Abstract
We examined the effect of high pressure on the sol–gel phase transition of a resorcinol–formaldehyde (RF) aqueous solution to a RF hydrogel, which was hold under a certain pressure varied in the range from the ambient pressure to 400 MPa. By employing the dynamic light scattering method, we analyzed the scattered intensity of a He–Ne laser which was irradiated to the RF solution to determine the hydrodynamic diameter of the structure formed during the phase transition. Before the completion of the phase transition, we measured the transient change in the size of colloidal particles, i.e. the unit of the porous structure formed in the RF hydrogel. It was revealed that the size of the colloidal particles increased with the increase in the pressure added to the RF solution. After the completion of the phase transition, we prepared a RF cryogel by removing solvent from a RF hydrogel by freeze-drying. The specific surface area of the RF cryogel was found to decrease with the increase in the added pressure, as a result of the increase in the size of the unit of the porous structure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The sol–gel method has extensively been applied to prepare porous materials from a homogeneous solution (Sakka 1985). A resorcinol–formaldehyde (RF) hydrogel, which possesses three-dimensional network structure composed of interconnected nanometer-sized particles, can be synthesized by the sol–gel reaction of resorcinol with formaldehyde in a slightly basic aqueous solution (Pekala 1989). If such a RF hydrogel is dried under ambient pressure and temperature, capillary force acts on a liquid–vapor interface and the network structure of the hydrogel is largely deformed. To prepare porous materials from such RF hydrogel, supercritical drying or freeze-drying is effective to maintain the network structure during drying. Freeze-drying is more useful than supercritical drying from the viewpoint of the cost of the drying apparatuses. A highly porous organic polymer gel can be obtained by removing solvent from a frozen RF hydrogel under reduced pressure. The porous structure of thus obtained RF cryogel, i.e. freeze-dried RF hydrogel, highly depends on the network structure of the parent RF hydrogel, because of the small shrinkage of the porous structure during freeze-drying (Tamon and Ishizaka 1999).
A carbon cryogel with numerous mesopores can be obtained by carbonization of a RF cryogel in an inert atmosphere (Tamon and Ishizaka 1999). So far, a number of attempts have been made for the application to adsorbents, supporting materials for catalysts, electrode materials of electric double-layer capacitors, etc. It is possible to obtain a carbon cryogel with desired mesoporosity, by adjusting the preparation condition of the starting RF solution (Yamamoto et al. 2001). The preparation method as well as the raw materials also influences on the porous structure of the resulting carbon gels (Maldonado-Hódar et al. 1999; Mukai et al. 2005; Elsayed et al. 2007; Yoshida et al. 2016). However, as far as the authors have investigated, the effect of high pressure on the structure formation during the sol–gel phase transition in a RF solution has not yet been reported. Addition of high pressure to a solution significantly affects the physical properties, such as the density of a solvent or solubility of a solute. In several industries, high pressure processes have been applied to drying of foods (Haas and Prescott 1972) or crystallization of chemicals (Moritoki 1979).
In this study, we examine the structure formation during the sol–gel phase transition of a RF solution under high pressure. By employing the dynamic light scattering (DLS) method, we measure the transient change in the size of the colloidal particles which are formed in the RF solution. After the completion of the sol–gel phase transition, we evaluate the porous structure of a RF cryogel by the nitrogen gas adsorption method. The porous structure of a carbon cryogel, which we prepare by carbonization of the RF cryogel in an inert atmosphere, is also observed by a scanning electron microscope (SEM). Based on the obtained results, we consider and discuss the relation between the size of the colloidal particles with the porous structure of the resulting RF cryogel or carbon cryogel.
2 Experimental
We purchased resorcinol, formaldehyde, and sodium carbonate in the research grade from the Sigma-Aldrich Co. Resorcinol and formaldehyde were the raw materials, while sodium carbonate was the catalyst to initiate the sol–gel phase transition, respectively. Distilled and deionized water was also used as the solvent. We summarized the conditions to prepare a RF solution in Table 1, where R/F is the molar ratio between resorcinol with formaldehyde, R/C is the molar ratio between resorcinol with catalyst, and R/W is the ratio between resorcinol with water, respectively. The mixture containing the above chemicals was agitated using a magnetic stirrer to obtain a homogeneous RF solution.
Figure 1 shows the schematic image of the high pressure apparatus employed in this study. The solution was put in the glass cell which was set in the high pressure vessel and was then kept at 298 K to proceed the sol–gel phase transition. Then, the pressure inside the vessel was elevated to the desired pressure in the range from 100 to 400 MPa and was kept constant during the DLS measurement. The nucleation and growth of colloidal particles in the RF solution was detected employing a DLS apparatus equipped with He–Ne laser (λ = 632 nm). The intensity of the scattered laser was transiently measured using a CCD detector to obtain the correlation function of the scattered intensity, G2(τ). Then, the auto-correlation function, G1(τ), can be calculated by analyzing G2(τ) based on the following Eq. (1):
where β is a constant. For the system in which uniform particles are dispersed, G1(τ) can be expressed by an exponential function as follows:
where Γ is the decay rate. Γ can be related to the diffusion coefficient, D, of the particles dispersed in a liquid phase and scattered wave vector, q, as follows:
here q can be calculated from the following equation:
where n and θ are the refractive index (= 1.386) of the RF solution and scattered angle (= 90°), respectively. Under the assumption that the translational diffusion of the particles is essentially governed by the viscosity of the solvent, the hydrodynamic diameter, dh, of the particles can be determined by the Stokes–Einstein equation as follows:
here kB, T and η indicate the Boltzmann constant, absolute temperature and viscosity of the solvent, respectively. By employing a RF solution just after the preparation, we determined the value of η as 2.03 using a viscometer (Model BL, Tokimec Inc.)
