Abstract
Using efficient and stable solid amine adsorbents to capture CO2 has been considered as an effective technology to reduce the CO2 concentration in the atmosphere. In this paper, a series of solid amine-containing fibrous adsorbent for CO2 capture were prepared by direct modification of polyacrylonitrile (PAN) fibers with amination reagents, including diethylenetriamine (DETA), triethylenetetramine (TETA), tetraethylenepentamine (TEPA) and polyethyleneimine (PEI). Abundant amine groups can be introduced on the PAN surface owing to the reactivity between cyano group and amino group. The effects of the type and structure of amination reagents on the swelling properties and CO2 adsorption capacities of the as-prepared adsorbents were investigated. The results indicated that chemical modification of PAN fibers with amine compounds could greatly increase the CO2 adsorption capacity of the aminated adsorbents. The adsorption capacities of the adsorbents were correlated well with the content of amino groups. PAN–TETA and PAN–TEPA showed higher CO2 adsorption capacities and better stability than PAN–DETA and PAN–PEI. Besides, as water molecules could take part in and facilitate the CO2 adsorption, the CO2 adsorption capacity of amine-modified fibers would be strongly dependent on the swelling property.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Atmospheric CO2 concentration has increased rapidly over the past century owing to the increase of consumption of fossil fuels, and as one of the primary greenhouse gases (GHGs), CO2 with overhigh concentration could lead to serious environmental issues such as global warming (Li et al. 2011) and natural disasters (i.e. widespread melting of ice and snow, floods, droughts and increasingly average sea levels) (Parry 2007; Kenarsari et al. 2013). Therefore the development of efficient and selective CO2 capture techniques to reduce atmospheric CO2 concentration has attracted great research interest. Quantities of processes have been developed for the removal of carbon dioxide from flue gases and other gas streams, including physical/chemical absorption (Rubin et al. 2012; Padurean et al. 2012), adsorption (Gargiulo et al. 2014; He et al. 2016), and membrane separation technology (Geyer et al. 2017). Among them, the most widely applied technology is to use liquid amine sorbents with high CO2 absorption capacity to absorb CO2. However, there are some obvious inherent drawbacks, such as high absorbent consumption caused by its high volatility, serious reactor equipment corrosion caused by its high alkalinity and high energy consumption to regenerate the absorbents. (Chaffee et al. 2007; Kang et al. 2016). In order to avoid these disadvantages, many researchers have tried to develop some promising adsorbents to adsorb CO2. The solids containing amines that prepared through bonding active amine materials to high surface area supports like carbon based materials (Chowdhury et al. 2015; Creamer and Gao 2016), zeolite (Song et al. 2017; Daeho et al. 2017), metal organic frameworks (MOF) (Masala et al. 2017) and polymers resin (Chen et al. 2013; Liu et al. 2017) can be the competitive adsorbents. Compared with liquid amine absorbents, these amine-modified materials could exhibit many merits, including high adsorption capacity, low cost, low pressure, well regeneration performance and low regeneration energy consumption (Samanta et al. 2012).
There are two methods to introduce amine groups to the substrates to prepare amine-modified solid adsorbents. One method is to physically impregnate amine materials into suitable support materials. But generally the recycle performance of the adsorbents prepared with this method are not good enough, because the physically impregnated amine could leak easily in the adsorption and regeneration processes. Another method is to chemically graft amine groups to the substrates. Due to their wide range of sources, light weight, plentiful functional groups, short transfer distance and highly modifiable surface, the polymeric fibers have been considered as good substrates for preparation of amine-modified solid adsorbents for CO2 capture. In comparison with impregnated-amine adsorbents, the most important advantages of amine-modified fibrous adsorbents are their high stability during adsorption-regeneration process owing to covalent bonding of organoamine through polymerization method, as well as their high CO2 adsorption capacity for the high density of grafted amino groups on the surface of the fibrous adsorbent.
