Abstract
Three types of agricultural waste, citrus maxima peel (CM), passion fruit shell (PF) and sugarcane bagasse (SB), were used to produce biosorbents for removing the heavy metal ions of copper(II), cadmium(II), nickel(II) and lead(II) from a pH 5.0 solution. The properties of biosorbents were characterized using scanning electron microscopy (SEM), zeta potential analyzer, Fourier transform infrared (FTIR) spectroscopy, elemental analyzer and tests of cation exchange capacity (CEC). The result indicated that the selected biosorbents possess rich carboxyl (COOH) and hydroxyl (OH) groups to produce a complexation with the heavy metals. Moreover, the negative surface charge of the biosorbent might adsorb the metal ions through the ion exchange. All of the adsorption isotherms indicated that L-type characters represented complexation and ion exchanges that were the adsorption mechanisms of biosorbents toward heavy metals. Biosorbents with higher oxygen content might generate high adsorption capacities. The adsorption capacities of CM and PF, estimated from the fitting to the Langmuir isotherm, are similar to those reported by others regarding biosorbents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Industrial processes can generate a large amount of heavy metal that is discharged into wastewater treatment plants (WWTPs). Several methods have been applied to remove heavy metal ions from the wastewater, including precipitation, flotation, ion exchange, membrane-related process, and electrochemical technique (González-Muñoz et al. 2006; Satapathy and Natarajan 2006; Bessbousse et al. 2008). Precipitation is used when the concentration is high, but must be followed by advanced treatments to further reduce the effluent concentration and its impact on the environment and human health. For wastewater with low concentration of heavy metal, ion-exchange is the most common treating method in WWTPs. However, ion exchange resins need high production costs (Kurniawan et al. 2006). A number of researchers have intended to develop biosorbents with low costs, and high adsorption capacities to remove the harmful heavy metals in recent years (Febrianto et al. 2009; Ofomaja and Naidoo 2010).
Two types of biosorbents were frequently used to remove heavy metal ions in the solution: agricultural wastes, including fruit peels, straw, coconut coir, and so on (Rocha et al. 2009; Zheng et al. 2009; Reddy et al. 2011); and microorganisms, such as bacteria, yeasts, fungi, and algae, were attached on mediums to form biosorbents (Deng et al. 2006, 2007; Dursun 2006; Vilar et al. 2007; Hanif et al. 2007; Preetha and Viruthagiri 2007; Bhainsa and D’Souza 2008). The agricultural wastes can be converted to biosorbents through a simple pretreatment process. Therefore, the obtained biosorbents possess the advantage of low cost. The agricultural wastes frequently possess specific functional groups, such as OH or COOH, which may produce high adsorption capacities toward heavy metals. The main mechanisms of heavy metals on biosorbents may include ion exchanges, complexation, adsorption, and/or microprecipitation (Febrianto et al. 2009). The adsorption capacities were dependent of the functional groups on the adsorbent surface and the properties of adsorbents and adsorbates. The microorganism attached on the medium to form the biosorbents also possesses high adsorption capacities for various heavy metals. However, the biosorbents with the microorganism need complex operational skills if they are used in the WWTPs. The biosorbents produced from agricultural wastes can offer a low-cost and simple approach to remove the heavy metals in wastewater.
In this study, we selected fibrous materials such as citrus maxima peel (CM), passion fruit shell (PF), and sugarcane bagasse (SB) to produce biosorbents with bio-rich carboxyl and hydroxyl groups by using the pretreatment processes of drying, grinding, and degreasing. The obtained adsorbents were applied to adsorb the heavy metal ions of copper (Cu), lead (Pb), nickel (Ni), zinc (Zn), and cadmium (Cd) in the pH 5.0 solution. In this study, we discuss the potential adsorption capacities and mechanisms. The obtained results can offer a reference to determine the appropriate biosorbents.
2 Material and methods
The selected materials of biosorbents consisted of citrus maxima peel (CM), passion fruit shell (PF), and sugarcane bagasse (SB). They were regarded as the agriculture wastes. The raw materials needed to be pretreated, including drying, grinding, heating, and degreasing, based on the characteristics of the materials before the adsorption process. The preparing processes of CM and SB are shown in Fig. 1(a). The preparation process of PF is shown in Fig. 1(b). In Fig. 1(a), the surfaces of materials were washed with distilled water to remove dirt. The materials were air-dried under room temperature until water evaporated. The materials were then placed in a beaker with a 50 °C water bath for 48 h. To obtain uniform size, the materials were dried in a 50 °C oven and then ground to pass a 100 mesh screen. The obtained adsorbents need to be degreased using Soxhlet extraction. For CM, the extracting solvent is n-hexane and ethanol with a 1:1 ratio (V/V). The solvent of chloroform and methanol with the 1:1 ratio (V/V) was used to remove grease of SB. After the extraction process, the adsorbents were rinsed with pure water and dried in a 50 °C oven for 24 h. The obtained biosorbents were stored in a brown bottle until use.
