Abstract
The rates of upstream and downstream range expansion of the round goby (Neogobius melanostomus) were examined in the Trent-Severn Waterway in 2009 and 2010. Relative abundance, demographics, and habitat use were also compared between areas of range expansion and a longer established area to determine how these characteristics varied along the invasion pathway. Round gobies were sampled using an angling removal method in May and August of 2009 and 2010 at 75 sites at each area of range expansion and 25 sites near the center of their range where they first became established in the waterway. Areas of range expansion had initially low abundance and low site occupancy in May 2009 relative to the longer established area. Large increases in abundance and site occupancy were observed over the first summer of occupation, but with limited range expansion. Rapid range expansion was observed during the non-reproductive season at the upstream edge of range. Individuals sampled in the expanded upstream range were small and female biased relative to other range locations. Round gobies also exhibited higher habitat selectivity for rocky substrates at range edges than in the longer established area. These characteristics of seasonal dispersal, biased demographics of dispersers, and high habitat selectivity may be important components of successful range expansion of the round goby in invaded ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasions by non-indigenous species (NIS) can be generally described by three phases: introduction, establishment, and spread (Jerde and Lewis 2007). The spread of NIS is an important aspect of invasions because the degree of their impact is a function of the spatial extent of their range (Johnson and Padilla 1996; Nentwig 2007). Important characteristics for successful establishment and range expansion in new environments can include aspects of population structure, behavior, life history traits, genetics, and habitat use (Mooney and Drake 1989; Shigesada and Kawasaki 1997; Sakai et al. 2001). Knowledge of dispersal characteristics is important for controlling the spread of NIS, as well as for predicting potential high-risk species (Sakai et al. 2001; Kolar and Lodge 2002).
The round goby is native to the Ponto-Caspian region of Eastern Europe and has become established in non-indigenous areas of Europe and North America (Corkum et al. 2004). Its spread has been attributed to a combination of ballast water introductions, bait bucket transfers, hull transfers, and natural population expansion (Charlebois et al. 1997; Tsepkin et al. 1992). The mechanism of ‘natural’ range expansion is likely related to its aggressive nature, where large round gobies force smaller individuals from preferred rocky substrates to less ideal habitats, from which they disperse (Ray and Corkum 2001; Johnson et al. 2005a). The round goby was first discovered in North America in the St. Clair River, Michigan in 1990 (Jude et al. 1992). It now occupies all of the Laurentian Great Lakes (Charlebois et al. 1997) and continues to expand its range into many of their tributaries (Phillips et al. 2003; Carman et al. 2006; Poos et al. 2009; Raby et al. 2010).
The round goby has been a highly successful invader in both North American and European ecosystems, making it ideal for understanding mechanisms of species invasions. A number of characteristics have been identified to contribute to its successful range expansion. It is known to exhibit a high reproductive output, especially in more recently invaded areas, where individuals mature earlier and allocate a greater amount of energy to reproduction (Gutowsky and Fox 2012). Recently invaded areas also appear to have a lower proportion of sites containing round gobies, with lower densities, larger individuals, and more male biased sex ratios than in longer established areas of their range in a fluvial ecosystem (Gutowsky et al. 2011; Gutowsky and Fox 2011), but little is known about the population dynamics of round goby in areas where actual range expansion is occurring.
Habitat can be an important factor limiting the spread of invasive species, one that may dictate their dispersal rate, distribution, and degree of ecosystem disturbance (Johnson and Padilla 1996; Shigesada and Kawasaki 1997). The round goby is known to inhabit nearly all habitat types in invaded ecosystems including rock, sand, and mud substrates, and those with macrophyte cover (Jude 2001; Johnson et al. 2005a; Taraborelli et al. 2009). However, habitat does affect round goby distribution and abundance, as it shows some affinity for rocky substrates, inhabits mud substrates less frequently, and wetlands appear most resistant to its invasion (Ray and Corkum 2001; Cooper et al. 2007; Young et al. 2010; Borcherding et al. 2011). While these habitat associations are known through studies in areas where round goby are well established, little is known about habitat use at the time and location of actual range expansion.
The goal of this study was to characterize the process of round goby range expansion at the pioneering upstream and downstream fronts in a fluvial ecosystem. Our specific objectives were to determine rates of range expansion, population dynamics, demographics, and habitat use of the round goby in the areas where actual range expansion was occurring, and to compare round goby demography and habitat use in these areas with those of longer established sites in the same system. We predicted that newly expanded areas would be characterized by lower abundance, a lower proportion of occupied sites, larger individuals, and more male-biased sex ratios, based on patterns observed in recently established areas in the Trent-Severn Waterway in 2007/2008 (Gutowsky and Fox 2011; Gutowsky and Fox 2012). We also predicted that round gobies would occupy rocky substrates more often than softer substrates such as sand and mud (Ray and Corkum 2001; Johnson et al. 2005a), and that this habitat preference would be more pronounced in newly occupied areas than in longer established areas.
Methods
Study location
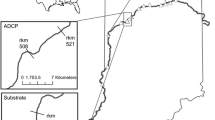
The Trent-Severn Waterway is a system of connected lakes, rivers, and canals in a 12,550-km2 watershed in Southeastern Ontario, Canada (Minns et al. 2004). It is a navigational waterway that contains many dams and locks, connecting Georgian Bay, Lake Huron to the Bay of Quinte, Lake Ontario. Study sites within the waterway were located in the Trent River, Rice Lake, the Otonabee River, and the Indian River (Fig. 1).