Our group has systematically studied the preparation of fibrous adsorbents and their CO2 adsorption performance, proving that fibers have great potential as a matrix for CO2 adsorption (Luo et al. 2016; Xu et al. 2015; Zhuang et al. 2013; Yang et al. 2010). Luo et al. 2016 chose natural sisal fiber (SF) as matrix to prepared amine modified adsorbent through grafting acrylamide and then aminating. The adsorption capacity of SF-AM-TETA could reach 4.20 mmol/g. Xu et al. 2015 has used polypropylene (PP) fibers as substrate to prepare hyperbranched solid amine fibers through co-irradiation grafting copolymerization with glycidyl methacrylate and then amination with ethylenediamine. The hyperbranched solid amine PP fibers adsorbent showed a CO2 adsorption capacity of 5.35 mmol/g. Yang et al. 2010 have grafted allylamine onto polyacrylonitrile (PAN) fibers through preirradiation grafting copolymerization. The CO2 adsorption capacity of this amine-modified PAN adsorbent reached up to 6.22 mmol/g. Although all of them showed a good CO2 adsorption performance, we noticed that they require introducing intermediate active groups or pretreatment in order to graft amine functional groups, which have the relatively complicated synthesis steps and restrict their application in the CO2 adsorption field.
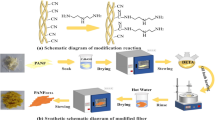
Zhao and Liu 2014 have prepared an amino-modified PAN (AMPAN) fiber through the reaction of PAN fiber and triethylenetetramine (TETA) in the presence of water. Although the highest adsorption capacity of the AMPAN fibers was only 0.09 g/g even at 15 bar and 50 °C, it showed that the polymeric PAN fiber contains a large number of cyano groups which could be aminated by amination reagent under appropriate reaction conditions. Moreover, in addition to inherent merits of fibers, PAN fibers even show excellent characteristics such as commercial availability, desirable chemical resistance, high thermal stability, good mechanical properties and non-toxicity in nature (Nirmala et al. 2013; Patel and Hota 2016). Therefore, in this paper, we consider that cyano groups on PAN fibers could directly react with amino groups of amination reagents at an appropriate temperature in order to reduce energy consumption and lower processing cost. Different amination reagents, including diethylenetriamine (DETA), triethylenetetramine (TETA), tetraethylenepentamine (TEPA) and polyethyleneimine (PEI), were applied to react with PAN fibers. The factors that would determine the adsorption property of PAN fibers modified with amine have been investigated.
2 Experimental section
2.1 Materials and reagents
Polyacrylonitrile (PAN) fiber was provided by Lanzhou Refinery, China. CO2 and N2 were purchased from Guangzhou Zhuorui Gas Company (Guangdong, China). Diethylenetriamine (DETA), triethylenetetramine (TETA), tetraethylenepentamine (TEPA) and acetophenone were purchased from Guangzhou Reagent Company (Guangzhou, Guangdong, China). Polyethyleneimine (PEI) was purchased from Aladdin Industrial Corporation (Shanghai, China). All chemicals were purchased as analytical grade and used without further purification. Distilled water was used to prepare all solution in the study.
2.2 Synthesis of amine-modified PAN fibrous adsorbents
In a general preparation procedure, 3.0 g PAN fibers were immersed with 120 mL 70 vol% TETA aqueous solution in a round-bottom flask, and heated at 120 °C for 6 h to complete the amination reaction. After that, the amine-modified fibers were rinsed with cold water and hot water (treating them into boiling water about 30 min) repeatedly until the filtrate was neutral. The washed samples were then dried under vacuum at 90 °C.The obtained amine-modified fiber was named as PAN–TETA. DETA, TEPA and PEI were also used to prepare amine-modified fibers, and the resulting fibers were labeled as PAN–DETA, PAN–TEPA and PAN–PEI, respectively. All these amine-modified fibers are generally called as solid amine fibers, or solid amine adsorbents. In order to study the effect of concentration and co-solvent on the amination degree and adsorption performance of PAN–TETA, three reaction systems including pure TETA, TETA aqueous solution and TETA mixed with acetophenone were used in the preparation of PAN–TETA. The reaction scheme is shown in Fig. 1 (taken TETA as an example).
