Abstract
The spatial and temporal differences in the structure and composition of benthic invertebrates were studied at three sites of the Samborombón River, which is an important tributary of the Río de la Plata Estuary (Argentina), having a low slope and brackish drainage. Biological samples were taken during each season. Physico-chemical variables were measured to determine their association in the benthic fauna distribution. Site 1, in the headstream, was characterized by freshwater Pampean organisms; site 2 showed the highest density, taxa diversity, and richness; brackish species, e.g., Laeonereis culveri, were found here. Site 3, close to the Samborombón Bay, was characterized by an unstable taxonomic composition that is strongly influenced by the estuary. The lowest density and taxonomic diversity of organisms were registered and distinguished by estuarine species. The multivariate method (redundancy analysis) showed the benthic groups having an important spatial variability, superimposed on the temporal variability, associated with the salinity gradient of the river.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Researches conducted in rivers and streams around the world show complex spatial and temporal patterns in the composition of the aquatic invertebrates. Lotic ecosystems support a longitudinal distribution of benthic community whose attributes (abundance, biomass, dominance, richness, and diversity) are used to analyze the ecological quality. Seasonal factors related to the hydrological cycle and environmental parameters have also been used to determine in the structure and function of benthic fauna (Coimbra et al. 1996; Rundle et al. 1998; Ogbeibu and Oribhabor 2002; Boyle and Fraleigh 2003).
Studies on benthic community ecology in streams and rivers of Buenos Aires province (Argentina) are limited (e.g., Fernández and Schnack 1977; Rodrigues Capítulo 1999; Rodrigues Capítulo et al. 2001; Graça et al. 2002; Ocon and Rodrigues Capítulo 2004). They all describe the aquatic invertebrates as bioindicators of the water quality in the Pampean freshwater bodies.
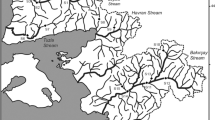
The Samborombón River forms an important drainage in the depressed Pampa (Buenos Aires province) and has a high salinity level. It is a tributary of the Río de la Plata Estuary in its external zone. Based on geological, hydrological, and biological criteria, the Río de la Plata Estuary can be divided into three main zones based on salinity level: internal zone (salinity: 0.2–0.5 PSU), intermediate zone (salinity: 0.5–5 PSU), and external zone (salinity: 5–25 PSU) (Boschi 1988; Wells and Daborn 1997; see Fig. 1). The ecological studies of Samborombón River are limited to the planktonic and phytobenthic communities (Claps 1996; Solari and Claps 1996; Modenutti 1998). Other studies describe physical, chemical, and geomorphologic characteristics (Conzonno and Fernández Cirelli 1991; Bauer et al. 2002). Until now, studies are limited to describing the spatial and temporal variability of the macrobenthos in the southern coastline of the Samborombón Bay (Ieno and Bastida 1998; Martin et al. 2004) and in the open water of Río de la Plata Estuary (external zone) (Giberto et al. 2004). The Samborombón Bay is a mesohaline area (10–15 PSU) characterized by a mixture of freshwater species and marine fauna intrusions (Boschi 1988; Guerrero et al. 1997). In this Bay, several aquatic migratory birds complete their life cycles. It is also a feeding site for different types of fish and brackish invertebrates (Boschi 1988; Ieno and Bastida 1998). The Bay was declared a Ramsar site in 1997 by UNESCO (Ramsar Convention Bureau 2001).
Salinity in rivers, brackish streams, and wetlands is increasing in many regions of the world, which is strongly impacting the biota. There have been several recent field and laboratory studies describing the effect of rising salinity levels on freshwater invertebrates [e.g., Kefford et al. (2003) in Victoria streams (southeast Australia), Horrigan et al. (2005) in Queensland streams (northeast Australia), Goetsch and Palmer (1997) in the Sabie River (South Africa), Piscart et al. (2005) in the Meurthe River (northeastern France), and Blasius and Merritt (2002) in Michigan streams (midwestern USA)]. The objective of this work was to (a) analyze the spatial and temporal changes of the benthic invertebrates in a low-lying salty river (Samborombón River) and (b) describe the structure and composition of its benthic community.