The source of the Trent River is at the eastern end of Rice Lake, stretching 90 km in length to the outlet in the Bay of Quinte (Fig. 1). In the area that center of range (CORE) sites were sampled (44.28319° N, 78.01102° W to 44.32097° N, 77.94218° W) the Trent River has a mean channel depth of 4.4 m and mean width of 150 m estimated from Navionics 2010 navigation charts. In the area of downstream expansion (DE) sites, the Trent River has a mean channel depth of 4.8 m and a mean width of 460 m estimated from Navionics 2010 navigation charts. Rice Lake is the furthest downstream of the Kawartha Lakes, located between the Otonabee River and the Trent River. It has a surface area of 10,018 ha, a mean depth of 2.6 m, and a mean width of 3204 m (Lester et al. 2004). Upstream expansion (UE) sites were located throughout most of Rice Lake, from near the Indian River, upstream to the Otonabee River (44.21999° N, 78.11313° W to 44.13430° N, 78.24733° W). The Otonabee River commences at the outlet of Katchewanooka Lake and flows 50 km to its mouth in Rice Lake. UE sampling sites were also located in the downstream 12.5 km of the Otonabee (44.20644° N, 78.27860° W to 44.15121° N, 78.22759° W). In this section, the mean channel depth is 5.4 m, and the mean width is 158 m estimated from Navionics 2010 navigation charts.
The round goby was first reported in the Trent River in 2003 at a location downstream of Lock 18 in the town of Hastings (44.31078° N, 77.952872° W) (Raby et al. 2010) (Fig. 1). Its introduction was likely due to one or more bait bucket releases of fish collected from infested waters (Raby et al. 2010). By 2008, the round goby had expanded its range upstream, with sightings 16 km up the Trent River and into Rice Lake (44.24136° N, 78.11561° W) (Fig. 1) (Raby et al. 2010). It had also spread downstream, with sightings 38 km down the Trent River into Percy’s Reach (44.23433° N, 77.79835° W) (Fig. 1). This is the only area of this system known to be occupied by the round goby prior to the initiation of this study.
Site selection and sampling procedure
Sampling sites were selected using a random point generator (www.geomidpoint.com) in three areas of the Trent-Severn Waterway to characterize range expansion and compare the population parameters of recently invaded areas to a longer established area. Twenty-five sites were selected near the center of round goby range in the system (hereafter referred to as the CORE area), near its point of introduction in 2003 (Fig. 1). Seventy-five sites were selected in both the areas of upstream expansion (UE) and downstream expansion (DE) in the system. These areas were determined by surveying sites near the locations identified as the upstream and downstream extent of round goby distribution in 2008 (Fig. 1). As sampling progressed in May 2009, sites were generated further upstream and downstream until no round gobies were consistently detected. Sites were also generated beyond the detected edges of the round goby’s range in May 2009 in the areas where range expansion was predicted to occur during additional sampling periods. In the area of upstream expansion, an additional 20 sites were generated for 2010 sampling to better characterize range expansion, which was more rapid than expected. In the area of downstream expansion, an additional 10 sites were generated for May 2010 sampling to confirm upstream movement from the Bay of Quinte round goby population (see Results). Sites were selected in a stratified design, where the majority of sites (≈75% of each sampling area) were selected in areas predicted to contain high-quality round goby habitat based on waterbody characteristics.
Round goby relative abundance was assessed in May and August of 2009 and 2010 using the angling removal method described in Gutowsky et al. (2011). This method provided the ability to sample round gobies in a wide range of environmental conditions, including complex rocky substrates that are most problematic for other sampling techniques (Gutowsky et al. 2011). The technique involves angling by two individuals within a 2-m2 floating barrier for 20 min at each site. The floating barrier was clamped to the side of a 16-foot Jon boat, which was anchored at both ends to remain on the site. Angling was conducted with micro-light rods and braided fishing line (0.15 mm diameter). Size 20 hooks were baited with a scented plastic maggot imitation, and small weights were attached to the line. All fish caught were identified, and round gobies were measured for total length (mm), sexed, and retained until the end of the sampling period. Sex was determined by examining the urogenital papilla, which is triangular on males and rectangular on females (Miller 1984). The time of catch was also recorded. All round gobies were returned to the site after sampling was completed. The size of round gobies captured with this method was restricted mainly to individuals larger than 45 mm total length (TL). These individuals would be almost entirely age 1 and older individuals, based on previous aging of this population (Gutowsky and Fox 2012).
Sites were located using a Garmin Oregon 400t Global Positioning System unit (Garmin International Inc., Olathe, KS). Additional parameters measured at each site included substrate composition, depth, current, and temperature. Substrate was quantified at each site by visual estimation of the percentage of rock, sand, and mud (in 10% intervals) within the 2 m2 sampling area using an Aquaview S-series color underwater video camera (Nature Vision Inc., Brainerd, MN). The dominant rock size was also estimated based on particle sizes defined by Krumbein and Sloss (1951). Substrate was not sampled in May 2009. In all other sampling dates, substrate composition was visually estimated from roughly 0.5 m above the bottom. Depth was measured using an Eagle Cuda 168 depth sounder (Eagle Electronics, Catoosa, OK). River velocity was measured by timing the horizontal movement of a float.
Data analysis
Statistical tests were conducted using Statistica (version 6.1, StatSoft Inc., Tulsa, OK). Data were checked for normality and homogeneity of variance and transformed when necessary. When parametric assumptions could not be met, nonparametric tests were used. Round goby catch per unit effort (CPUE) from 20-min angling trials (expressed as catch per m2) was used to compare round goby densities between range locations (CORE, DE, and UE) and over sampling periods (May and August, 2009 and 2010) using a two-way fully factorial ANOVA or its nonparametric Kruskal–Wallis equivalent, followed by Tukey HSD tests to examine range location differences within sampling periods when significant effects were found. The same tests were also used to compare the lengths and sex ratios (% male) of round gobies between range locations and over sampling periods. All three parameters were analyzed by calculating a respective mean for each occupied site.
To examine the demographics of dispersing round gobies, those that occupied the expanded upstream range in 2010, upstream of occupied sites in 2009, were compared to other range locations. Expanded upstream range was the focus of this comparison because these individuals were likely to have actively moved into this area, as opposed to downstream, where larval drift may have ultimately contributed to range expansion (see Hensler and Jude 2007). Mean round goby TL and sex ratio (% male) were calculated for each sampling site and compared between range locations (CORE, DE, all sampling dates included), UE sampled in 2009, sites occupied in 2010, upstream of the range edge determined in August 2009 (denoted area of upstream expansion in 2010), and upstream sites occupied in May or August of 2009 and resampled in 2010 (denoted area of upstream expansion in 2009) using Kruskal–Wallis ANOVAs.