2.3 Determination of aminating degree of fiber
The aminating degree of fiber was characterized by the weight gain rate (G, %) of the fiber after amine modification, which was calculated with the following Eq. (1) :
where W1 (g) and W2 (g) are weights of PAN and PAN-X (X = DETA, TETA, TEPA or PEI), respectively.
2.4 Determination of the content of amino groups
0.50 g Solid amine fibers were put into 75.0 mL 0.050 mmol/L hydrochloric acid standard solution in a conical flask and oscillated at room temperature for 24 h. After that, 10 mL of solution was taken into the conical triangular bottle. The unreacted hydrochloric acid was titrated with 0.050 mmol/L sodium hydroxide standard solution using phenolphthalein as indicator. The content of amino groups of the solid amine fiber was calculated by by subtracting unreacted hydrochloric acid amount from total hydrochloric acid amount.
2.5 Physical and chemical characterization
The elemental analysis were obtained using an Analysen systeme GmbH Elementar Vario EL (Germany) to analyze the elemental composition of the fibers. The Fourier transform Infrared (FTIR) spectra of fibers were detected by using FTIR spectrometer (Tensor-27, Germany) equipped with an attenuated total reflection (ATR) objective and FTIR-Raman Spectrometer (Nicolet NXR 9650, America). X-ray powder diffraction (Empyrean, Netherlands) were conducted to determine the crystal structure of fibers. The 13C nuclear magnetic resonance (NMR) spectra of fibers were recorded using a solid NMR (Bruker, Germany) at 400 MHz. The Ultra-depth three-dimensional microscope (VHX-1000C) were employed to observe the morphology and measure the diameter of fibers.
2.6 CO2 adsorption experiment
The adsorption experiment was conducted in a fixed bed packed with adsorbents in the presence of water. The CO2 adsorption performances of all samples were characterized by using breakthrough curves. A desired amount of adsorbent was packed into a glass column (1.3 cm inner diameter), from which the air and excess water in the column was removed by introducing dry N2 with a flow rate of 30 mL/min for 0.5 h before starting detection. Then, the CO2/N2 mixture gas (10% CO2/90% N2) with a flow rate of 30 mL/min was introduced into the adsorption column. The influent/effluent concentration of CO2 were tested every 2 min, using a gas chromatograph (GC, D7900, Techcomp, China) with a thermal-conductivity detector (TCD). After adsorption, pure N2 gas at a flow rate of 30 mL/min was introduced through the column at 90 °C to regenerate the spent adsorbent sample.
The adsorption capacity of CO2 was calculated as follows:
where Q represents the adsorption capacity of the adsorbent (mmol CO2/g), t is the adsorption time (min), and Cin and Ceff were the influent and effluent concentrations of CO2 (mL/min), respectively. V is the total flow rate, 30 ml/min; W and 22.4 are the weight of the fiber sample (g) and molar volume of gas (mL/mmol), respectively.