Materials and methods
Study area
The Samborombón River is located in the NE of Buenos Aires province (Argentina) and belongs to the Del Plata basin (Fig. 1). The headwaters of the river lie in the territories of San Vicente and Brandsen. The midstream crosses the territories of Chascomús and Magdalena and ends at the Samborombón Bay (external zone of the Río de la Plata Estuary). The drainage covers an area of 5,090 km2. The river is ca. 140 km long, about 13 m wide, and varies in its depth between 0.5 and 2 m. The water velocity in the river varies from 0.31 to 0.35 m s−1 with very low gradient (0.13 m km−1) (Solari and Claps 1996). The water flow is regulated by both rain water and underground water. The bottom sediments are soft, fine, and highly muddy and contain a high percentage of calcium carbonate “caliche”, mainly in the downstream. The regional biome is the steppe grassland with marginal leguminous forests, such as Gleditsia triacanthos Linnaeus 1753, scattered throughout the landscape. Aquatic vegetation as Potamogeton striatus Ruiz et Pavón (1798), Chara sp., Althernanthera sp., Scirpus americanus Persoon (1805), and Ludwigia peploides (Kunth) Raven 1963, are observed in the headwaters. The midstream contains Juncus sp. in the littoral. The downstream is characterized by Salicornia ambigua Michaux 1803, Spartina densiflora Brongniart (1829), Scirpus californicus (Meyer) Steudel 1841, and Juncus sp. (Cabrera and Zardini 1993; Claps 1996; Modenutti 1998). Small rivers surround the downstream as hydrological nets and are permanently flooded by tidal salt-water from Río de la Plata Estuary. The groundwater rises to the surface due to the low altitude and forms an alluvial, wetland valley in the bay. The estuary has a semi-diurnal tidal regime with tidal amplitude varying from 0.3 to 1 m (Wells and Daborn 1997).
Sampling collections
Due to a rather poor accessibility of the Samborombón River, only three sampling sites were chosen: site 1, in the surroundings of the city of Brandsen (35°07′ S–60°30′ W); site 2, in Chascomús territory (35°34′ S–58°02′ W); and site 3, close to the Samborombón Bay (35°50′ S–57°23′ W) (Fig. 1). Four seasonal samples were taken: summer (December 1997), autumn (April 1998), winter (July 1998), and spring (October 1998). Each sample of benthic fauna on soft substrates from each site on the littoral of the river was taken in replicate (2×) with an Ekman grab (100 cm2). During floods Van Veen grab (470 cm2) was used for sampling from over a bridge in the center of the river. Samples were fixed with 5% formaldehyde in situ and then sorted with a sieve (150 μm) in the laboratory. The individuals were stained with erythrosine-B and observed under microscope and magnifying glass as has been used earlier for the Pampean freshwater bodies (Rodrigues Capítulo et al. 1995, 2001). Benthic fauna were identified to different taxonomic levels, using references books and keys (Lopretto and Tell 1995; Merrit and Cummins 1996; Fernández and Domínguez 2001).
The following parameters of the river were measured in situ: water temperature (T), dissolved oxygen (DO), pH, transparency with Secchi-disc depth (SeD), and conductivity (Cond). The principal ions in total dissolved solids (TDS) were taken from Solari and Claps (1996). TDS was used to express conductivity, being a measure of salinity. Biochemical oxygen demand (BOD5) and chemical oxygen demand (COD) were also measured. Sediment samples (50 g fresh weight) were collected to analyze organic matter in the sediment (OM), which was derived from the weight loss after ignition method (LOI) at 500°C during 4 h by previously drying for 48 h at 60°C (APHA 1998). Depth and width of each site of the stream were also measured.
Statistical analysis
Each sample (ind m−2) was averaged for statistical analysis. The following ecological parameters were analyzed: frequency (Fr) of benthic organisms in seasons of presence and in % of occurrence [constant = 100% (in 4 seasons), very common = 75% (in 3 seasons), common = 50% (in 2 seasons), rare = 25% (1 season), and absent = 0%]; dominance (Do) in % (most abundant taxa numerically); density (N) in ind m−2; taxa richness (S), i.e., total number of taxa per site; taxonomic diversity expressed as Shannon–Wiener Index (H′) with logarithm base e; Equitability Index (J′) and Jaccard Index (I J) at the taxa level (Moreno 2001). We also calculated richness and diversity indices with taxa grouped at a family level to compare the same hierarchical rank. All these parameters further interpret the taxonomic and ecological response to the changes in the physical and chemical variables in the environment (Boyle and Fraleigh 2003).