To determine whether round gobies occupied higher quality habitats at the edges of their range than in the CORE area, chi-square goodness of fit tests were used to compare the proportion of sites occupied by one or more round gobies in sites classified by proportion of rock substrate. Also to examine round goby habitat use along the invasion pathway, Kruskal–Wallis tests were used to compare round goby abundance between four substrate types (boulder, cobble, gravel, and sand) within each range location. The level of significance for all tests was P < 0.05.
Results
Site occupancy and range expansion
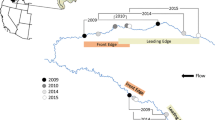
Round gobies were present at the center of their range in the Trent-Severn in 84–88% of sites across the four sampling periods (Fig. 2). In the upstream expansion area determined in May 2009, round gobies were distributed widely in Rice Lake and were found as far upstream as the mouth of the Otonabee River, indicating a 14.2 km upstream advance from 2008 sightings (Fig. 2). However, they only occupied 26% of upstream edge sampling sites within their detected range and were limited to a few rocky shoals. By August 2009, round gobies had spread to many sites within the detected upstream expansion area and occupied 47% of sample sites, but little upstream range expansion occurred during this time period (<1 km). By the following spring (May 2010), round gobies were detected in the Otonabee River 9.14 km upstream of the population edge in August 2009, indicating rapid range expansion over the 8-month period (Fig. 2). Site occupation within their detected range remained similar (49%) to the previous sampling period. Between May and August 2010, changes in round goby distribution were similar to those observed over the summer of 2009, with limited range expansion (1.13 km upstream from the May 2010 edge) and a higher site occupancy rate (58%).
Round goby relative abundance (catch/m2) in three range locations in the Trent-Severn Waterway, sampled using the angling removal method in a May 2009, b August 2009, c May 2010, and d August 2010. Stars indicate detected population fronts. For location refer to Fig. 1
In the area of downstream expansion, round gobies were detected as far downstream as Percy’s Reach in May 2009 (Fig. 2). They occupied 27% of 75 downstream sites within their detected range and were mainly confined to a large rocky shoal near the start of the reach, with the exception of one individual caught 4 km downstream of any other catch site (Fig. 2). No other round gobies were caught in the other 20 sampling sites in this 4-km stretch. Including this individual, the advance was 5.2 km from previous downstream sightings in the summer of 2008. Similar to the area of upstream expansion, by August 2009, little range expansion occurred (0.5 km from the lone individual), but round gobies occupied 55% of sites within their range. In May 2010, round gobies occupied sites from the previous detected population front in 2009 all the way south to the Bay of Quinte. Therefore, the amount of downstream range expansion could not be determined. In May 2010, round gobies occupied 48% of downstream sampling sites, which increased to 53% in August 2010.
Abundance in sites occupied by round goby
In CORE area sites, round goby abundance was relatively consistent over time in comparison with areas where range expansion was occurring (Figs. 2, 3). Mean CPUE was lowest in May 2009 (3.9/m2) and increased to its maximum in August 2009 (5.8/m2). Mean CPUE dropped to 5.1/m2 by May 2010 and again to 4.4/m2 by August 2010.
In the area of upstream expansion, round goby abundance was very low in May 2009, with a mean CPUE of 1.7/m2 and a maximum of 4/m2 (Figs. 2, 3). In August 2009, round goby CPUE increased dramatically to 7.8/m2 with a maximum CPUE of 17/m2. CPUE declined in May 2010 and remained consistent in 2010 (Fig. 3).
In the area of downstream expansion, abundance was also very low in May 2009, with a mean CPUE of 1.5/m2 (in catch sites), and a maximum of 3/m2 (Figs. 2, 3). Mean CPUE doubled in August 2009, increased again in May 2010, and dropped in August 2010 (Fig. 3).
There was a significant difference in round goby CPUE among range locations (Table 1a), with downstream expansion sites being significantly lower in abundance than both CORE and upstream expansion sites (Tukey HSD, P ≤ 0.05). There was also a significant date effect, as well as a nearly significant date by area interaction. Further investigation showed that CPUE varied significantly between range locations in May and August 2009 (P < 0.004 in both cases). As predicted, CPUE was significantly lower at both the upstream and downstream expansion areas than in the CORE area in May 2009 (Tukey HSD, P ≤ 0.05). In August 2009, downstream expansion sites had significantly lower CPUE than both CORE and upstream expansion sites (Tukey HSD, P ≤ 0.05). However, by May 2010, there was no significant difference among range locations in round goby CPUE (P > 0.30 in both May and August).
Length distribution
In CORE area sites, the mean TL of round gobies caught was highest in May 2009 (89 mm ± 2.3 SE, Fig. 4). Mean TL decreased in every subsequent sampling period, which reflects a decrease in 80+ mm and an increase in <70 mm round gobies (Fig. 4). In upstream expansion sites in May 2009, the mean TL of round gobies was 79 mm ± 2.6 SE (Fig. 4). Mean TL increased dramatically in August 2009, peaking after the first summer of occupation, and declining in 2010 (Fig. 4). Very similar sizes and temporal patterns were observed in the area of downstream expansion (Fig. 4).
Overall, there was no significant difference in the mean length of round gobies among range locations, but there was a significant date effect and a significant date by area interaction (Table 1b). In May 2009, there was a significant difference in mean length between range locations (F 2,43 = 7.0, P = 0.0023), with CORE area sites having significantly larger gobies than both areas of recent expansion (Tukey HSD, P < 0.05). There was also a significant difference in mean length among range locations in August 2009 (F 2,74 = 6.1, P = 0.0036); however, during this period, gobies in the upstream expansion sites were significantly larger than those in the CORE area (Tukey HSD, P < 0.05). There was a significant difference in mean lengths in May 2010 as well (F 2,101 = 3.9, P = 0.023), with CORE area sites once again having significantly larger gobies than both areas of recent expansion (Tukey HSD, P < 0.05). However, there was no significant difference in mean lengths between range locations in August 2010 (F 2,112 = 1.2, P = 0.29).