3 Results and discussion
3.1 Structure and morphology of PAN and amine-modified PAN
The ATR-FTIR spectra of PAN, PAN–DETA, PAN–TETA, PAN–TEPA and PAN–PEI and the FTIR-Raman of PAN and PAN–TETA are shown in Fig. 2. For the pristine PAN fibers, the prominent peaks in Fig. 2a at 2243, 2925 and 1452 cm−1 can be ascribed to the stretching vibrations of cyano groups (–C≡N), stretching and bending vibrations of methylene groups (–CH2–), respectively. Besides, the absorption peak at 1738 cm−1 is the stretching vibration of bonding carbonyl groups (C=O), which is the second monomer ester in PAN fibers. After reaction with different amine agents, it can be found that the –C≡N absorption peak of PAN-X (X = DETA, TETA, TEPA or PEI) at 2243 cm−1 was weaken in varying degrees for the different chemical reactivity of their cyano groups with amino groups. It is noteworthy that the peaks of –C≡N and –C=O at 2243 cm−1 and 1738 cm−1 almost completely disappeared in the IR spectra of PAN–TETA and PAN–TEPA, which indicated that almost all cyano groups on PAN fibers have been converted into imino or amino groups by TETA and TEPA. The absorption peaks at 1555 and 1263 cm−1, corresponding to amide –N–H bending vibration and –C–N stretching vibration, were strengthened. A strong peak at 1647 cm−1, corresponding to –C=O or –C=N adsorption, appeared in the IR spectra, which indicated that cyano groups in PAN fibers have been converted into –CONH– through amidation reaction and/or into –C=N through addition reaction between –CN group and –NH group. In order to confirm the reactions, FTIR-Raman spectra of PAN–TETA and PAN were conducted, results in Fig. 2b showed that there were two new absorption peaks appearing at 3288 cm−1 and 1646 cm−1 in the FTIR-Raman spectrum of PAN–TETA, corresponding to the N–H and C=N, respectively. The result confirmed the addition reaction between –CN group and –NH group. On the other hand, elemental analysis results (Table 1) showed that percentage of oxygen atoms in PAN–TETA fibers obviously increased after amination with TETA (5.326 wt% for PAN, 17.452 wt% for PAN–TETA), indication –CN groups in PAN fiber were hydrolyzed into carboxyl groups, which then reacted with amino groups of TETA to form –CONH.
For DETA and PEI, they can hardly react with the cyano groups on PAN fibers, so that absorption peaks of cyano groups can still be detected evidently by ATR-FTIR. This is because that for DETA, its strong alkalinity could cause fiber degradation during the reaction process. Besides, owing to the limited chain flexibility and tendency to tangling of its macromolecules, PEI can hardly react with PAN fiber even under the same reaction conditions as small molecule amines like TETA and TEPA. Given the above, the desirable solid amine fibers will be prepared using TETA and TEPA for their suitable chain length and basicity.
The ultra-depth 3D microscope images (Fig. 3) showed that the diameter of PAN raw fiber was approximately 13 µm, while diameters of PAN–TETA and PAN–TEPA fibers both increased to nearly 30 µm, confirming the occurrence of a chemical reaction between polyacrylonitrile fibers and amine reagents. The weight gain degree of PAN–PEI was really low, thus the diameter of PAN–PEI was similar to raw fibers. Obviously, amine compounds in amination process had a significant effect on fiber diameter.
From Fig. 4 it could be found that untreated-PAN fibers showed two strong diffraction peaks at 18.6° and 29.8°, indicating the high crystallinity of raw fibers. But in amine-modified fibers, the above two peaks disappeared, instead the wide and weak peaks showed up at 10.0° or 23.0°, suggesting that the modification of TEPA and TETA could significantly damage crystalline structure of fibers. Under the reaction condition, PAN fibers began to relieve at order area segment, and the movement of their medial and branched macromolecule chains could facilitate the diffusion of amine solution into the inner part of the fibers, so as to enhance the interaction between amino groups and cyano groups.
3.2 Adsorption behavior of amino-modified adsorbent for CO2
The chemical compositions of the solid amine fibers were analyzed by elemental analysis, and the results are shown in Table 1. It could be seen that the percentage of oxygen atoms on the solid amine fibers obviously increased due to the hydrolyzation of cyano groups under alkaline conditions.