A biological and environmental matrix was built to apply a multivariate statistical method. The Cochran’s test was used to examine the heterogeneity of variances. As significant heterogeneity was detected, the abundance and physico-chemical variables were transformed (except pH) with log10 (X + 1) (Underwood 1997). An indirect ordination was performed: the detrended correspondence analysis (DCA; with detrending by segments) to obtain the gradient length of taxa in the environmental space (Ter Braak and Prentice 1988). This value did not exceed 2 units of standard deviation (SD). Therefore, a linear direct ordination was applied: the redundancy analysis (RDA) (Ter Braak and Smilauer 2002). Only taxa with >1% of the average abundance in the sample was considered in this analysis. Forward selection option was used to identify and select the best environmental variables. The value of the variance inflation factor (VIF) >10 excluded variables that caused multicolinearity effects. Statistical significance was analyzed with the Monte Carlo test using non-restricted permutations (n = 199 permutations, P < 0.05). The ordination was performed using statistical package of Ter Braak and Smilauer (2002).
Results
Physical–chemical characteristics
Water temperature (T) varied temporally and had a maximum (27.8°C) in summer (December) at site 2 and a minimum (12°C) in winter (July) at site 1. pH was slightly alkaline (8.03) at all sites (Table 1). Dissolved oxygen (DO) showed the highest values at site 2 and in all seasons (8.25 ± 0.83 mg l−1). Transparency (SeD) differed in each site and season: site 3 had the lowest average value and least variations (16.5 ± 4.73 cm). Organic matter in the sediment (OM) was high at all sites, with site 3 containing the highest percentage (10.9 ± 0.78%). The BOD5 decreased from site 1 (8.5 ± 4.12 mg l−1) to site 3 (5.25 ± 2.5 mg l−1); also the COD5 decreased from site 1 (44.5 mg l−1) to site 3 (18.5 mg l−1). Conductivity (Cond) was high and more than doubled from site 1 (2,800 μS cm−1) to site 3 (5,900 ± 5,540 μS cm−1), where seasonal variability was high.
Benthic community patterns
Table 2 shows the frequency and dominance of the faunistic groups. Site 1 had a total taxonomic richness of 33. Constants faunistic groups (Fr = 100%) were the Nematoda, Naididae, Cyprideis sp., Hyalella curvispina Shoemaker, 1942, Berosus sp., and Chironomidae. Numerically dominant taxa belonged to Ostracoda (Do = 26%), Gastropoda (Do = 21.5%), and Chironomidae (Do = 14.7%). Groups such as Caenis sp. (Fr = 25%), Erythrodiplax nigricans (Rambur, 1842) (Fr = 25%), and Hydroptila sp. (Fr = 50%) appeared solely at this site and in a very low density. Total taxonomic richness at site 2 was 38. The most frequent groups (Fr = 100%) found were Nematoda, Naididae, Tubificidae, Harpacticoidea, Cyclpoidea, Cytherideidae (Cyprideis sp.), Lymnocythere sp., and Chironomidae. Ostracoda (Do = 30%) was the most abundant group followed by Chironomidae (Do = 15%). Species of Hirudinea, and Oxyagrion hempeli Calvert, 1909, were only found at this sampling site with low density and low frequency. Site 3 had a total taxonomic richness of 25. Nematoda and Ostracoda appeared in all samples (Fr = 100%), being the most abundant (Do = 24.5% and 34.5%, respectively). Species such as Kalliapseudes schubarti Mañé-Garzon, 1949; Corophium rioplatense Giambiagi, 1926; and Rotalia sp. only appeared in samples from site 3 as rare taxa (Fr = 25%) and were in low abundance (Table 2). The site 2 had highest density of organisms and highest fluctuations in abundance (48,829 ± 22,918 ind m−2), and site 3 had the lowest (12,661 ± 8,256 ind m−2) (Fig. 2). The Shannon–Wiener Index at taxa level increased from site 1 to site 2 and decreased to site 3 (highest value at site 2 in autumn was H′ = 3.43), and the lowest at site 3 in spring was H′ = 1.2) (Fig. 3). Table 3 summarizes richness and diversity indices of family level as homogenous taxonomic group. The highest richness and diversity of families at site 1 and site 3, respectively, in winter were exceptional. The Equitability Index illustrated the site 2 with the highest uniformity (J′ = 0.81) in autumn and site 3 with the lowest value (J′ = 0.43) in spring. The Jaccard Index (I J) indicated low similarities between sampling sites: sites 1 and 2 = 0.37; sites 2 and 3 = 0.24; sites 1 and 3 = 0.23.