With the progress of the phase transition as depicted in Fig. 2, the viscosity of the solution gradually increased, followed by the transition from a solution to a transparent RF hydrogel. To stiffen the network structure, the prepared RF hydrogel was kept at 323 K for 1 day, followed by 363 K for 3 days in a sealed vial. The RF hydrogel was then immersed in t-butanol for 1 day to exchange the pore-filling solvent. The volume of t-butanol is more than 20 times of that of the RF hydrogel. t-butanol is a useful solvent for freeze-drying because the change in the density by solidification is much smaller than that of water, and the vapor pressure is much larger than that of water. We repeated the rinse at least 3 times. The rinsed RF hydrogel was slowly frozen at 243 K and was then placed in a glass chamber under vacuum. After outgassing the chamber for 1 day to sufficiently sublimate t-butanol from the hydrogel, we could obtaine the RF cryogel. A carbon cryogel was also prepared by carbonizing thus obtained RF cryogel at 1273 K in an inert atmosphere.
The porous structure of the freeze-dried RF hydrogel, i.e. RF cryogel, was evaluated by the nitrogen gas adsorption method by employing an automatic gas adsorption and desorption apparatus (Belsorp-mini, Bel Japan, Inc.). The adsorption and desorption isotherms on the RF cryogel were measured at 77 K after outgassing the cryogel under vacuum. Then, the BET specific surface area, SBET, of the RF cryogel was determined by applying the BET equation to the measured isotherms. The cross section of a carbon cryogel was observed using a SEM (JEOL Ltd.).
3 Results and discussion
With the progress of the sol–gel reaction, the color of the RF solution transiently changed from light yellow to dark red. Just after the preparation of the RF solution, the scattered intensity from the solution was too weak to collect the data. However, we observed the enough scattered intensity to calculate G1(τ) after the reaction time of 60 min when the homogeneous nucleation of the colloidal particles in the RF solution was assumed to occur. As depicted in Fig. 1, the time of the nucleation indicates the time when the colloidal particles were generated from the condensation products of substituted resorcinol. With regard to the mechanism of the nucleation, we assume that generation of substituted resorcinol is the rate-determining step. The nucleation rate may be related to the added pressure, because we consider that the diffusion of resorcinol molecules to react with formaldehyde molecules in the RF solution depends on the viscosity of water (The viscosity of water gradually increases with the increase in the added pressure). However, under the examined conditions, it is difficult to observe the significant effect of pressure on the time of the nucleation.
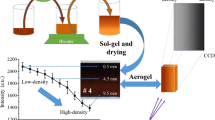
Figure 3 shows a typical example of the transient change of lnG1(τ) with the progress of the sol–gel reaction of the RF solution. Since the colloidal particles formed in the RF solution is assumed to be poly-dispersed because of the difference in the time of nucleation between the particles, it is difficult to apply the Eq. (2) to analyze G1(τ) in the entire range of τ. Therefore, as shown in Fig. 3, we estimated the slope, Γ, of the lnG1(τ) against τ at the decay time of zero, i.e. τ = 0. We determined dh, as the representative size of the colloidal particles formed in the RF solution, from thus estimated Γ based on the Eqs. (3)–(5). Figure 4 shows the transient change in the size of the colloidal particles with the progress of the sol–gel reaction under the different pressure. It should be noted that the growth of the particles is found to be accelerated by adding high pressure. This is probably because the aggregation of the particles is enhanced under high pressure. We determined the final size, dh,final, of the colloidal particles as the size when the RF solution lost the fluidity.
Since the shrinkage of the porous structure of the RF hydrogel can be restrained during freeze-drying, we assume that the specific surface area of the RF cryogel almost equals to that of the parent RF hydrogel. Figure 5 shows the adsorption and desorption isotherms of nitrogen on the RF cryogels, which we have prepared by adding the different pressure during the sol–gel phase transition of the RF solution. According to the IUPAC classification, the isotherms can be classified into the type-IV, indicating the presence of mesopores. Figure 6 shows the relation between SBET of the RF cryogel and dh,final. As depicted in Fig. 1, dh,final can be interpreted as the unit size of the particles forming the network structure of the resulting RF hydrogel. The specific surface area of the RF cryogel is almost unchanged when the pressure added to the RF solution is lower than 200 MPa. On the other hand, the specific surface area significantly decreases when the added pressure is greater than 300 MPa. It can be roughly seen that SBET decreases with the increase in dh,final. As discussed in the previous section, we consider that aggregation of the colloidal particles in the RF solution is enhanced by adding high pressure, which results in the increase of the size of the unit structure of the RF hydrogel. As a result, the surface area of the RF cryogel where nitrogen molecules are accessible is assumed to decrease. However, the highest pressure (400 MPa) does not correspond to the smallest specific surface area of the RF cryogel. This implies that the effect of high pressure on the specific surface area of the RF cryogel cannot be simply related to the hydrodynamic diameter of the colloidal particles. The most probable reason for this is the difference in the degree of the coordination of the colloidal particles forming the network structure. However, at this moment, we cannot provide any experimental data to support the assumption.
