, over time it may be possible to project each spring if conditions are evolving toward conditions favorable for bloom formation in the shoals by using the washout criterion and a simplification of the loading and concentration criteria. First, the delta outflow should be used to evaluate if flow is <1100 m3s−1 (the washout criterion) and if so then the loading and concentration criteria need to be assessed. For the loading criterion, the NH4 loading to the shoal or channel is easily calculated (Eqs. 1–5) using the delta outflow and NH4 concentration at the entrance to Suisun Bay. Then, this is compared with either a revised criteria of depth-integrated NH4 uptake for wet or dry years or directly measured uptake data. The loading and uptake are then compared, and when the uptake rate exceeds the loading, there is a strong possibility of a bloom. NH4 concentration will start to decrease as the productivity sequence commences, and as it continues to go down, it should reach <4 µmol L−1 (the concentration criterion) and chlorophyll will increase to bloom levels (>10 µg L−1). This assumes no other sources of NH4 within Suisun Bay.
Conclusion
This study of the low-salinity zone of the nSFE covered both low and high river flow conditions and showed that high river flow to an estuary will cause dilution and washout of phytoplankton resulting in lower nutrient uptake and chlorophyll. In contrast, low flow conditions lead to higher chlorophyll and nutrient uptake rates, especially in a well-lit shoal location. In both situations, the low primary productivity and HNLG condition of the nSFE were affirmed. We confirmed that the sequence observed in enclosures for NH4 drawdown, NO3 and C uptake and chlorophyll accumulation occurs in the field in Suisun Bay, enabling high rates of NO3 and C uptake to fuel blooms. The depth-integrated uptake data obtained support our original criteria for bloom formation (Dugdale et al. 2012), with the simplest predictor being washout flow, which when exceeded prevents blooms and results in export of nutrients and seed phytoplankton to the ocean. The other criteria proposed were less straightforward to assess when applied to situations with different flow conditions. However, using simple parameters (delta outflow, NH4 and estimates of NH4 uptake) it may be possible to make projections of the likelihood of blooms, successful lower trophic level growth and improved conditions for the delta fish.
This study points to the importance worldwide of considering studies of transport and tidal processes simultaneously with phytoplankton rate processes to understand bloom dynamics in estuaries. In order to assess and predict the impacts of nutrient enrichment and cultural eutrophication on ecosystem function in any aquatic body, dynamic relationships between nutrients and microbial consortia need to be considered. Uptake rate data reflects phytoplankton nutrient physiology and can be used to test mechanistic hypotheses such as the “NH4 hypothesis” (Dugdale et al. 2007). In this study, there was uncoupling of phytoplankton N and C uptake from the nutrient concentrations such that uptake and growth did not match the availability of N in the system, and contributed to lower productivity than was expected from the ambient nutrients. Such a holistic approach incorporating rate processes provides an improvement on the typical nutrient standing stock relationships that are used to build simple models for management.
References
Alpine AE, Cloern JE (1992) Trophic interactions and direct physical effects control phytoplankton biomass and production in an estuary. Limnol Oceanogr 37:946–955
Arar E, Collins GB (1992) In vitro determination of chlorophyll a and phaeophytin in marine and freshwater phytoplankton by fluorescence. USEPA methods for determination of chemical substances in marine and estuarine environmental samples. Cincinnati, OH, USEPA