The relationships between the environmental and biological variables that were analyzed with the RDA are demonstrated in the triplot (Fig. 4). The first two axes explained 65.5% of the total accumulated variance. The correlation coefficient between the distribution of taxa and the environmental variables was 0.97 for the horizontal Axis I and 0.91 for the vertical Axis II. The sum of all the canonical axes was 0.73. The negative side of Axis I is correlated with the high organic matter in sediment percentages and higher temperature. The opposite side of this axis was correlated with the water transparency and dissolved oxygen. The conductivity and pH were more associated with the negative side of the Axis II. Samples from site 3 from winter through summer were positioned in the lower left quadrant. They were associated with low taxonomic diversity and poor dominance of estuarial taxa, for example, Rotalia sp., C. rioplatense, and Centropagidae. The lower right quadrant demonstrates the majority of sampling of site 1 (summer, autumn, and winter) and the sampling of winter at site 2. They were associated with sensitive species, such as Hydroptila sp, Caenis sp., O. hempeli, E. nigricans, Berosus sp., H. curvispina, and Collembola, and the water transparency. The remaining organisms from site 2 were positioned at the upper left quadrant (highest taxonomic diversity) associated with taxa like Ostracoda, Cyclopoida, Harpacticoida, Nematoda, Tubificidae, Laeonereis culveri (Webster, 1879), Hirudinea, and Cladocera (Daphnia sp., Leydigia sp., and Macrothrix sp.).
Triplot showing the results of the redundancy analysis (RDA). Environmental data selected (arrows): Temperature (T), dissolved oxygen (DO), conductivity (Cond), pH, Secchi disc (SeD), organic matter in sediment (OM). Sampling sites and seasons (circle): 1 (site 1), 2 (site 2), 3 (site 3), Su (summer), Au (autumn), Wi (winter), and Sp (spring). Principal taxa (triangular): Rot (Rotalia sp.), Cr (Corophium rioplatense), Ce (Centropagidae), Coll (Collembola), Hydr (Hydroptila sp.), Ber (Berosus sp.), Hcu (Hyallela curvispina), Ca (Caenis sp.), Od (Odonata), Cd (Cletocamptus deitersi), Gast (Gastropoda), Chir (Chironomidae), Cy (Cytheridae), Cp (Cypretta sp.), Naid (Naididae), Lym (Lymnocythere sp.), Ac (Hidrachnidia), Clad (Cladocera), Hir (Hirudinea), CCyc (Copepoda Cyclopoida), Tub (Tubificidae), Cerat (Ceratopogonidae), Nem (Nematoda), Emp (Empididae), Ksc (Kalliapseudes schubarti), Lc (Laeoneris culveri), Ost und (Ostracoda undetermined), Ha und (Harpacticoida undetermined)
Discussion
Changes of the environmental variables
The physical–chemical characteristics and the biological parameters in the Samborombón River vary between sites and seasons such that the different stream sections are distinguishable. The connection between the Samborombón River and the Río de la Plata Estuary (external zone) facilitates mixing of salt water and freshwater, creating a saline wedge over the sediment and causing a complex salinity gradient (Boschi 1988). The content of major cations (potassium, sodium, magnesium) and the increase of conductivity levels along the river support the concept of salinity gradient (Table 1). According to Solari and Claps (1996) and Modenutti (1998), there is a considerable increase in salinity downstream, i.e., from site 1 to site 3. The measured values of conductivity registered are higher than in other streams located in the NE of Buenos Aires province like Juan Blanco, Buñirigo, or El Pescado, small bodies of water (20–25 km) with low flow, low dissolved oxygen, and high biological oxygen demand (Bauer et al. 2002). The highest levels and fluctuations observed in both BOD5 and COD upstream coincide with the lowest values in DO. The littoral of the midstream had lower OM and higher DO values. In the downstream, the lowest BOD5 and COD corresponded to the highest values of OM in the sediment. The dominant aquatic vegetation of Juncus sp. and S. alterniflora in the littoral may contribute significantly to supply OM, as also observed in other similar estuarine ecosystems in South America, e.g., Solís Grande Stream (south Uruguay), (Muniz and Venturini 2001) and the Paranaguá Bay (Southeastern Brasil) (Netto and Lana 1999). The tidal intrusions together with littoral silts and the flocculation of seston can cause a permanent deposit of fine sand, mire, and slime as reported for the Solís Grande Stream (Muniz and Venturini 2001). A high abundance of calcareous material from the biodeposition of Ostracoda valves, Gastropoda shells, and Foraminifera tests was observed in the sediment of the midstream section. It is well known that the NE of Buenos Aires province and the study area studied were covered by salt water ca. 6,000–3,500 years BP (Mid Holocene marine transgression) (Cavallotto 2002; Fucks et al. 2005).