The preparation process of PF was similar to that of CM and SB. The main difference is that the preparation of PF did not require extracting grease.
The surface properties of the 3 biosorbents were analyzed using scanning electron microscopy (SEM, Hitachi S-3000N) and Fourier transform infrared (FTIR, Perkin Elemer Model 1600) spectroscopy. The biosorbents were examined using a zeta potential analyzer (ZEN3600, MALVERN Nano-ZS) to determine the surface charges and an elemental analyzer (HORIBA 7021H) to determine the compositions of the major elements. In addition, the cation exchange capacities (CEC) of the biosorbents were also measured.
The test heavy metal ions included copper(II), lead(II), nickel(II), and cadmium(II). All of the solutions of the heavy metal ions were prepared from a 1000 mg/L of stock solutions. To avoid competitive adsorption, each adsorption experiment was run using only a single test heavy metal. To avoid system errors, the added concentrations of heavy metals were controlled below 1000 mg/L. The maximum adsorption amount is less than 80 % of the initial concentrations of heavy metals in the solution. The 0.2 g of biosorbent was added in a 50 ml Teflon centrifuge tube. The 40 ml solution containing various metal ion concentrations was added into the tube. The pH of the solution can affect the adsorption capacities of the tested heavy metals on the biosorbents. According to references, the biosorbents possessed the maximum adsorption capacities for the heavy metals in a solution of pH 5.0–6.0 (Vaghetti et al. 2009). In addition, the investigators presented that the copper, zinc and cadmium may form Cu(OH)+, Zn(OH)+, and Cd(OH)+ species under a pH higher than 5.0 condition (Rocha et al. 2009). Thus, the pH of the solution was adjusted at 5.0±0.1 before the adsorption process.
The centrifuge tubes were equilibrated for 24 h in a reciprocating shaker at 180 rpm at 25 °C. After completing the adsorption process, each solution was centrifuged for 30 min at 9000 rpm (9020 g) and filtered through a 0.45-μm filter. The amount of metals adsorbed on the biosorbents was determined by the difference between the metal concentrations in the solution before and after equilibration. The concentrations of heavy metals were determined using atomic absorption spectrometry (Avanta/AAS, GBC).
Each experiment was duplicated and the data were averaged. If the bias of the repeated experiments exceeded 15 %, triplicate repetitions were performed. Blank experiments without biosorbents were performed for the test compounds for each batch experiment; the recoveries ranged from 90 % to 100 %. The measured equilibrium concentrations were not adjusted for the recoveries.
The Freundlich and Langmuir adsorption equilibrium equations were typically used to explain the characteristics for the adsorbates and adsorbents. The Freundlich equation can be expressed as
where x is the amount of the organic compound sorbing onto the zeolite (mg); m is the zeolite weight (kg); K is the equilibrium constant (mg/kg(L/mg)1/n); C is the concentration (mg/L) of the organic compound in a solution at equilibrium; and n is an empirical constant. When n was greater than 1, the isotherm was concave downward; when n was less than 1, the isotherm was concave upward; and when n was equal to 1, the isotherm was linear.
The Langmuir equation can be expressed as
where x, m, and C were defined as above, K is the Langmuir adsorption constant (L/mg), and b is the maximum sorption capacity (mg/kg). When an adsorbate with a low concentration exists in the system, the Langmuir equation appears in the linear adsorption isotherm x/m=K H C. The K H is the Henry adsorption constant. The Henry adsorption constant is commonly used to indicate the affinity of an adsorbate toward a solid surface.
Although the Freundlich and Langmuir equations were most commonly applied to determine the characteristics of an adsorbate relative to an adsorbent in solution, complex adsorption behavior may result in poor data approximation. In this study, the biosorbents may uptake heavy metal ions through a complexation or the ion exchange. The adsorption characteristics were discussed herein according to obtained isotherms and adsorption capacities.