Ball MD, Arthur JF (1979) Planktonic chlorophyll dynamics in the Northern San Francisco Bay and Delta. In: Conomos J (ed) San Francisco Bay: the urbanized estuary investigations into the natural history of San Francisco bay and delta with reference to the influence of man. Pacific Division, AAAS, San Francisco, pp 265–286
Boynton WR, Kemp WM, Keefe CW (1982) A comparative analysis of nutrients and other factors influencing estuarine production. In: Kennedy VS (ed) Estuarine comparisons
Bran Luebbe, Inc. (1999a) Bran Luebbe autoanalyzer applications: autoanalyzer method No. G-177-96 silicate in water and seawater. Bran Luebbe, Buffalo Grove
Bran Luebbe, Inc. (1999b) Bran Luebbe autoanalyzer applications: autoanalyzer method No. G-175-96 phosphate in water and seawater. Bran Luebbe, Buffalo Grove
Bran Luebbe, Inc. (1999c) Bran Luebbe autoanalyzer applications: autoanalyzer method No. G-172-96 nitrate and nitrite in water and seawater. Bran Luebbe, Buffalo Grove
Bricker, SB, Clement CG, Pirhalla DE, Orlando SP, Farrow DRG (1999) National estuarine eutrophication assessment: effects of nutrient enrichment in the nation’s estuaries. NOAA, National Ocean Service, Special Projects Office and the National Centers for Coastal Ocean Science, Silver Spring
Cloern JE (2001) Our evolving conceptual model of the coastal eutrophication problem. Mar Ecol Prog Ser 210:223–253
Cloern JE, Jassby AD (2012) Drivers of change in estuarine-coastal ecosystems: discoveries from four decades of study in San Francisco Bay. Rev Geophys 50:RG4001. doi:10.10292012RG000397
Cloern JE, Foster SQ, Kleckner AE (2014) Phytoplankton primary production in the worlds estuarine ecosystems. Biogeosciences 11:2477–2501
Cole BE, Cloern JE (1984) Significance of biomass and light availability to phytoplankton productivity in San Francisco Bay. Mar Ecol Prog Ser 17:15–24
Cox T, Maris T, Soetaert K, Conley D, Van Damme S, Meire P, Middelburg J, Vos M, Struyf E (2009) A macro-tidal freshwater ecosystem recovering from hypereutrophication; the Schelde case study. Biogeosciences 6:2935–2948
Cuetara J, Bureau J (2007) Drifter studies in open shallow water habitats of the San Francisco Bay and Delta. http://sfbay.wr.usgs.gov/watershed/drifter_studies/index.html
Dugdale RC, Wilkerson FP (1986) The use of 15N to measure nitrogen uptake in eutrophic oceans: experimental considerations. Limnol Oceanogr 31:673–689
Dugdale RC, Wilkerson FP, Hogue VE, Marchi A (2007) The role of ammonium and nitrate in spring bloom development in San Francisco Bay. Estuar Coast Shelf Sci 73:17–29
Dugdale RC, Wilkerson FP, Parker AE, Marchi A (2012) River flow and ammonium concentration determines spring phytoplankton bloom in an urbanized estuary. Estuar Coast Shelf Sci 115:187–199
Dugdale RC, Wilkerson FP, Parker AE (2013) A biogeochemical model of phytoplankton productivity in an urban estuary: a biogeochemical model of phytoplankton productivity in an urban estuary: the importance of ammonium and freshwater flow. Ecol Model 263:291–307
Edwards VR, Tett P, Jones KJ (2003) Changes in the yield of chlorophyll a from dissolved available inorganic nitrogen after an enrichment event—applications for predicting eutrophication in coastal waters. Cont Shelf Res 23:1771–1785
Eppley RW, Peterson BJ (1979) Particulate organic matter flux and planktonic new production in the deep ocean. Nature 282:677–680
Fisher TR, Hagy J III, Boynton WR, Williams MR (2006) Cultural eutrophication in the Choptank and Patuxent estuaries of Chesapeake Bay. Limnol Oceanogr 51:435–447
Friederich GE, Walz PM, Burczynski MG, Chavez FP (2002) Inorganic carbon in the central California upwelling system during the 1997–1999 El Niño-La Niña event. Prog Oceanogr 54:185–203
Glibert PM, Madden CJ, Boynton W, Flemer D, Hei C, Sharp J (2010) Nutrients in estuaries: a summary report of the national estuarine experts workgroup 2005–2007. United States Environmental Protection Agency, Washington, DC