Sex ratio
The mean sex ratio of round gobies caught at angling sites followed a similar pattern over time in upstream expansion and CORE range locations, with a peak in the proportion of males in August 2009 (Fig. 5a). At the downstream edge, the opposite pattern was observed, with a drop in the proportion of males in August 2009. The sex ratio was the most heavily male biased in CORE area sites (63% vs. 53 and 57%, in UE and DE sites, respectively); however, the difference was not significant (Table 1c). There was also no significant difference in sex ratio among sample dates, but there was a significant range location by date interaction. In this case, significant differences among locations were not found in May of either year (P > 0.38 in both cases), but CORE area sites had a significantly higher proportion of males than downstream sites in August 2009, and downstream sites had a significantly higher proportion of males than upstream sites in August 2010 (Tukey HSD, P < 0.05).
a Round goby sex ratios in angling sites in three range locations in the Trent-Severn Waterway. b The mean percentage of males and total length (TL, males and females) of round goby at CORE, UE (2009), DE, UE 2009 (occupied in 2009, resampled in 2010), and UE 2010 range locations; May and August of 2009 and 2010 (±SE)
Upstream dispersers
There was a significant difference in the mean length of round gobies between the area of upstream expansion in 2010, the area of upstream expansion in 2009 (resampled in 2010), the area of upstream expansion in 2009, the CORE area, and the area of downstream expansion (H 4,431 = 21.3, P < 0.001; Fig. 5b). A post hoc analysis showed that round gobies occupying the expanded upstream range in 2010 were smaller than those from all other sites except for the resampled former upstream expansion area. Individuals occupying the area of upstream expansion in 2010 were also female biased (58%), unlike the other sample areas (Fig. 5b). The difference in sex ratio among range locations was significant (F 4,379 = 6.2, P < 0.001), with the expanded upstream edge in 2010 being different from all other areas (Tukey HSD, P < 0.03 in all cases). Despite these differences, a few very large females were sampled in the expanded range in 2010, and the largest female caught in this study (128 mm TL) was located at the most upstream site in May 2010 at the very edge of the detected population front.
Habitat
Round gobies occupied between 88 and 100% of sites with 40% or more rock substrate in all range locations in sampled sites within their detected range (Fig. 6a). In sites with less than 80% rock substrate, both areas of range expansion had lower site occupancy than CORE area sites. There was a significant difference in the distribution of occupied sites across substrate classes between both areas of range expansion and the CORE area (χ 2 > 18.5, P < 0.001, df = 4 in both cases), and the largest differences occurred in sites with less than 20% rock substrate.
a Occupancy of round goby by proportion of rock substrate in three range locations in the Trent-Severn Waterway. b Mean relative abundance (catch/m2 in occupied sites) of round goby in five substrate types (boulder, cobble, gravel, sand, and mud) in three range locations in the Trent-Severn Waterway; May and August 2009 and 2010 (±SE)
Round goby abundance in occupied sites was higher in all three types of rock substrate (boulder, cobble, and gravel) than in sand or mud substrates in all three range locations (Fig. 6b). Both areas of range expansion had the highest abundance in gravel-dominated substrate, while in the CORE area, abundance was highest in boulder substrate. In CORE area sites, there was no significant difference in round goby abundance between substrate types (H 3,62 = 2.5, P = 0.47). In contrast, round goby abundance varied significantly between substrate types in upstream expansion sites (H 3,125 = 48.3, P < 0.001), with all three types of rock substrate having significantly higher abundance than sand substrate (post hoc analysis, P < 0.001 in all cases). Abundance also varied significantly between substrate types in downstream expansion sites (H 3,107 = 18.3, P < 0.001), with cobble substrate having significantly higher abundance than sand (Tukey HSD, P = 0.002).
Discussion
Range expansion population dynamics
As predicted, both the areas of upstream and downstream expansion in the Trent-Severn Waterway were characterized by low site occupancy and low relative abundance during the spring of the first year of occupation in comparison with sites at the center of their range. Lower abundance in more recently invaded areas has been observed previously in the Trent-Severn round goby population (Raby et al. 2010; Gutowsky et al. 2011), as well as in Lake Ontario, Lake Michigan, and Lake Huron (Bergstrom et al. 2008; Lederer et al. 2008; Taraborelli et al. 2009).
However, the lower density in recently invaded areas was only temporary. Over the first summer of occupation, major increases in site occupancy rate and relative abundance of occupied sites were observed in both areas of range expansion. This was particularly true upstream, where mean CPUE increased almost fourfold from May, peaking in August 2009 at the highest observed in this study at any range location. CPUE doubled in downstream expansion sites during the same time period, but did not peak until May 2010. This late peak can be attributed to the coalescence of the Trent-Severn population with the upstream expansion of the Bay of Quinte population. Evidence for this coalescence comes from the sudden, consistent presence, and high abundance of round gobies from the edge of the sample area to the Bay of Quinte. Such sudden increases in site occupancy and abundance are inconsistent with that typically observed in recently invaded areas, especially at the downstream edge of their range (Fig. 2). If this range expansion was occurring from upstream in the Trent-Severn, a gradient from high to low abundance would be expected at the range edge, as observed at both upstream and downstream edges of their range in 2009 (Fig. 2). This was not the case from the downstream edge to the Bay of Quinte.
By 2010, upstream expansion sites decreased significantly in relative abundance, and all three range locations had similar mean abundances in both May and August of that year (Fig. 3). While recently invaded areas are generally considered to have lower abundance than longer established areas (Raby et al. 2010), during this study period in the Trent-Severn, this phenomenon was very brief, and round goby abundance in areas of range expansion became fairly homogeneous with a well-established area just 1 year after first detection (Fig. 3). Rapid increases in recently invaded areas are likely the result of a combination of high resource availability due to the low initial goby density, fast somatic growth, early maturity, and high reproductive output (Raby et al. 2010; Gutowsky and Fox 2012). Survival may also be high at range edges due to low predation rates (Brownscombe 2011).
