Abstract
Two series of zeolite X/activated carbon composites with different ratios of zeolite and activated carbon were prepared through a combination process of CO2 activation of the mixtures of elutrilithe and pitch and subsequent hydrothermal crystallization in alkaline solution. An additional surface modification was achieved in diluted NH4Cl solution. CO2 and N2 uptakes on the composites before and after modification were determined for pressures up to 101 kPa at 273 and 298 K, respectively. Langmuir-Freundlich and Toth adsorption models were used to describe the adsorption isotherms of CO2 and the corresponding heats of adsorption were estimated with the Clausius-Clapeyron equation. Both before and after modification, all composites exhibited a remarkable preferential adsorption of CO2 compared to N2, with the modified composites showing a higher adsorption selectivity to CO2 over N2 than the unmodified composites. With an increasing ratio of zeolite in the composites, adsorption capacity and adsorption heat of CO2 on the composites increased simultaneously. Lower adsorption heat was observed both before and after modification especially at the low-loading region and when there was less energetic heterogeneity on the surface of the modified composites. The results may be attributed to the elimination of strong basic sites on the modified composites, which is favorable for desorption of CO2 on the adsorbents and application in pressure swing adsorption processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Since fossil fuel is still the world’s primary energy source, its combustion is one of the major sources of the green house gas carbon dioxide (CO2). CO2 has been known as one of the causes of global warming, but the purified and concentrated CO2 stream can be used as an important carbon source to synthesize clean fuels and fine chemicals. Due to the easy applicability of CO2 over a relatively wide range of temperatures and pressures and its high-energy efficiency, the pressure-swing adsorption (PSA) process has become a preferred technology in the recovery of CO2 from gas mixture streams. Using this technology, gas species are separated from a mixture of gases under pressure according to their molecular characteristics and affinity for an adsorbent material. A key aspect in separating CO2 of PSA is to identify a suitable adsorbent. Although several types of adsorbents, such as zeolites, activated carbon, and silica, etc. may be employed for the adsorption of CO2, the development of regenerable adsorbents with high selectivity and high adsorption capacity for CO2 is a critical point for an efficient PSA process.
Zeolite has shown promising results for separating CO2 from gas mixtures and can potentially be used in the PSA processes (Gomes and Yee 2002; Siriwardane et al. 2005). On the basis of the advanced analysis of several important studies concerning CO2 adsorption using zeolites (Bonenfant et al. 2008; Walton et al. 2006), it appears that the surface basicity and pore size of zeolites, as well as the strength of the electric fields caused by the presence of exchangeable cations in the frameworks and cavities of zeolites are the essential factors for the CO2 adsorption on zeolites. Synthetic consideration of all of these factors seems to be necessary to optimize an appropriate zeolite for CO2 adsorption. It has been found that zeolite 13X (common name of zeolite NaX) with large pore diameter of 0.74 nm and Na/Si ratio of close to 1.5 is one of the most suitable zeolite adsorbents for the recovery and capture of CO2 due to its high adsorption capacity, high selectivity and rapid mass transfer (Harlick and Handan-Tezel 2004). Alternatively, activated carbon is also proposed to be a suitable candidate for CO2 capture because of its high adsorption capacity at ambient pressures, easy regeneration and low-cost (Schell et al. 2012). Activated carbon materials can be obtained from almost any carbonaceous product through carbonization followed by an activation step. The adsorption capacity of activated carbon is mainly governed by its textural property, but also strongly influenced by its surface chemistry (Shafeeyan et al. 2010). In general, zeolites have higher adsorption capacities for CO2 as well as a higher equilibrium selectivity for CO2 over N2 than activated carbons, but the heat of adsorption of CO2 on activated carbons is lower than on zeolites, so that the use of activated carbon in the PSA process may result in less severe heat effects on the PSA performance (Chue et al. 1995; Siriwardane et al. 2001). Desorption by pressure swing is often economical if the heat of adsorption is less than about 30 kJ per mole of adsorptive (Bart and von Gemmingen 2005). The temperature excursion due to heats of adsorption and desorption is detrimental to the separation performance of PSA process (Yang and Cen 1986).
In recent years, zeolite/activated carbon composites have been prepared and their environmental application for gas separation and wastewater treatment have been explored to overcome inherent drawbacks of the sole zeolite and activated carbon in adsorptive properties (Foo and Hameed 2011). Elutrilithe is a kaolinite-rich gangue containing mainly aluminosilicate and organic carbons. It is found that such novel porous composite of zeolite A/activated carbon extrudates can be prepared from elutrilithe based on its mineralogical and chemical composition. Moreover, the ratio of zeolite to activated carbon in the composites can be adjusted by addition of pitch powder into elutrilithe as a raw material (Ma et al. 2010).