Glibert PM, Fullerton D, Burkholder JM, Cornwell J, Kana TM (2011) Ecological stoichiometry, biogeochemical cycling, invasive species, and aquatic food webs: San Francisco Estuary and Comparative Systems. Rev Fish Sci 19:358–417
Glibert PM, Dugdale RC, Wilkerson FP, Parker AE, Alexander J, Antell E, Blaser A, Johnson A, Lee J, Lee T, Strong Murasko S (2014) Major—but rare—spring blooms in 2014 in San Francisco Bay Delta, California, a result of the long-term drought, increased residence time, and altered nutrient loads and forms. J Exp Mar Biol Ecol 460:8–18. doi:10.1016/j.jembe.2014.06.001
Gowen RJ, Tett P, Jones KJ (1992) Predicting marine eutrophication: the yield of chlorophyll from nitrogen in Scottish coastal waters. Mar Ecol Prog Ser 85:153–161
Hager S, Schemel L (1992) Sources of nitrogen and phosphorus to northern San Francisco Bay. Estuaries 15:40–52
Holm-Hansen O, Lorenzen CJ, Holmes RW, Strickland JDH (1965) Fluorometric determination of chlorophyll. J Conseil 30:3–5
Jassby AD (2008) Phytoplankton in the upper San Francisco Estuary: recent biomass trends, their causes and their trophic significance. San Franc Estuary Watershed Sci 6:1–24. http://www.escholarship.org/uc/item/71h077r1
Jassby AD, Cloern JE, Cole BE (2002) Annual primary production: patterns and mechanisms of change in a nutrient-rich tidal ecosystem. Limnol Oceanogr 47:698–712
Kimmerer WJ (2004) Open water processes of the San Francisco Estuary: from physical forcing to biological responses. San Franc Estuary Watershed Sci (Online Serial) 2(1). http://repositories.cdlib.org/jmie/sfews/vol2/iss1/art1
Kimmerer WJ, Thompson JK (2014) Phytoplankton growth balanced by clam and zooplankton grazing and net transport into the low-salinity zone of the San Francisco Estuary. Estuaries Coasts. doi:10.1007/s12237-013-9753-6
Kimmerer WJ, Ferm N, Nicolini MH, Peñalva C (2005) Chronic food limitation of egg production in populations of copepods of the genus Acartia in the San Francisco Estuary. Estuaries 28:541–550
Kimmerer WJ, Parker AE, Lidström UE, Carpenter EC (2012) Short-term and interannual variability in primary production in the low-salinity zone of the San Francisco Estuary. Estuaries Coasts 35:913–929
Legendre L, Gosselin M (1996) Estimation of N or C uptake rates by phytoplankton using 15 N or 13C: revisiting the usual computation formulae. J. Plankt Res 19:263–271
Lucas LV, Koseff J, Cloern JE, Monismith SG, Thompson JK (1999) Processes governing phytoplankton blooms in estuaries. I: the local production-loss balance. Mar Ecol Prog Ser 187:1–15
MacIsaac JJ, Dugdale RC, Huntsman SA, Conway HL (1979) The effect of sewage on uptake of inorganic nitrogen and carbon by natural populations of marine phytoplankton. J Mar Sci 37:51–66
Müeller-Solger A, Jassby AD, Muller-Navarra D (2002) Nutritional quality of food resources for zooplankton (Daphnia) in a tidal freshwater system (Sacramento-San Joaquin River Delta). Limnol Oceanogr 47:1468–1476
Nixon SW (1988) Physical energy inputs and the comparative ecology of lake and marine ecosystems. Limnol Oceanogr 33:1005–1025
Nixon SW (1990) Eutrophication and the macroscope. In: Andersen J, Conley DJ (eds) Eutrophication in coastal ecosystems: towards a better understanding. Springer, Berlin, pp 5–21
Nixon SW (1995) Coastal marine eutrophication: a definition, social causes, and future concerns. Ophelia 41:199–219
OECD (1982) Eutrophication of waters, monitoring, assessment and control. Organization for Economic Cooperation and Development, Paris
Painting SJ, Devlin MJ, Rogers SI, Mills DK, Parker ER, Rees HL (2005) Assessing the suitability of OSPAR EcoQOs for eutrophication vs ICES criteria for England and Wales. Mar Pollut Bull 50:1569–1584
Painting SJ, Devlin MJ, Malcolm SJ, Parker ER, Mills DK, Mill C, Tett P, Wither A, Burt J, Jones R, Winpenny K (2007) Assessing the impact of nutrient enrichment in estuaries: susceptibility to eutrophication. Mar Pollut Bull 55:74–90
Parker AE (2005) Differential supply of autochthonous organic carbon and nitrogen to the microbial loop of the Delaware Estuary. Estuaries 286:856–867