The high abundance observed in August 2009 in the area of upstream expansion, and subsequent lower abundance in 2010, similar to a well-established area in the system, is suggestive of a peak in abundance during the first summer of occupation in a new area. This peak occurred much faster than in previous examples in the Great Lakes. A peak in round goby abundance was observed 2 years after detection in Eastern Lake Michigan (Clapp et al. 2001), while in Duluth Harbour, Lake Superior, a peak in abundance occurred 6 years post-detection (Bergstrom et al. 2008). There are likely differences in biotic and abiotic conditions between the Trent-Severn, Lake Michigan, and Lake Superior, which may have resulted in differences in the rate of round goby population growth. One of these differences is that the Great Lakes are much larger ecosystems, with more area for individuals to disperse before densities become high. However, these differences may also be related to sampling technique. The previously mentioned studies sampled with bottom trawls, which are often limited to less complex substrates (Johnson et al. 2005a; Bergstrom et al. 2008). These substrates are associated with lower abundance, smaller individuals, and a high proportion of females, indicating that they are less favorable habitats (Ray and Corkum 2001; Johnson et al. 2005a; Bergstrom et al. 2008). During initial stages of invasion, round gobies appear to occupy mainly high-quality habitats, and over time individuals occupy habitats of lower quality. Differences in sampling techniques may help explain the later abundance increases observed in Lake Michigan and Lake Superior, as trawling targets less ideal habitats, which probably have more delayed increases in abundance than the complex, rocky habitats sampled in this study using the angling removal method. By sampling with angling in late spring and summer, we have shown that round goby abundance can increase in ideal habitats of recently invaded areas much faster than previously known.
Range expansion
Range expansion occurred in similar patterns at both upstream and downstream edges of round goby range in the Trent-Severn in 2009 and 2010. Little range expansion occurred during the primary reproductive seasons (May to August), while large-scale range expansion occurred during the non-reproductive seasons (September to April). The round goby is known to spread by bait bucket transfer in North America (Raby et al. 2010), and in Europe, they have been suspected to spread by attaching to the hulls of vessels (Tsepkin et al. 1992; Sokolov et al. 1994). However, these types of introductions often result in large gaps between infested zones (Wiesner 2005; Raby et al. 2010; Bronnenhuber et al. 2011). The consistent presence and abundance of round gobies from their range edge in August of 2009, throughout the Otonabee River, to their expanded range edge in May 2010 strongly suggests that their dispersal in this study was primarily natural.
When considering the characteristics of the round goby, it is not surprising that limited range expansion occurred during reproductive seasons. Round goby exhibits high reproductive energy allocation, especially at the edges of its range, as well as a long reproductive season, multiple spawning by females, and nest guarding by males (MacInnis and Corkum 2000; Jude 2001; Gutowsky and Fox 2012). It appears that round gobies allocate more energy to reproduction than to movement during the late spring/summer months. Some smaller-scale movement did occur during these months into sites within their range. These movements may have been density driven by large, aggressive gobies forcing smaller individuals into new areas (Ray and Corkum 2001; Johnson et al. 2005a).
While round gobies are considered relatively immobile, with very small home range sizes (Skora 1996; Wolf and Marsden 1998; Ray and Corkum 2001), it is evident from their distribution in the Trent-Severn that they incurred a large-scale seasonal range expansion upstream into the Otonabee River sometime between autumn 2009 and early spring 2010. During the winter months, temperatures are very low in Ontario, and at these temperatures, round gobies are relatively inactive (Lee and Johnson 2005), so it is unlikely that large-scale movements were occurring during this season. Spring and autumn are more likely the potential seasons for round goby range expansion. Round gobies are known to migrate into deeper overwintering habitats in the fall and return to shallow areas in the spring (Knight 1997; Pennuto et al. 2010). This dispersal appears to be the primary mode by which the round goby expands its range during the early spring and/or fall seasons, whereas little large-scale range expansion occurred during the late spring/summer. However, it is interesting that the large increases in abundance in expansion sites during the summer of 2009 were accompanied by a peak in mean body size, where a large proportion of the sampled population was age 2 or 3 based on length at age data from this system (Gutowsky and Fox 2012). If no large-scale dispersal was occurring during this time period, it would be expected that an increase in abundance would be accompanied by smaller body sizes, as young-of-year and age 1 fish grew large enough to become recruited to the sampling gear (45+ mm). Because there was a high proportion of large round goby in the samples in August 2009, individuals were likely migrating into areas of range expansion from closer to the population core. Dispersal may also have been occurring during the late spring/summer months, but none was detected beyond their population front.
The rates of spread observed in this study were rapid, but not extraordinary for the round goby. An average of 25 km/year of range expansion was previously observed in round goby moving through Chicago inland waterways from Lake Michigan to the Mississippi River (Steingraeber and Thiel 2000). Round gobies have been collected up to 120 km further downstream of where they were detected the year before in the Illinois Waterway (Irons et al. 2006). However, the only previously documented rates of upstream range expansion were much slower, with a maximum 1 km/year from 1998 to 2003 through Duluth Harbour, Lake Superior, into the St. Louis River (Bergstrom et al. 2008). There are likely a number of differences between the conditions of the Trent-Severn and Duluth Harbour/the St. Louis River, the most important of which may be productivity level, as Lake Superior is oligotrophic (Urban et al. 2004), whereas the Trent-Severn is mesotrophic (Gutowsky and Fox 2011), and Rice Lake is eutrophic (Nurnberg 1999).
Population dynamics
Round gobies occupying the CORE area showed some indication of a population decline despite the fact that abundance did not vary significantly over time. During the reproductive season of 2009, mean round goby abundance increased by nearly 50% in CORE area sites. An increase in abundance is expected in a stable population during the summer months due to growth and reproduction. Individuals born within the reproductive season may not be included, but summer growth would be expected to recruit many age 1 fish into the sample (Gutowsky and Fox 2012). A subsequent 12% decline in abundance was observed by May 2010. This would also be expected, as most temperate fishes exhibit low levels of growth and some mortality over the winter season (Wootton 1998). However, by August 2010, abundance did not rebound as expected, but decreased by another 14%. In addition, the mean length of CORE area gobies declined consistently throughout the sampling period (17% decrease from May 2009 to August 2010). A similar trend was observed in Hamilton Harbour, Lake Ontario, where a 72% decrease in round goby abundance coincided with a 10% decrease in body length from 2002 to 2008, less than a decade from its detection in Lake Ontario (Mills et al. 2003; Young et al. 2010). In Lake Erie, round goby abundance peaked in 1999, 5 years after its first detection (Johnson et al. 2005b; Bunnell et al. 2005). Invasive species in general are often characterized by an initial rapid increase in population size, followed by a decline to lower abundance (Parker et al. 1999; Arin et al. 2005). The round goby may be showing early signs of this decline roughly 7 years after its detection in the Trent-Severn.