Changes of the benthic community
The benthic community in the Samborombón River has considerable spatial and temporal differences, a complex structure, and composition; the variations of the invertebrates from upstream to downstream are associated with the fluctuations of the environmental variables. The Jaccard Index showed very few taxa in common between sites. The headstream had similar taxa [e.g., Hydra sp., Bratislavia sp., Heleobia parchappei (d’Orbigny, 1835), H. curvispina, Caenis sp., Berosus sp.] to other rivers studied in the NE of Buenos Aires province, with conductivity recorded between 150 and 3,000 μS cm−1 (Rodrigues Capítulo et al. 2001; Ocon and Rodrigues Capítulo 2004). We found the highest number of organisms in the midstream; a high density of planktonic Rotifera has been reported by Modenutti (1998), and benthic algae are shown to develop (Solari and Claps 1996) at the same site. We found the midstream to show a higher richness and taxonomic diversity and greater homogeneity in the biota (Equitability Index) than the other sites. The high conductivity in the midstream, due to the underground saline intrusion and the brackish water from the estuary, allows the occurrence of some organisms with estuarine characteristics (e.g., L. culveri). Cyprideis sp. are generally abundant with different types of tubers and thorns in their valves as a reaction to the high water salinity (Whatley et al. 1997). Also in Samborombón Bay, Cyprideis sp. has been reported to be abundant in the deposits of Mid Holocene (Fucks et al. 2005). The hydrodynamics of the drainage and the high turbidity in the downstream contribute to the poor development in the temporal patterns of the benthic community. Some brackish species of the Río de la Plata Estuary appear in this unstable section of Samborombón River in low density (e.g., Rotalia sp., K. schubarti, and C. rioplatense). Even L. culveri, a Polychaete tolerant to high salinity and organic matter in sediment, was found in low abundance. This species is sensitive to the fluctuating salinity in the estuary (Ieno and Bastida 1998). The abundance, richness, and taxonomic diversity in the downstream were low and influenced constantly by the estuary. Low values of these indices are frequent in mixohaline ecosystems in the world and often signify an environmental stress (Rundle et al. 1998). The total taxa richness in the downstream site was the lowest (25), compared with the upstream (33) and midstream (38) sites. The taxa richness increases (>37 taxa) in the open water of the Río de la Plata Estuary (external zone) (Giberto et al. 2004) as well as in the south of the Samborombón Bay (>43 taxa) (Vallarino et al. 2002), although the fauna is totally different in the marine coastline.
Salinity tolerance of the benthic fauna: implications
In the present article, the ecological quality of this saline river was evaluated with univariate (e.g., richness, diversity) and multivariate (RDA) methods. Several workers (Goetsch and Palmer 1997; Blasius and Merritt 2002; Horrigan et al. 2005; Piscart et al. 2005; Kefford et al. 2007) who have evaluated the response of some benthic species to the salinization both experimentally and in the field found different results. Piscart et al. (2005) described species of Trichoptera as bioindicators of salinity level in the Meurthe River; factories producing chemical wastes discharge salt water to this stream, causing conductivity values to increase to up 3,422 μS cm−1. In the Meurthe River the highest diversity, equitability, and bio-ecological trait combinations were observed in the intermediate salinity level. According to Piscart et al. (2005), intermediate levels of disturbance maximize species diversity because competitively dominant species exclude subordinate species at lower disturbance levels. In the same way, a high constraint level leads to local extinctions. Hence, there exists a trade-off between a species ability to tolerate disturbance and to compete. The diversity was the highest at an intermediate level of salinity because both halo-tolerant species and freshwater species co-occurred [Intermediate Disturbance Hypothesis (IDH) by Connell 1978]. Blasius and Merritt (2002) examined the effects of road salt on aquatic macroinvertebrates (e.g., Amphipoda, Trichoptera, and Ephemeroptera) in Michigan streams. Their studies focused on the drift, behavior, and survival of benthic community. In Sabie River, a salty river in Kruger National Park (Africa), Goetsch and Palmer (1997) selected macroinvertebrates like Ephemeroptera: Baetidae to perform toxicity tests and assess the salinity tolerance of the taxa. These authors discussed the difficulty of reaching a general conclusion about several ions and dissolved solids (TDS) in water that contribute to mortality of organisms. In some streams of Australia, with very high conductivity (35,000 μS cm−1), Kefford et al. (2003) studied the tolerance of some invertebrates like Hirudinea, Crustacea, or Chironomidae to different salinity concentration. Kefford et al. (2006) also studied the tolerance of salinity by Coenagrionidae (O. hempeli was found in the midstream of the Samborombón River and belongs to this family). Kefford and Nugegoda (2005) applied toxicity test to measure the salinity effect on the growth, reproduction, and development of freshwater Physa acuta (Gastropoda: Physidae). These authors showed that individuals of Physidae thrive best in intermediate salinity and worst in high or low salinity. This is a case of an inverted ‘U’- (or bell-) shaped salinity concentration–response curve (Kefford and Nugegoda 2005). While there are several potential physiological mechanisms that explain this response, the hypothesis of the inverted ‘U’-shaped curve may explain the richness and diversity of taxa found for the Samborombón River (highest values in the midstream). Horrigan et al. (2005) worked in streams of Queensland with benthic macroinvertebrates as bioindicators and applied the salinity index to determine the conductivity impacts (salinity range 6,000–12,000 μS cm−1).