The objective of this work is to study the adsorption of CO2 on a series of zeolite X/activated carbon composites and to examine the effects of the relative proportion of zeolite X and activated carbon in the composites and their surface modification upon CO2 adsorption in order to develop a novel CO2 adsorbent with the advantages of both zeolite and activated carbon. The composites of zeolite X/activated carbon are prepared from elutrilithe by adding solid silicon dioxide and pitch powder as an additional silica and carbonaceous source, respectively. Through a two-step process of CO2 activation followed by hydrothermal transformation in an alkali-solution, the composites are surface modified by ammonium chloride solution. After obtaining unmodified and modified composites, CO2 and N2 adsorption isotherms on two series of the composites before and after modification at 273 and 298 K up to 101.3 kPa are measured, and fitted with the Langmuir-Freundlich and Toth models, respectively, so that corresponding heats of adsorption can be calculated.
2 Experimental
2.1 Preparation of zeolite X/activated carbon composites
The composition of Elutrilithe employed was 41.0 wt% SiO2, 35.5 wt% Al2O3 and 7.0 wt% carbon. It was ground to below 200 meshes and mixed with SiO2 and pitch powder to make up a final composition of 25, 35, or 50 wt% carbon with a SiO2/Al2O3 molar ratio of 4.5. The resulting mixture was kneaded with water and extruded to a cylinder shape with dimensions of Ø2.0 mm×6.0 mm. The extrudates were dried overnight and thereafter placed inside a tubular furnace for calcinations and activation. The samples were heated to 450 °C for 2 h and then to 850 °C for an additional 2 h under N2 flow to carry out the carbonization; after which, the nitrogen gas was shut off and the activating agent CO2 was introduced into the furnace. The total duration of activation was 24 h. The activated samples were treated in a NaOH hydrothermal system (Na2O/SiO2=1.5, H2O/Na2O=3). When crystallization was finished, the samples were removed from the reactor, filtered and washed with hot water until the pH of the filtrate reached 6–7. The composite materials obtained were denoted as ZCC-n (n=1,2,3), in which the mass percentage of activated carbon in each composite is 12.5 %, 18.4 % and 29.4 %, respectively.
The surface modification of the composites was carried out by using 0.3 mol/L ammonium chloride solution. Approximately 5 g of powder was treated with 125 ml of the solution for 0.5 h under constant agitation at 340 K. The treated samples were filtered and washed with distilled water and activated in a tube furnace at 623 K under vacuum. The corresponding samples after modification were denoted as MZCC-n (n=1,2,3).
2.2 Characterization of composites
The phase of the samples was checked by X-ray powder diffraction (XRD-6000, Shimadzu) using Cu Kα radiation operated at 40 kV and 30 mA. The BET specific surface area (S BET) was calculated by N2 adsorption isotherms measured on an automatic adsorption instrument (Nova1200e, Quantachrome) at 77 K. The total pore volume (V total) was calculated from the amount of N2 adsorbed at P/P 0=0.98. Micropore volume (V mic), and surface area (S mic) were determined by using the t-plot method. The external surface area (S ext), which includes the contribution of mesopores, macropores and the external surface of the particles, was obtained from the difference between BET and microporous surface area. The mass percent of carbon in the sample was analyzed on a TG209/3/F Thermogravimetric Analyzer (Netzsch). The analysis was carried out with a ramp of 10 °C/min in air atmosphere.
2.3 Measurement of adsorption isotherms
The adsorption isothermal data of carbon dioxide on the composites were measured using a NOVA 1200e instrument that is a static volumetric type of apparatus. Prior to the adsorption measurement, the samples were activated under vacuum at 623 K for at least 4 h. Isotherms were obtained at two temperatures (273 K and 298 K) to determine the isosteric heat of adsorption.