Range location demographics
Round gobies sampled in this study were male biased at all range locations, which is consistent with findings in other invaded areas (Corkum et al. 2004; Young et al. 2010; Gutowsky and Fox 2011). However, counter to predictions, CORE area sites were the most heavily male biased (1.7:1), while both areas of range expansion had a more even distribution of sexes (1.1:1 at upstream expansion and 1.2:1 at downstream expansion). While this finding appears to be inconsistent with that of a previous round goby study in the Trent-Severn Waterway in which more recently invaded areas were more male biased than in a longer established area, the difference may be explained by the much broader definition of the edge of range in that study. Gutowsky and Fox (2011) examined areas of recent range expansion where a moderate abundance of round gobies were located, whereas the current study utilized areas of current range expansion that were invaded less than 1 year prior to the initiation of the study and were at very low abundance.
The length of round gobies sampled in the Trent-Severn varied from 43 to 135 mm TL with a mean of 79 mm. These sizes are similar to those found throughout North American ecosystems, which are generally smaller than those found in their native range (Charlebois et al. 1997; Taraborelli et al. 2010; Gutowsky and Fox 2011). Contrary to our prediction that areas of range expansion would consist of larger individuals based on previous research in the Trent-Severn, they consisted of slightly smaller individuals. This again probably relates to our use of areas currently undergoing range expansion. Smaller individuals at pioneering range edges may be the most mobile members of the population, as previously speculated by Ray and Corkum (2001), Johnson et al. (2005b), and Bergstrom et al. (2008). Within range locations, there was high variability in round goby body size over time. Round gobies were significantly larger in August 2009 than at other sample dates in both areas of range expansion. This was likely due to high resource availability when round goby density was low, contributing to high growth rates in these areas (Raby et al. 2010; Gutowsky and Fox 2011) during the first summer of occupation.
Demographics of dispersers
Round gobies sampled in the Otonabee River in 2010 showed a significantly higher proportion of females (0.7 M:F ratio) than other range locations in the system, which suggests that a higher proportion of females were contributing to range expansion. Dispersal is male biased in most polygynous fishes (Gros et al. 2009), in contrast to our findings. Sex-biased dispersal is known to evolve for various reasons, including inbreeding depression avoidance, asymmetry in the costs of dispersal, and mating system characteristics (Gros et al. 2009). While they usually work in conjunction, the more dispersive sex is often decided by the two latter factors. In some bird species, the benefits for males in dominating a natal territory are higher than for females, leading to female-biased dispersal (Gros et al. 2009). This may also be the case for the round goby. Males that secure high-quality nesting sites in a high-density area have the potential benefit of multiple females contributing to their nest (MacInnis and Corkum 2000), which may be the greatest limitation to male reproductive success. Conversely, resource availability likely limits egg production in females, and resources are high at range edges (Raby et al. 2010; Gutowsky and Fox 2012). While dispersing females have less choice in a sexual partner, the most prominent round goby nest predators are male conspecifics (Wickett and Corkum 1998), so at range edges in low-density conditions, nesting success may still be high despite a lack of large males.
The dispersing individuals were also significantly smaller than those of all other range locations, but they were not smaller than occupied upstream expansion sites in 2009 when they were resampled in 2010. While there may have been a regional or temporal effect, a greater proportion of small individuals likely contributed to range expansion. In a previous study, more recently colonized areas of Lake St. Clair and surrounding waterbodies were also comprised of smaller individuals (Ray and Corkum 2001). As first speculated by Ray and Corkum (2001), the primary mechanism of round goby range expansion is likely intraspecific competition, which forces smaller individuals into less ideal habitats, from which they disperse. Our findings are consistent with this hypothesis, although some large individuals, particularly females, were also found in the expanded upstream range area of the Trent-Severn, and large-scale movements are not limited to small round gobies. The movement of these large females may be important for range expansion, as establishment is more probable with adults present (Vélez-Espino et al. 2010), and these females likely have high reproductive output (MacInnis and Corkum 2000) contributing to propagation in new areas.
Habitat
As predicted, round gobies were more abundant on all types of rocky substrates (boulder, cobble, and gravel) than on sand, and very few were caught on mud-dominated substrate. Round gobies are known to have an affinity for rocky substrates because they provide complex benthic structure that they use for refuge (Ray and Corkum 2001; Erős et al. 2005; Johnson et al. 2005a). This affinity was very pronounced in this study, which is likely because the sampling method targeted mainly mature individuals during the reproductive season, and the complex rocky habitats of the Trent-Severn provide ideal nesting sites in addition to refuge (Miller 1984; MacInnis and Corkum 2000; Gutowsky et al. 2011). In both areas of range expansion, round goby abundance was highest on gravel-dominated substrate, while in the CORE area, it was highest on boulder. This difference may be related to predation risk, which appears higher in established areas than recently invaded areas of this system (Brownscombe 2011). As predators adapt to feeding on round gobies and predation risk becomes higher, habitat preference may shift to more complex rocky substrates, which likely provide better refuge from predators.
As predicted, round gobies showed a more pronounced habitat preference in recently invaded areas than at the center of their range, with larger differences in site occupation and abundance in high-quality habitats (rock) than low quality (sand or mud). It appears that individuals migrating into new areas are in search of these high-quality habitats. In longer established areas, round gobies are known to occupy more marginal habitats (Johnson et al. 2005a; Bergstrom et al. 2008; Taraborelli et al. 2009). As the population density increases, intraspecific competition likely forces individuals into neighboring, poorer-quality substrates.