The cited literature on the impact of salinity and our research shows that it is necessary to perform salinity bioassays (e.g., tolerance test) in the field and laboratory, in Samborombón River and other brackish streams of Argentina with suitable native benthic species. This would allow us to describe the ecological water quality. Some of the cited authors (e.g., Goetsch and Palmer 1997; Blasius and Merritt 2002; Piscart et al. 2005) have tested the tolerance to salinity of Trichoptera, Ephemeroptera, and Odonata based on different criteria (e.g., salt-sensitive, abundance, or trophic levels). In our work, the abundance and frequency of occurrence of these taxa were low, and organisms involved may not be the ones that reflect tolerance to the salt in this Pampean river. The use of benthic invertebrates as salinity bioindicators would be very helpful to determine if urban, natural, or industrial waste affect sections of the Samborombón River. Approximately 60,000 people inhabit the Brandsen and San Vicente headstream area, and their number continues to increase due to the urbanization and the industrial activities in the region (INDEC 2001). Consequently, the potential impact of discharge of organic waste and nutrients release will also increase. Cattle activities frequently exist in the midstream along with the use of fertilizers in agriculture. This could reach the river by leaching. The downstream, a Ramsar site, is rich in salty clay sediments with shallow sandy areas where the agriculture and urban activity barely exist.
Conclusion
The spatial scale defines the variability of the community of benthic invertebrates in the Samborombón River over the temporal scale. Thus, some taxa or community assemblage characterizes each section of the river. Redundancy analysis shows that the seasonal samplings from each site tend to group together. The most important taxa are defined mainly by the conductivity and by organic matter in sediment. Ostracoda, Cladocera, and Oligochaeta are the most significant and diversified faunistic groups in the different sections of the river. Both richness and diversity analyses at taxa and family level show the same tendency in the changes between the three sites and seasons. Overall, the abundance, diversity, and richness have increased from upstream to midstream and then decreased at the downstream site (U-shaped curves and ID Hypothesis).
References
APHA (American Public Health Association) (1998) Standard methods for examination of water and wastewater, 20th edn. Washington, DC, 1325 pp
Bauer DE, Donadelli J, Gómez N, Licursi M, Ocon C, Paggi AC et al (2002) Ecological status of the pampean plain streams and rivers (Argentina). Verh Int Verein Limnol 28:259–262
Blasius BJ, Merritt RW (2002) Field and laboratory investigations on the effects of road salt (NaCl) on stream macroinvertebrate communities. Environ Pollut 120:219–231. doi:10.1016/S0269-7491(02)00142-2
Boschi EE (1988) El ecosistema estuarial del Río de la Plata (Argentina y Uruguay). An Inst Cienc del Mar y Limnol, Univ Nac Auton Mex 15:159–182
Boyle TP, Fraleigh HD Jr (2003) Natural and anthropogenic factors affecting the structure of the benthic macroinvertebrate community in an effluent-dominated reach of the Santa Cruz River, AZ. Ecol Indic 3:93–117. doi:10.1016/S1470-160X(03)00014-1
Cabrera AL, Zardini EM (1993) Manual de la flora de los alrededores de Buenos Aires. ACME, Buenos Aires, 755 pp
Cavallotto J (2002) Evolución holocena de la llanura costera del margen sur del Río de la Plata. Rev Asoc Geol Argent 57:376–388
Claps MC (1996) Structure and dynamics of epipelic algae from a plain river (Samborombón River, Buenos Aires, Argentina). Arch Hydrobiol 137:251–263
Coimbra CN, Graça MAS, Cortes RM (1996) The effects of a basic effluent on macroinvertebrate community structure in a temporary Mediterranean River. Environ Pollut 94:301–307. doi:10.1016/S0269-7491(96)00091-7