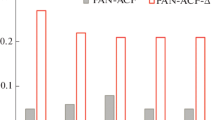
The actual content of amino groups in amine-modified PAN fibers were determined by acid-base titration, and the results are shown in Fig. 5. Meanwhile, their corresponding adsorption amount and amino efficiency are compared in Fig. 5. It can be clearly seen that PAN–DETA and PAN–PEI contain much lower alkyl amino groups than PAN–TETA and PAN–TEPA. As discussed in the above section, the low alkyl amino group contents of PAN–DETA and PAN–PEI can be attributed to the strong alkalinity of DETA and the low reactivity of PEI with PAN. Due to low amino contents, PAN-DETA (1.95 mmol amino/g) and PAN–PEI (0.28 mmol amino/g) showed very low CO2 adsorption capacities. In contrast, the CO2 adsorption amount of PAN–TETA and PAN–TEPA could reach 5.44 mmol/g and 4.35 mmol/g as their amino content could be as high as 7.20 mmol amino/g and 5.81 mmol amino/g, respectively. Their utilization efficiencies of amino, which is defined as the percentage ratio of CO2 adsorption amount to amino content of the adsorbent, were all approximately 75%.
We focused on the study of the CO2 adsorption behavior of PAN–TETA and PAN–TEPA. A series of PAN–TETA and PAN–TEPA samples with different weight gain rates (from 10 to 140 wt%) were prepared and then applied to CO2 adsorption. Their breakthrough curves of CO2 adsorption are shown in Fig. 6. It can be seen that PAN–TETA and PAN–TEPA samples showed similar adsorption behavior. All of them were able to thoroughly adsorb CO2 at the initial phase to keep their effluent CO2 concentration at zero, and then a breakthrough occurred. It was found that the higher weight gain rate could lead to a longer time to breakthrough, and thus increase the CO2 adsorption capacities of the amine-modified PAN fibers. The increased content of amino group would be beneficial to the diffusion of CO2 gas and interaction between CO2 and amino groups. For example, the PAN–TETA with the highest amino content (124 wt%) showed the highest adsorption capacity among these different PAN–TETA samples, reaching up to 5.44 mmol/g.
3.3 Effect of swelling property of PAN–TETA on its adsorption performance
It is found that the swelling property of amine-modified PAN fibers has been remarkably improved after amination, and the swelling capacity of amine-modified fibers could significantly affect their CO2 adsorption property. In order to further study the influence of preparation process of fibers on its swelling and adsorption performance, PAN fibers have been aminated in three reaction media, including pure TETA, TETA aqueous solution, and the mixture of TETA and acetophenone. The swelling and adsorption properties of the resulting samples of PAN–TETA were compared.
The spectra of TETA-modified PAN fibers prepared in different media are shown in Fig. 7. It should be noted that the deformation and stretching vibrations of amino groups (~ 3379 cm−1) were identical, indicating that the reaction medium had little effect on the species of amino groups of PAN–TETA fibers. However, it could determine the species and contents of other groups. As shown in Fig. 7, the positions and relative absorption intensities of –C=N and –C=O groups of the samples have changed markedly. In TETA aqueous solution medium, the PAN could be hydrolyzed in the presence of water and the cyano groups could be converted into carbonyl groups. Therefore, the intensity of absorption peak at 1653 cm−1 that corresponding to the –C=N and –C=O groups significantly increased, compared with that of PAN–TETA prepared in pure TETA medium. For TETA-acetophenone medium, acetophenone with high enough boiling point can decrease the viscosity of TETA, and efficiently promote the amination reaction, so that a fair high amination degree can be obtained.
The element analysis of the three PAN–TETA samples prepared in different media were listed in Table 2. It is found that the three samples had different nitrogen contents and amino group densities. PAN–TETA (H2O) showed the highest amino group content among the three sample, reaching up to 7.20 mmol/g. The hydroscopicity of the origin PAN fibers and the three amine-modified PAN–TETA fibers prepared in different reaction media are compared in Table 2. A comparison of the CO2 adsorption capacity on these samples are also illustrated in Fig. 8.