The round goby expanded its range in the Trent-Severn Waterway by seasonal dispersal during the non-reproductive season and large increases in abundance during the reproductive season. In addition, dispersers contributing to range expansion were small and female biased relative to other range locations and also exhibited high habitat selectivity. Range expansion likely occurs as demographically biased individuals are forced from occupied areas by intraspecific competition and migrate in search of alternate high-quality habitats. These findings provide insight into the mechanisms by which the round goby expands its range in non-indigenous areas, and these should be considered for other invasive species both for understanding invasion patterns and predicting potential high-risk species.
References
Arin M, Abades SR, Neill PE, Lima M, Marquet PA (2005) Spread dynamics of invasive species. Proc Natl Acad Sci USA 103:374–378
Bergstrom MA, Evrard LM, Mensinger AF (2008) Distribution, abundance, and range of the round goby, Apollina melanostoma, in the Duluth-Superior Harbor and St. Louis River Estuary. J Great Lakes Res 34:535–543
Borcherding J, Staas S, Krüger S, Ondračková M, Šlapanský LS, Jurajda P (2011) Non-native Gobiid species in the lower River Rhine (Germany): recent range extensions and densities. J Appl Ichthyol 27:153–155
Bronnenhuber JE, Dufour BA, Higgs DM, Heath DD (2011) Dispersal strategies, secondary range expansion and invasion genetics of the nonindigenous round goby, Neogobius melanostomus, in Great Lakes tributaries. Mol Ecol. doi:10.1111/j.1365-294X.2011.05030.x
Brownscombe JW (2011) Invasion dynamics of the round goby (Neogobius melanostomus) in the Trent-Severn Waterway. Dissertation, Trent University
Bunnell DB, Johnson TB, Knight CT (2005) The impact of introduced round gobies (Neogobius melanostomus) on phosphorus cycling in central Lake Erie. Can J Fish Aquat Sci 62:15–29
Carman SM, Janssen J, Jude DJ, Berg MB (2006) Diel interactions between prey behaviour and feeding in an invasive fish, the round goby, in a North American river. Freshw Biol 51:742–755
Charlebois PM, Marsden JE, Geottel RG, Wolfe RK, Jude DJ, Rudnicka S (1997) The round goby, Neogobius melanostomus (Pallas), a review of European and North American literature. Illinois-Indiana Sea Grant Program and Illinois Natural History Survey. INHS Special Publication. No. 20
Clapp DF, Schneeberger PJ, Jude DJ, Madison G, Pistis C (2001) Monitoring round goby (Neogobius melanostomus) population expansion in Eastern and Northern Lake Michigan. J Great Lakes Res 27:335–341
Cooper MJ, Ruetz CR III, Uzarski DG, Burton TM (2007) Distribution of round gobies in coastal areas of Lake Michigan: are wetlands resistant to invasion? J Great Lakes Res 33:303–313
Corkum LD, Sapota MR, Skora KE (2004) The round goby, Neogobius melanostomus, a fish invader on both sides of the Atlantic Ocean. Biol Invasions 6:173–181
Erős T, Sevcsik A, Tóth B (2005) Abundance and night-time habitat use patterns of Ponto-Caspian gobiid species (Pisces, Gobiidae) in the littoral zone of the River Danube, Hungary. J Appl Ichthyol 21:350–357
Gros A, Poethke HJ, Hovestadt T (2009) Sex-specific spatio-temporal variability in reproductive success promotes the evolution of sex-biased dispersal. Theor Popul Bio 76:13–18
Gutowsky LF, Fox MG (2011) Occupation, body size, and sex ratio of round goby (Neogobius melanostomus) in established and newly invaded areas in an Ontario river. Hydrobiologia 671:27–37
Gutowsky LF, Fox MG (2012) Intra-population variability of life history traits and growth during range expansion of the invasive round goby (Neogobius melanostomus). Fish Manage Ecol 19:78–88
Gutowsky LF, Brownscombe JW, Fox MG (2011) Angling to estimate the density of large round goby (Neogobius melanostomus). Fish Res 108:228–231
Hensler SR, Jude DJ (2007) Diel vertical migration of round goby larvae in the Great Lakes. J Great Lakes Res 33:295–302
Irons KS, McClelland MA, Pegg MA (2006) Expansion of round goby in the Illinois Waterway. Am Midl Nat 156:198–200
Jerde CL, Lewis MA (2007) Waiting for invasions: a framework for the arrival of nonindigenous species. Am Nat 170:1–9
Johnson LE, Padilla DK (1996) Geographic spread of exotic species: ecological lessons and opportunities from the invasion of the zebra mussel, Dreissena polymorpha. Biol Conserv 78:23–33
Johnson TB, Allen M, Corkum LD, Lee VA (2005a) Comparison of methods needed to estimate population size of round gobies (Neogobius melanostomus) in western Lake Erie. J Great Lakes Res 31:78–86
Johnson TB, Bunnell DB, Knight CT (2005b) A potential new energy pathway in central Lake Erie: the round goby connection. J Great Lakes Res 31:238–251
Jude DJ (2001) Round and tubenose gobies: 10 years with the latest great lakes phantom menace. Dreissena! 11:1–14
Jude DJ, Reider RH, Smith GR (1992) Establishment of Gobiidae in the Great Lakes basin. Can J Fish Aquat Sci 49:416–421
Knight C (1997) The round goby in the central basin of Lake Erie: range expansion and size-selective predation on zebra mussels. In: Charlebois PM, Marsden JE, Goettel RG, Wolfe RK, Jude DJ, Rudnicka S (ed) The Round goby, Neogobius melanostomus (Pallus): a review of European and North American literature. Urbana, IL, pp 45–46
Kolar CS, Lodge DM (2002) Predictions and risk assessment for alien fishes in North America. Science 298:1233–1236
Krumbein WC, Sloss LL (1951) Stratigraphy and sedimentation. Freeman, San Francisco