Connell JH (1978) Diversity in tropical rain forests and coral reefs. Science 199:1302–1310. doi:10.1126/science.199.4335.1302
Conzonno VH, Fernández Cirelli A (1991) Aggregation of soluble humic substance from Río Samborombón (Buenos Aires province). Rev Bras Biol 51:487–493
Fernández HR, Domínguez E (eds) (2001) Guía para la determinación de artrópodos bentónicos sudamericanos. EudeT. Serie: investigaciones de la UNT, 282 pp
Fernández L, Schnack JA (1977) Estudio preliminar de la fauna bentónica de tramos poluídos de los arroyos Rodríguez y Carnaval (provincia de Buenos Aires). Ecosur Argent 4:103–115
Fucks E, Aguirre M, Deschamps CM (2005) Late Quaternary continental and marine sediments of northeastern Buenos Aires province (Argentina): fossil content and paleoenvironmental interpretation. J S Am Earth Sci 20:45–56. doi:10.1016/j.jsames.2005.05.003
Giberto DA, Bremec CS, Acha EM, Mianzan H (2004) Large-scale spatial patterns of benthic assemblages in the SW Atlantic: the Río de la Plata estuary and adjacent shelf waters. Estuar Coast Shelf Sci 61:1–13. doi:10.1016/j.ecss.2004.03.015
Goetsch P-A, Palmer CG (1997) Salinity tolerances of selected macroinvertebrates of the Sabie River, Kruger National Park, South Africa. Arch Environ Contam Toxicol 32:32–41. doi:10.1007/s002449900152
Graça MAS, Rodrigues Capítulo A, Ocon C, Gómez N (2002) In situ tests for water quality assessment: a case study in pampean rivers. Water Res 36:4033–4040. doi:10.1016/S0043-1354(02)00132-X
Guerrero RA, Acha EM, Framiñan MB, Lasta CA (1997) Physical oceanography of the Río de la Plata estuary, Argentina. Cont Shelf Res 17:727–742. doi:10.1016/S0278-4343(96)00061-1
Horrigan N, Choy S, Marshall J, Recknagel F (2005) Response of stream macroinvertebrates to changes in salinity and the development of a salinity index. Mar Freshw Res 56:825–833. doi:10.1071/MF04237
Ieno EN, Bastida RO (1998) Spatial and temporal patterns in coastal macrobenthos of Samborombon Bay, Argentina: a case study of very low diversity. Estuaries 21:690–699. doi:10.2307/1353273
INDEC (2001) http://www.indec.mecon.ar/censo2001s2_2/ampliada_index.asp?mode=06
Kefford BJ, Nugegoda D (2005) No evidence for a critical salinity threshold for growth and reproduction in the freshwater snail Physa acuta. Environ Pollut 134:377–383. doi:10.1016/j.envpol.2004.09.018
Kefford BJ, Papas PJ, Nugegoda D (2003) Relative salinity tolerance of macroinvertebrates from the Barwon River, Victoria, Australia. Mar Freshw Res 54:755–765. doi:10.1071/MF02081
Kefford BJ, Zalizniak L, Nugegoda D (2006) Growth of the damselfly Ischnura heterosticta is better in saline water than freshwater. Environ Pollut 141:409–419. doi:10.1016/j.envpol.2005.08.064
Kefford BJ, Nugegoda D, Zalizniak L, Fields E, Hassell K (2007) The salinity tolerance of freshwater macroinvertebrate eggs and hatchlings in comparison to their older life-stages: a diversity of responses. Aquat Ecol 41:335–348. doi:10.1007/s10452-006-9066-y
Lopretto E, Tell G (eds) (1995) Ecosistemas de aguas continentales. Metodología para su estudio. Ediciones Sur Tomo II, 895 pp & Tomo III, 1401 pp
Martin JP, Bastida R, Trassens M (2004) Polychaete assemblages of intertidal mixohaline flats of Bahía Samborombón (La Plata River estuary-Argentina). Thalassas Int J Mar Sci 20:9–16
Merrit RW, Cummins KW (eds) (1996) An introduction to the aquatic insects of North America. Kendall/Hunt Publishing Company, Iowa, 862 pp
Modenutti BE (1998) Planktonic rotifers of Samborombón River Basin (Argentina). Hydrobiologia 387/388:259–265. doi:10.1023/A:1017045317756
Moreno CE (2001) Métodos para medir la biodiversidad. M&T Manuales y Tesis SEA, vol. 1, Zaragoza, 84 pp
Muniz P, Venturini N (2001) Spatial distribution of macrozoobenthos in the Solís Grande stream estuary (Canelones-Maldonado, Uruguay). Braz J Biol 61:409–420. doi:10.1590/S1519-69842001000300010