Although the reaction medium had almost no effect on functional species as demonstrated above, the swelling property of these amine modified PAN fibers had changed (Table 2). Owing to the hydrogen bonding between amino groups and water, the hydroscopicity of all amine-modified PAN fibers were obviously increased compared with origin PAN fibers. The water absorption capacity of origin PAN fiber was just 37.62%, but that of PAN–TETA (H2O) fibers prepared in TETA aqueous system significantly increased to 97.90%, which was also higher than that of PAN–TETA (acetophenone) (84.74%) and PAN–TETA (pure) (56.50%). Based on the essence of the amino-CO2 interactions, the CO2 adsorption capacity is actually enhanced by the presence of moisture. Under humidity conditions, one mole of amino groups could theoretically react with one mole of CO2 to form ammonium bicarbonate. On the other hand, the swollen fiber made CO2 molecules easy to diffuse into the inner part of the fiber to react with amino groups, which would also increase the utilization efficiency of amino groups in CO2 adsorption, and further increase the CO2 adsorption amount.
4 Conclusions
A series of fibrous amine adsorbents were prepared by amination of PAN fiber with different amine agents, including DETA, TETA, TEPA and PEI. The alkalinity of amine reagents would significantly determine the aminating degree of PAN fiber. Amine reagents could react with cyano groups to introduce amino groups onto the fiber, but overhigh alkalinity would break PAN chains and result in the degradation of PAN fibers. TETA and TEPA were appropriate reagents for the amination of PAN fiber because of their moderate alkalinity and high reactivity, and the as-prepared PAN–TETA and PAN–TEPA fibers showed remarkable CO2 adsorption capacity (5.44 mmol/g, 4.35 mmol/g, respectively). It is also found that high swelling capacity of fibers could significantly improve the CO2 adsorption capacity as it can promote the CO2 diffusion and mass transfer during the CO2 adsorption process. These results demonstrated that the solid amine-modified PAN fibers would have great potential in the practical application of CO2 adsorption.
References
Chaffee, A.L., Knowles, G.P., Liang, Z.: CO2 capture by adsorption: materials and process development. Int. J. Greenh. Gas Control 1, 11–18 (2007)
Chen, Z., Deng, S., Wei, H., et al.: Polyethylenimine-impregnated resin for high CO2 adsorption: an efficient adsorbent for CO2 capture from simulated flue gas and ambient air. ACS Appl. Mater. Interfaces 5, 6937–6945 (2013)
Chowdhury, S., Parshetti, G.K., Balasubramanian, R.: Post-combustion CO2 capture using mesoporous TiO2/graphene oxide nanocomposites. Chem. Eng. J. 263, 374–384 (2015)
Creamer, A.E., Gao, B.: Carbon-based adsorbents for postcombustion CO2 capture: a critical review. Environ. Sci. Technol. 50, 7276–7289 (2016)
Gargiulo, N., Pepe, F., Caputo, D.: CO2 adsorption by functionalized nanoporous materials: a review. J. Nanosci. Nanotechnol. 14, 1811–1822 (2014)
Geyer, F., Schönecker, C., Butt, H., et al.: Enhancing CO2 capture using robust superomniphobic membranes. J. Adv. Mater. 29, 1–6 (2017)
He, H., Zhuang, L., Chen, S., et al.: A solid amine adsorbent prepared by molecularly imprinting and its CO2 adsorption properties. Chem. Asian J. 11, 3055–3061 (2016)
Kang, J.L., Wong, S.H., Jang, S.S., et al.: A comparison between packed beds and rotating packed beds for CO2 capture using monoethanolamine and dilute aqueous ammonia solutions. Int. J. Greenh. Gas Control 46, 228–239 (2016)