3 Results and discussion
3.1 Characterization of composites
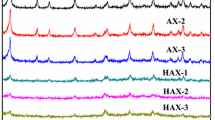
Figure 1 shows the representative XRD patterns and N2 adsorption/adsorption isotherms of the zeolite X/activated carbon composites before and after modification, respectively. From the XRD patterns, it can be seen that all samples exhibit characteristic peaks of zeolite X without other impurity phases. Upon surface modification by the diluted ammonium chloride solution, there is a relative decrease in the intensity of the peaks, indicating a slight loss of crystallinity during modification. The decrease in intensity is almost 18 %. In addition, the N2 adsorption/desorption isotherms of the composites before and after modification at 77 K display a combination of type I and IV isotherms (Yoo et al. 2012), reflecting the coexistence of micropores and mesopores in the composites. The characterization from XRD and N2 adsorption shows the formation of zeolite X/activated carbon composites with developed microporous and mesoporous porosity from elutrilithe by adding pitch powder and solid SiO2. Table 1 lists the pore structure parameters of the zeolite X/activated carbon composites before and after modification with different ratios of zeolite X and activated carbon. Because zeolite X is the sole microporous and activated carbon and its hierarchical porosity ranges from micropore to mesopore, the microporous surface area of the composites decreases with the increase of the proportion of activated carbon in the composites, whereas the external surface area and S ext/S BET ratio increases. Additionally, modification by NH4Cl results in some changes in pore distribution between the micropores and mesopores of composites, i.e., the microporous surface area decreases while the external surface area increases thereby resulting in an S ext/S BET ratio increase.
3.2 CO2 adsorption on composites
3.2.1 CO2 adsorption on synthetic composites
Families of isotherms for CO2 and N2 adsorption on zeolite X/activated carbon composites before modification with different ratios of zeolite X and activated carbon at temperatures of 273 K and 298 K are presented in Fig. 2(a, b) and (c). The isotherm for CO2 is much higher than that for N2 indicating that there is a preferential adsorption of CO2 on the composites. The CO2 experimental isotherms obtained for the three samples are the combination of type I and IV according to the IUPAC classification. With the increase of CO2 pressure, the adsorbed CO2 volume increases sharply at low pressures before a slower increase occurs in the medium- and high-pressure ranges, especially for ZCC-1 with its higher proportion of zeolite. This result suggests that a CO2 molecule preferably adsorbs at the high binding energy sites in micropores of the composite. Moreover, adsorbed CO2 volume decreases when the adsorption temperature is increased. However, it is remarkable that the difference in adsorbed CO2 volume at different temperatures is small under the low-pressure region yet it becomes obvious under the high-pressure region, and the temperature affects the volume adsorbed at a higher surface coverage where CO2 may adsorb on the external surface of the composites with multilayer coverage. On the other hand, it is also shown that the adsorbed CO2 volume decreases with an increasing of the relative proportion of activated carbon in the composites at 273 K and 298 K over the entire range of pressure studied. The corresponding CO2/N2 selectivity is summarized in Table 2. The highest adsorption capacity and CO2/N2 selectivity are obtained for ZCC-1, which is in agreement with it having the highest proportion of zeolite in the composites.
It is well known that an accurate correlation of experimental data for the equilibrium adsorption isotherms of carbon dioxide in adsorbents is required for the reliable modeling and simulation of the different PSA separation processes of CO2. The collected experimental adsorption isotherm data need to be fitted to an isotherm model to extend its utility. In this work, conventional Langmuir-Freundlich (Eq. (1)) and Toth (Eq. (2)) adsorption models were used to fit experimental data of CO2 adsorption onto various composites at different temperatures.


where q is the amount adsorbed and p the pressure; q m the maximum adsorption capacity, and b,n and t are model parameters to be determined by a data fitting method, b is the adsorption affinity, and n is a parameter that qualitatively characterizes the heterogeneity of the adsorbate-adsorbent system, and the deviation of n from unity may be taken as a measure of the deviation from the Langmuir isotherm.
It is found that both equations provide a very good fit to the data over the entire pressure range studied. Table 2 lists fitted parameters for all composites before modification. By inspecting the table, it is apparent that b and n values decrease with an increasing proportion of activated carbon in the composites, indicating lower affinity and less heterogeneity of surface on the activated carbon than zeolite towards CO2. In addition, Henry’s law constants K H (=q m b) derived from the Toth isotherm (Harlick and Handan-Tezel 2005) also decrease in the order of ZCC-1<ZCC-2<ZCC-3, implying their decreasing affinity of adsorption at low surface coverage.
The adsorption isotherms of CO2 on the composites studied at 273 K and 298 K are employed for evaluating the adsorption enthalpy. For this purpose, the Clausius-Clapeyron equation is applied to the isotherm data for the purpose of calculating the isosteric heats of adsorption. The isosteric heat of CO2 adsorption at different loadings on the three samples is shown in Fig. 2(d). In general, it is observed that the heat of adsorption maintains a considerable dependence on surface coverage in the three composites due to surface energetic heterogeneity, and the heat of adsorption on the composites decrease with an increasing proportion of activated carbon in the composites over the entire range studied. The heat of adsorption is quite high and decreases rapidly at low surface coverage and then decrease gradually at high surface coverage, implying that the number of basic adsorption sites that can strongly interact with CO2 is limited with the strong adsorption sites being saturated first. With an increase in loading, some weaker adsorption sites will be in operation resulting in a decrease in adsorption heat.