Carbon cryogel also possesses the porous structure which is composed of the semi-spherical unit structure as shown in Fig. 7. It can be found that the size of the unit structure of the carbon cryogel prepared by the sol–gel polycondensation under 400 MPa is greater than that prepared under ambient pressure. Thus, we have confirmed that the addition of high pressure during the sol–gel phase transition of a RF solution affects the porous structure of a resulting RF cryogel and a carbon cryogel.
4 Conclusion
In this study, we employed the DLS method to study the effect of high pressure on the growth of the colloidal particles, which were formed during the sol–gel phase transition from a RF solution to a RF hydrogel. As a result of the DLS measurements, it was revealed that the addition of high pressure to the RF solution enhanced the growth of the colloidal particles during the phase transition. It was also confirmed that the specific surface area of a RF cryogel, which could be prepared from a RF hydrogel by freeze-drying, decreased with the increase in the pressure added to the solution. In the subsequent study, we will examine a kinetic model to clarify the mechanism of the sol–gel phase transition forming a network structure.
Abbreviations
- D :
-
diffusion coefficient (m2 s−1)
- d h :
-
hydrodynamic diameter (nm)
- d h,final :
-
final hydrodynamic diameter (nm)
- k B :
-
Boltzmann constant (J K−1)
- n :
-
refractive index (-)
- p/p 0 :
-
relative pressure of nitrogen gas (-)
- q :
-
scattered wave vector (m−1)
- q N2 :
-
adsorbed amount of nitrogen gas (cm3(stp) g−1-adsorbent)
- S BET :
-
BET specific surface area (m2 s−1)
- T :
-
absolute temperature (K)
- β :
-
constant (-)
- Г :
-
decay rate (s−1)
- η :
-
viscosity (Pa s)
- λ :
-
wavelength (m)
- θ :
-
scattered angle (°)
- τ :
-
decay time (s)
References
Elsayed, M.A., Hall, P.J., Heslop, M.J.: Preparation and structure characterization of carbons prepared from resorcinol-formaldehyde resin by CO2 activation. Adsorption 13, 299–306 (2007)
Haas, G.J., Prescott, H.E. Jr.: Freezing under gaseous pressure: effect on cells and proposed mechanism. Cryobiology 9, 101–106 (1972)
Maldonado-Hódar, F.J., Ferro-García, M.A., Rivera-Utrilla, J., Moreno-Castilla, C.: Synthesis and textural characteristics of organic aerogels, transition-metal-containing organic aerogels and their carbonized derivatives. Carbon 37, 1199–1205 (1999)
Moritoki, M.: Fractional crystallization process applying high pressure. Kagaku Kogaku Ronbunshu (Jpn.) 5, 79–84 (1979)
Mukai, S.R., Tamitsuji, C., Nishihara, H., Tamon, H.: Preparation of mesoporous carbon gels from an inexpensive combination of phenol and formaldehyde. Carbon 43, 2628–2630 (2005)
Pekala, R.W.: Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mater. Sci. 24, 3221–3227 (1989)
Sakka, S.: Sol-gel synthesis of glasses. Present and future. Am. Ceram. Soc. Bull. 64, 1463–1466 (1985)
Tamon, H., Ishizaka, H.: Preparation of organic mesoporous gel by supercritical/freeze drying. Dry. Technol. 17, 1653–1665 (1999)
Yamamoto, T., Nishimura, T., Suzuki, T., Tamon, H.: Control of mesoporosity of carbon gels prepared by sol–gel polycondensatino and freeze-drying. J. Non-Cryst. Solids 288, 46–55 (2001)
Yoshida, S., Iwamura, S., Ogino, I., Mukai, S.R.: Adsorption of phenol in flow systems by a monolithic carbon cryogel with a microhoneycomb structure. Adsorption 22, 1051–1058 (2016)
Acknowledgements
The supports of the travel expenses from the Université Claude-Bernard Lyon 1 and Hyogo Overseas Research Network (HORN) program are gratefully acknowledged. The authors thank for the JSPS Grants-in-Aid for Scientific Research (Grant No. 16K06832).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamamoto, T., Tayakout-Fayolle, M., Iimura, K. et al. Effect of high pressure on growth of colloidal particles during sol–gel phase transition of resorcinol–formaldehyde solution. Adsorption 25, 1115–1120 (2019). https://doi.org/10.1007/s10450-019-00042-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-019-00042-4