Kenarsari, S.D., Yang, D., Jiang, G., Zhang, S., Wang, J., Russell, A.J., Wei, Q., Fan, M.: Review of recent advances in carbon dioxide separation and capture. RSC Adv. 3, 22739–22773 (2013)
Ko, D., Siriwardane, R., Biegler, L.T.: Optimization of a pressure-swing adsorption process using zeolite 13X for CO2 sequestration. Ind. Eng.Chem. Res. 42, 339–348 (2017)
Li, J.R., Ma, Y., Mccarthy, M.C., Sculley, J., Yu, J., Jeong, H.K., Balbuena, P.B., Zhou, H.C.: Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 255, 1791–1823 (2011)
Liu, F., Chen, S., Gao, Y., Xie, Y.: Synthesis and CO2 adsorption behavior of amine-functionalized porous polystyrene adsorbent. J. Appl. Polym. Sci. 134, 1–7 (2017)
Luo, S., Chen, S., Chen, Y., et al.: Sisal fiber-based solid amine adsorbent and its kinetic adsorption behaviors for CO2. RSC Adv. 6, 72022–72029 (2016)
Masala, A., Vitillo, J.G., Mondino, G., Grande, C.A., Blom, R., Manzoli, M., Marshall, M., Bordiga, S.: CO2 capture in dry and wet conditions in UTSA-16 metal organic framework. Appl. Mater. Interfaces 9, 455–463 (2017)
Nirmala, R., Jeon, K., Navamathavan, R., Kim, B.S., Khil, M.S., Kim, H.Y.: Fabrication and characterization of II–VI semiconductor nanoparticles decorated electrospun polyacrylonitrile nanofibers. J. Colloid Interface Sci. 397, 65–72 (2013)
Padurean, A., Cormos, C.C., Agachi, P.S.: Pre-combustion carbon dioxide capture by gas–liquid absorption for integrated gasification combined cycle power plants. Int. J. Greenh. Gas Control 7, 1–11 (2012)
Parry, M.L., Climate change 2007: impacts, adaptation and vulnerability: working group II contribution to the fourth assessment report of the IPCC, Cambridge University Press, 2007
Patel, S., Hota, G.: Iron oxide nanoparticle-immobilized PAN nanofibers: synthesis and adsorption studies. RSC Adv. 6, 15402–15414 (2016)
Rubin, E.S., Mantripragada, H., Marks, A., Versteeg, P., Kitchin, J.: The outlook for improved carbon capture technology. Prog. Energy Combust. Sci. 38, 630–671 (2012)
Samanta, A., Zhao, A., Shimizu, G.K.H., et al.: Post-combustion CO2 capture using solid sorbents: a review. Ind. Eng. Chem. Res. 51, 1438–1463 (2012)
Song, Z., Dong, Q., Xu, W.L., et al.: Molecular layer deposition-modified 5A zeolite for highly efficient CO2 capture. ACS Appl. Mater. Interfaces 10, 769–775 (2017)
Xu, T., Wu, Q., Chen, S., Deng, M.: Preparation of polypropylene based hyperbranched absorbent fibers and the study of their adsorption of CO2. RSC Adv. 5, 32902–32908 (2015)
Yang, Y., Li, H., Chen, S., Zhao, Y., Li, Q.: Preparation and characterization of a solid amine adsorbent for capturing CO2 by grafting allylamine onto PAN fiber. Langmuir 26, 13897–13902 (2010)
Zhao, W.B., Liu, B.: Preparation of amino-modified PAN fibers with triethylenetetramine as aminating reagents and their application in adsorption. J. Nanomater. 13, 24–33 (2014)
Zhuang, L., Chen, S., Lin, R.: Preparation of a solid amine adsorbent based on polypropylene fiber and its performance for CO2 capture. J. Mater. Res. 28, 2881–2889 (2013)
Acknowledgements
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (Grant No. 51873238), Science and Technology Project of Guangdong Province (Grant Nos. 2016A010103013, 2016B090930007, 2016A040403094).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuang, Y., He, H., Chen, S. et al. Adsorption behavior of CO2 on amine-functionalized polyacrylonitrile fiber. Adsorption 25, 693–701 (2019). https://doi.org/10.1007/s10450-019-00070-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-019-00070-0