The comparison between the adsorption of CO2 on molecular sieve 13X and activated carbon has shown preferential adsorption of CO2 on the adsorption materials, but the amount of CO2 adsorbed on the 13X zeolite is more than that of the activated carbon at pressures less than 50 psi indicating that the surface of 13X zeolite has a better affinity for CO2 than that of activated carbon. Moreover, isosteric heat of adsorption of 13X zeolite decreases from 50 to 10 kJ/mol with increasing coverage from 1 to 5 mol/kg, and for the activated carbon, the isosteric heat of adsorption decreases from 28 to 11 kJ/mol with increasing coverage, indicating that the 13X zeolite has a strong and wide distribution of active sites for CO2 adsorption (Siriwardane et al. 2001). As the above results show, it is reasonable for the amount of CO2 adsorbed and the heat of adsorption on the zeolite X/activated carbon composites to vary with the proportion of activated carbon in the composites.
3.2.2 CO2 adsorption on modified composites
The adsorption isotherms of CO2 and N2 on the modified composites at 273 K and 298 K are shown in Fig. 3(a, b) and (c). Preferential adsorption of CO2 on the modified composites is also observed, and CO2/N2 selectivity of the samples is higher than that of the corresponding unmodified samples. However, the shapes of the isotherms CO2 on the composites before and after modification appear to be very similar, i.e. CO2 adsorption increases rapidly when the pressure is increased in the lower pressure regions while CO2 adsorption at higher pressure regions increases gradually. However, the slopes of the isotherms in the low pressure range as well as the amounts of CO2 adsorption on the modified composites are lower than that of the corresponding unmodified composites.
The results of an experimental adsorbent screening study for CO2 removal from N2 (Harlick and Handan-Tezel 2004) have shown that the most promising adsorbent characteristics are a near linear CO2 isotherm. This is because a simple criterion for adsorbent selection in a PSA cycle is proposed based on the effective working capacities, i.e., the difference in the adsorption amounts between the high pressure adsorption step and the low pressure desorption step. A rectangular isotherm requires low pressure regeneration to realize a high effective working capacity, whereas a linear CO2 isotherm may obtain a high effective working capacity at higher regeneration (desorption) pressure in the PSA cycle, hence, the reducing CO2 isotherm curvature, that is, a lower slope of the isotherm is more favorable to its use in a PSA process due to the greater regenerability of the adsorption materials.
Similarly, as shown in Fig. 3(a, b), both the Langmuir-Freundlich and Toth models can also satisfactorily describe the experimental isotherm data of CO2 adsorption on the modified composites. The fitted parameters are represented in Table 3. Comparing the values of parameter b and n from the unmodified and modified composites, the latter composites have lower b values and deviation of n from unity than the corresponding former composites, implying a weaker interaction between adsorbate and adsorbent, at least at low coverage, and a lower degree of surface heterogeneity of the adsorption centers on the modified composites. In addition, a lower value of Henry’s law slope, K H, for the modified composite also indicates a weaker interaction because at low coverage there is very little interaction between adsorbate molecules and adsorbents.
Moreover, isosteric heats of adsorption of CO2 as a function of surface coverage on the modified composites are also calculated by adsorption isotherms at 273 K and 298 K using the Clausius-Clapeyron equation. As shown in Fig. 3(d), all modified composites show fundamentally the same trend in variation of adsorption heat with coverage. By comparing the curves of adsorption heats with the surface loading on the composites before and after modification, obvious differences are observed. First, the heat of adsorption presents a slower decrease in the low-loading region that remains essentially constant becoming nearly independent of loading, in the high-loading region. Second, the average heat of adsorption decreases after modification. These results indicate less surface heterogeneity and weaker adsorbate-adsorbent interactions on the modified composites than on unmodified ones. As a result of the above discussion, it can be noted that the modified composites are more promising judging from the shape of isotherms, equilibrium selectivity and heat of adsorption, though it is inferior with respect to adsorption capacity.
The amount of adsorption on an adsorbent depends on the available surface area and the affinity for an adsorption gas. Adsorbents used for gas separation should have the affinity based on physical interaction between the surface of the adsorbents and the adsorbate molecules since the adsorption-desorption processes should be reversible. Increasing physical affinity leads to the increase of adsorption capacity. Bonenfant et al. (2008) has summarized the influence of the structural characteristics of zeolites on the CO2 adsorption, and it is considered that the basic properties of zeolites generated by the different electron densities of the framework oxygen allow strong adsorption of CO2 acidic molecules (Bonenfant et al. 2008). This kind of adsorption usually involves a high heat of adsorption that is not desirable for CO2 capture due to a high-energy demand for adsorbent regeneration in desorption. For synthetic zeolite X/activated carbon composites studied in the present work, which have been prepared through hydrothermal treatment in alkali-solution, they have stronger basic surfaces including either zeolite X and activated carbon in the composites, while modification by the diluted NH4Cl solution has resulted in the decrease of basicity on the composite surfaces.