Parker AE, Fuller J, Dugdale RC (2006) Estimating dissolved inorganic carbon concentrations from salinity in San Francisco Bay for use in 14C-primary production studies. Interagency Ecol Prog San Franc Estuary Newslett 19:17–22
Parker AE, Hogue VE, Wilkerson FP, Dugdale RC (2012a) The effect of inorganic nitrogen speciation on primary production in San Francisco Estuary. Estuar Coast Shelf Sci 104:91–101
Parker AE, Kimmerer WJ, Lidström U (2012b) Re-evaluating the generality of an empirical model for light-limited primary production in the San Francisco Estuary. Estuaries Coasts 35:930–942
Parker AE, Wilkerson FP, Dugdale RC (2012c) Elevated ammonium concentrations from wastewater discharge depress primary productivity in the Sacramento River and the northern San Francisco Estuary. Mar Pollut Bull 64:574–586
Pennock JR (1987) Temporal and spatial variability in phytoplankton ammonium and nitrate uptake in the Delaware Bay. Estuar Coast Shelf Sci 24:841–857
Redfield AC, Ketchum BH, Richards FA (1963) The influence of organisms on the composition of sea water. In: Hill MN (ed) The Sea, vol 2., The composition of sea-water comparative and descriptive oceanographyInterscience Publishers, New York, pp 26–77
Sharp JH (2001) Marine and aquatic communities, stress from eutrophication. Encycl Biodivers 4:1–11
Slawyk G, Collos Y, Auclair JC (1977) The use of the 13C and 15 N isotopes for the simultaneous measurement of carbon and nitrogen turnover rates in marine phytoplankton. Limnol Oceanogr 22:925–932
Solorzano L (1969) Determination of ammonia in natural waters by the phenolhypochlorite method. Limnol Oceanogr 14:99–801
Sommer TR, Armor C, Baxter R, Breuer R, Brown L, Chotkowski M, Culberson S, Feyrer F, Gingas M, Herbold B, Kimmerer W, Müller-Solger A, Nobriga M, Souza K (2007) The collapse of the pelagic fishes in the upper San Francisco Estuary. Fisheries 32:270–276
Waiser MJ, Tumber V, Holm J (2010) Effluent-dominated streams. Part 1: presence and effects of excess nitrogen and phosphorus in Wascana Creek, Saskatchewan, Canada. Environ Toxicol Chem 30:496–507
Whitledge TE, Malloy SC, Patton CJ, Winck CD (1981) Automated nutrient analyses in seawater. Department of Energy and Environment, Brookhaven National Laboratory, Upton
Wilkerson FP, Dugdale RC, Hogue VE, Marchi A (2006) Phytoplankton blooms and nitrogen productivity in San Francisco Bay. Estuaries Coasts 29:401–4176
Winder M, Jassby AD (2010) Shifts in zooplankton community structure: implications for food-web processes in the upper San Francisco Estuary. Estuaries Coasts 34:675–690
Xu J, Glibert PM, Liu H, Yin K, Yuan X, Chen M, Harrison PJ (2012) Nitrogen sources and rates of phytoplankton uptake in different regions of Hong Kong waters in summer. Estuaries Coasts 35:559–571
Yoshiyama K, Sharp JH (2006) Phytoplankton response to nutrient enrichment in and urbanized estuary: apparent inhibition of primary production by overeutrophication. Limnol Oceanogr 51:424–434
Acknowledgments
This research was supported by the Surface Water Ambient Monitoring Program (SWAMP) of the SF Bay Regional Water Quality Control Board and the Central Contra Costa Sanitary District. We wish to thank Karen Taberski for intellectual support, Peter Otis and his crew for water sampling, Annika Anderson for analyzing the clam data, Jim Fuller and graduate students Erica Kress, Christina Buck, Jamie Lee and Allison Johnson for help with laboratory analyses, Victoria Hogue for data management and two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Bas W. Ibelings.
Rights and permissions
About this article
Cite this article
Wilkerson, F.P., Dugdale, R.C., Parker, A.E. et al. Nutrient uptake and primary productivity in an urban estuary: using rate measurements to evaluate phytoplankton response to different hydrological and nutrient conditions. Aquat Ecol 49, 211–233 (2015). https://doi.org/10.1007/s10452-015-9516-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-015-9516-5