Lederer AM, Janssen J, Reed T, Wolf A (2008) Impacts of the introduced round goby (Apollonia melanostoma) on dreissenids (Dreissena polymorpha and Dreissena burgensis) and on macroinvertebrate community between 2003 and 2006 in the littoral zone of Green Bay, Lake Michigan. J Great Lakes Res 34:690–697
Lee VA, Johnson TB (2005) Development of a bioenergetics model for the round goby (Neogobius melanostomus). J Great Lakes Res 31:125–134
Lester NP, Dextrase AJ, Kushneriuk RS, Rawson MR, Ryan PA (2004) Light and temperature: key factors affecting walleye abundance and production. Trans Am Fish Soc 133:588–605
MacInnis AJ, Corkum LD (2000) Fecundity and reproductive season of the round goby Neogobius melanostomus in the Upper Detroit River. Trans Am Fish Soc 129:136–144
Miller PJ (1984) The tokology of gobioid fishes. In: Potts JW, Wootton RJ (eds) Fish reproduction strategies and tactics. Academic Press, London, pp 119–153
Mills EL, Casselman JM, Dermott R, Fitzsimons JD, Gal G, Holeck KT (2003) Lake Ontario: food web dynamics in a changing ecosystem (1970–2000). Can J Fish Aquat Sci 60:471–490
Minns CK, Moore JE, Seifried KE (2004) Nutrient load and budgets in the Bay of Quinte, Lake Ontario, 1972–2001. Canadian Manuscript Report of Fisheries and Aquatic Sciences 2694. Great Lakes Laboratory for Fisheries and Aquatic Sciences, Fisheries and Oceans Canada, Burlington, ON
Mooney HA, Drake JA (1989) Biological invasions: a SCOPE program overview. Biological invasions: a global perspective. Wiley, New York, pp 491–508
Nentwig W (2007) Biological invasions. Springer, Berlin
Nurnberg GK (1999) Determining trophic state in experimental lakes. Limnol Oceonogr 44:1176–1179
Parker IM, Simberloff D, Londsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, Von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19
Pennuto CM, Krakowiak PJ, Janik CE (2010) Seasonal abundance, diet, and energy consumption of round gobies (Neogobius melanostomus) in Lake Erie tributary streams. Ecol Freshw Fish 19:206–215
Phillips EC, Washek ME, Hertel AW, Niebel BM (2003) The round goby (Neogobius melanostomus) in Pennsylvania tributary streams of Lake Erie. J Great Lakes Res 29:34–40
Poos M, Dextrase AJ, Schwalb AN, Ackerman JD (2009) Secondary invasion of the round goby into high diversity Great Lakes tributaries and species at risk hotspots: potential new concerns for endangered freshwater species. Biol Invasions 12:1269–1284
Raby GD, Gutowsky LFG, Fox MG (2010) Diet composition and consumption rate in round goby (Neogobius melanostomus) in its expansion phase in the Trent River, Ontario. Environ Biol Fish 89:143–150
Ray WJ, Corkum LD (2001) Habitat and site affinity of the round goby. J Great Lakes Res 27:329–334
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Shigesada N, Kawasaki K (1997) Biological invasions: theory and practice. Oxford University Press, New York
Skora KE (1996) In Report of the working group on introduction and transfers of marine organisms (WGITMO) ICES annual science conference. Reykjavik, Iceland, pp 96–107
Sokolov LI, Sokolova EL, Pegasov VA (1994) Ichthyofauna of the Moskva River within Moscow City and some data on its state. Voprosy Ikhtiologii 35:364–641
Steingraeber MT, Thiel PA (2000) The round goby (Neogobius melanostomus): another unwelcome invader in the Mississippi River Basin. In: Transactions of the 65th North American wildlife and natural resources conference, pp 328–344
Taraborelli AC, Fox MG, Schaner T, Johnson TB (2009) Density and habitat use by the round goby (apollonia melanostoma) in the Bay of Quinte, Lake Ontario. J Great Lakes Res 35:266–271
Taraborelli AC, Fox MG, Johnson TB, Schaner T (2010) Round goby (Neogobius melanostomus) population structure, biomass, prey consumption and mortality from predation in the Bay of Quinte, Lake Ontario. J Great Lakes Res 36:625–632
Tsepkin EA, Sokolov LI, Rusalimchik AV (1992) Ecology of the round goby Neogobius melanostomus (Pallas), an occasional colonizer of the basin of the Moskva River. Biologicheskie Nauki 1:46–51
Urban NR, Apul DS, Auer MT (2004) Community respiration rates in Lake Superior. J Great Lakes Res 30:230–244
Vélez-Espino LA, Koops MA, Balshine S (2010) Invasion dynamics of round goby (Neogobius melanostomus) in Hamilton Harbour, Lake Ontario. Biol Invasions 12:386–3875
Wickett RG, Corkum LD (1998) Nest defense by the non-indigenous fish, the round goby, Neogobius melanostomus (Gobidae), on a shipwreck in Western Lake Erie. Can Field Nat 112:653–656
Wiesner C (2005) New records of non-indigenous gobies (Neogobius spp.) in the Austrian Danube. J Appl Ichthyol 21:324–327
Wolf RK, Marsden JE (1998) Tagging methods for the round goby (Neogobius melanostomus). J Great Lakes Res 24:731–735
Wootton RJ (1998) Ecology of teleost fishes. Kluwer, Norwell
Young JAM, Marentette JR, Gross C, McDonald JI, Verma A, Marsh-Rollo SE (2010) Demography and substrate affinity of the round goby (Neogobius melanostomus) in Hamilton Harbour. J Great Lakes Res 36:115–122
Acknowledgments
This project was supported by a National Science and Engineering Council Discovery Grant to MGF. We thank O. Puckrin, E. Fobert, A. Rooke, and G. Meisner for assistance with field sampling, L. Gutowsky for helping design the sampling regime, and D. Beresford, D. MacKay and two anonymous reviewers for providing helpful comments on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Thomas Mehner.
Rights and permissions
About this article
Cite this article
Brownscombe, J.W., Fox, M.G. Range expansion dynamics of the invasive round goby (Neogobius melanostomus) in a river system. Aquat Ecol 46, 175–189 (2012). https://doi.org/10.1007/s10452-012-9390-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-012-9390-3