4 Conclusions
Zeolite X/activated carbon composites with different ratios of zeolite and activated carbon were prepared and then modified by diluted NH4Cl solution. The adsorption characteristics of CO2 on the composites before and after surface modification have been investigated. Adsorption equilibrium data at 273 K and 298 K up to 101 kPa were measured and modeled successfully using Langmuir-Freundlich and Toth isotherms. As shown there is a higher capacity and steeper nature of the CO2 isotherm on the unmodified composites and a lower capacity and lower slope of the isotherm on the modified composites. In general, the CO2 adsorption capacity and corresponding adsorption heat on both unmodified and modified composites decreased with increasing activated carbon in composites, indicating a weaker interaction between CO2 and activated carbon than with zeolite. After modification, higher adsorption selectivity of CO2/N2 and lower adsorption heat, especially in the low-loading regions, were obtained compared to unmodified composites which results in their favorable utilization in a PSA process.
References
Bart, H.-J., von Gemmingen, U.: Adsorption in Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim (2005). Published Online: 15 Jan. 2005. doi:10.1002/14356007.b03_09.pub2
Bonenfant, D., Kharoune, M., Niquette, P., Mimeault, M., Hausler, R.: Advances in principal factors influencing carbon dioxide adsorption on zeolites. Sci. Technol. Adv. Mater. 9, 013007 (2008)
Chue, K.T., Kim, J.N., Yoo, Y.J., Cho, S.H.: Comparison of activated carbon and zeolite 13X for CO2 recovery from flue gas by pressure swing adsorption. Ind. Eng. Chem. Res. 34, 591–598 (1995)
Foo, K.Y., Hameed, B.H.: The environmental applications of activated carbon/zeolite composite materials. Adv. Colloid Interface Sci. 162, 22–28 (2011)
Gomes, V.G., Yee, K.W.K.: Pressure swing adsorption for carbon dioxide sequestration from exhaust gases. Sep. Purif. Technol. 28, 161–171 (2002)
Harlick, P.J.E., Handan-Tezel, F.: An experimental adsorbent screening study for CO2 removal from N2. Microporous Mesoporous Mater. 76, 71–79 (2004)
Harlick, P.J.E., Handan-Tezel, F.: Equilibrium analysis of cyclic adsorption processes: CO2 working capacities with NaY. Sep. Sci. Technol. 40, 2569–2591 (2005)
Ma, J., Tan, J., Du, X., Li, R.: Effects of preparation parameters on the textural features of a granular zeolite/activated carbon composite material synthesized from elutrilithe and pitch. Microporous Mesoporous Mater. 132, 458–463 (2010)
Schell, J., Casas, N., Ronny, P.: Pure and binary adsorption of CO2, H2, and N2 on activated carbon. Adsorption 18, 49–65 (2012)
Shafeeyan, M.S., Daud, W.M.A.W., Houshmand, A., Shamiri, A.: A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 89, 143–151 (2010)
Siriwardane, R.V., Shen, M.S., Fisher, E.P., Poston, J.A.: Adsorption of CO2 on molecular sieves and activated carbon. Energy Fuels 15, 279–284 (2001)
Siriwardane, R.V., Shen, M.S., Fisher, E.P.: Adsorption of CO2 on zeolites at moderate temperatures. Energy Fuels 19, 1153–1159 (2005)
Walton, K.S., Abney, M.B., Douglas LeVan, M.: CO2 adsorption in Y and X zeolites modified by alkali metal cation exchange. Microporous Mesoporous Mater. 91, 78–84 (2006)
Yang, R.T., Cen, P.L.: Improved pressure swing adsorption processes for gas separation: by heat exchange between adsorbers and by high-heat-capacity inert additives. Ind. Eng. Chem. Process Des. Dev. 25, 54–59 (1986)
Yoo, W.C., Zhang, X., Tsapatsis, M., Stein, A.: Synthesis of mesoporous ZSM-5 zeolites through desilication and re-assembly processes. Microporous Mesoporous Mater. 149, 147–157 (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, J., Si, C., Li, Y. et al. CO2 adsorption on zeolite X/activated carbon composites. Adsorption 18, 503–510 (2012). https://doi.org/10.1007/s10450-012-9440-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-012-9440-0