Netto SA, Lana PC (1999) The role of above- and below-ground components of Spartina alterniflora (Loisel) and detritus biomass in structuring macrobenthic associations of Paranaguá Bay (SE, Brazil). Hydrobiologia 400:167–177. doi:10.1023/A:1003753001411
Ocon CS, Rodrigues Capítulo A (2004) Presence and abundance of Ephemeroptera and other sensitive macroinvertebrates in relation with habitat conditions in pampean stream (Buenos Aires, Argentina). Arch Hydrobiol 159:473–487. doi:10.1127/0003-9136/2004/0159-0473
Ogbeibu AE, Oribhabor BJ (2002) Ecological impact of river impoundment using benthic macro-invertebrates as indicators. Water Res 36:2427–2436. doi:10.1016/S0043-1354(01)00489-4
Piscart C, Lecerf A, Usseglio-Polatera P, Moreteau JC, Beisel JN (2005) Biodiversity patterns along a salinity gradient: the case of net-spinning caddisflies. Biodivers Conserv 14:2235–2249. doi:10.1007/s10531-004-4783-9
Ramsar Convention Bureau (2001) The annotated Ramsar list. The list of wetlands of international importance designated by the contracting parties to the convention on wetlands (Ramsar, Irán, 1971). Annotated version, update-August 6, 2001. Convention on wetlands, 300 pp
Rodrigues Capítulo A (1999) Los macroinvertebrados como indicadores de la calidad de ambientes lóticos en el área Pampeana. Rev Soc Entomol Argent 58:208–217
Rodrigues Capítulo A, Paggi AC, César I (1995) Composición del zoobentos de la laguna de Lobos, provincial de Buenos Aires, Argentina. Limnetica 11:29–37
Rodrigues Capítulo A, Tangorra M, Ocon C (2001) Use of benthic macroinvertebrates to assess the biological status of Pampean streams in Argentina. Aquat Ecol 35:109–119. doi:10.1023/A:1011456916792
Rundle SD, Attrill MJ, Arshad A (1998) Seasonality in macroinvertebrate community composition across a neglected ecological boundary, the freshwater-estuarine transition zone. Aquat Ecol 32:211–216. doi:10.1023/A:1009934828611
Solari LC, Claps MC (1996) Planktonic and benthic algae of a pampean river (Argentina): comparative analysis. Ann Limnol 32:89–95
Ter Braak CJF, Prentice IC (1988) A theory of gradient analysis. Adv Ecol Res 18:271–313. doi:10.1016/S0065-2504(08)60183-X
Ter Braak CJF, Smilauer P (2002) CANOCO reference manual and CanoDraw for Windows user’s guide: software for canonical community ordination (version 4.5). Microcomputer Power, Ithaca, 500 pp
Underwood AJ (1997) Experiments in ecology. Their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge, 504 pp
Vallarino EA, Rivero MS, Gravina MC, Elías R (2002) The community-level response to sewage impact in intertidal mytilid beds of the Southwestern Atlantic, and the use of the Shannon index to assess pollution. Rev Biol Mar Oceanogr 37:25–33
Wells PG, Daborn GR (eds) (1997) The Río de la Plata. An environmental overview. An EcoPlata project background report. Dalhousie University, Halifax, 256 pp
Whatley R, Moguilevsky A, Toy N, Chadwick J, Feijo Ramos M (1997) Ostracoda from the South West Atlantic. Part II. The littoral fauna from between Tierra del Fuego and the Río de la Plata. Rev Esp Micropaleontol 29(2):5–83
Acknowledgments
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) PIP 5305 and La Plata University (FCNyM-UNLP) no. 469. This article is a Scientific Contribution (no. 808) to the Instituto de Limnología “Dr. Raúl A. Ringuelet”. We are very grateful to Joanne Marciano for help with the English version of this text. Special thanks to the anonymous reviewers for their suggestions and critical comments that improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spaccesi, F., Rodrigues Capítulo, A. Benthic invertebrate assemblage in Samborombón River (Argentina, S. America), a brackish plain river. Aquat Ecol 43, 1011–1022 (2009). https://doi.org/10.1007/s10452-008-9212-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-008-9212-9