3 Results and Discussion
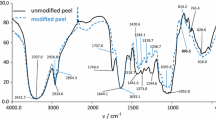
The particle size and morphology of biosorbents examined by SEM are shown in Fig. 2. In the SEM images, the obtained biosorbents lack the pore volume. Porous materials such as zeolite and bentonite can possess a large surface area and CEC. Thus, they can possess high adsorption capacities toward the heavy metals. The selected biosorbents are not porous materials, which means that they cannot provide a large number of exchangeable cation sites to adsorb the heavy metals. The CEC values of the biosorbents only result from their functional groups on the surfaces. The dissociation of hydrogen or other cation from the functional groups might generate the exchangeable cation under the specific pH condition. In addition, the functional groups, such as carboxyl, amino, and hydroxyl on the surface of biosorbents could provide complexation with the heavy metals (Zheng et al. 2009). Functional groups containing oxygen or nitrogen atoms can provide nonbonding electrons to coordinate with the divalent metals. In other words, the functional groups on the surface are considered as the dominant factors in determining adsorption capacities. To understand the presence of functional groups, the main element composites of the biosorbents are shown in Table 1. The carbon and oxygen atoms are the primary elements of the test biosorbents. The nitrogen atom is not shown in Table 1. The results indicate that the amino group is not a dominant functional group but that carboxyl and hydroxyl are the potential dominant groups. The functional groups of the biosorbents are able to be demonstrated using FTIR spectra. The FTIR spectra of 3 biosorbents are indicated in Fig. 3. The biosorbents possess similar functional groups on their surfaces. The absorbance near 3400 cm−1 demonstrates that the surfaces possess OH groups. The result indicates that selected biosorbents have OH groups on their surfaces. The weak absorbance of a signal near 1700 cm−1 for biosorbents indicates the presence of a C=O group on the surface. The result implied that the biosorbents may possess COOH groups on the surfaces. The other absorbance of a signal also illustrated that the selected biosorbents may possess rich OH and COOH groups. As expected, the FTIR spectra exhibited biorsorbents possessing rich hydroxyl and carboxyl groups. Consequently, the obtained results revealed that the complexation was a potential adsorption mechanism between the test heavy metals and the selected biosorbents.
Ion exchange is also a potential mechanism, although a complexation is regarded as the main mechanism of the heavy metals relative to the selected biosorbents. The zeta potential (ZP) values of the biosorbents under the various pH values are shown in Fig. 4 for observing the surface charge. A positive zeta potential value indicates the positive surface charge on the adsorbent surface and vice versa. Significantly, all of the zeta potential values under the given pH range are negative. This shows that surface charges of the biosorbents are negative and the biosorbents may possess exchangeable cations on their surfaces. The functional groups on the surface such as COOH and OH still possess the CEC under the specific pH conditions. Thus, we further examined the CEC values of 3 biosorbents under the pH=5.0 condition to understand the net surface negative charge. The results regarding CEC values of the CM, SB, and PF are 47.3, 11.8, and 26.9 (meq/100 g), respectively. The results indicated that the selected biosorbents possess the exchangeable cation sites on the surfaces. The CEC values of 3 biosorbents are in the following order: CM>PF>SB. When the biosorbents uptake the heavy metals, the ion exchange is a potential adsorption mechanism.
Figure 5 shows the isotherms of the adsorption of heavy metal ions (nickel, copper, cadmium, lead) on the selected biosorbents in pH 5.0 solutions. The adsorption isotherms exhibit similar profiles for test heavy metals relative to biosorbents. These isotherms rise sharply under low equilibrium concentrations and then reach a plateau after increasing the equilibrium concentration continuously. All of the isotherms have an L-type character (concave downward), revealing that the examined heavy metals have high affinity to the biosorbent surfaces. The results implying that the potential adsorption mechanisms between the heavy metals and the biosorbents are the ion exchange or the complexation. According to the previous description, the biorsorbents can allow exchangeable cation sites and functional groups to form the complexation with the heavy metals. Thus, both ion exchanges and complexations may become the adsorption mechanisms in the study.
From the isotherms, we found the adsorption capacities for the test heavy metals indicated the order: CM>PF>SB. However, the adsorption capacities of the 4 heavy metals on the different biosorbents exhibited an irregular order. For example, CM and SB possess the maximum adsorption amount of copper, but PF possesses the maximum adsorption amount of cadmium. The reason may result from the difference in the adsorption mechanism. If the ion exchange is the primary mechanism, the adsorption capacities of the test heavy metals should be proportional to the ion radii. That is the decreasing order: Pb>Cd>Cu>Ni. According to the CEC values, the maximum adsorption capacities of divalent metals on biosorbents ranged from 59 to 237 mmol/kg through the ion exchange. The obtained values are far lower than the maximum adsorption amount shown in Fig. 5. The results implied that the ability to form complexations became a key factor in determining adsorption capacities. However, the complexation abilities for the heavy metals are a function of the properties of heavy metals, functional groups, and of the pH of the solution. Generally, copper(II) and nickel(II) possess a relatively higher potential to form such complexes. Conversely, lead(II) and cadmium(II) generate relatively higher adsorption amounts through the ion exchange. Consequently, the maximum adsorption amounts of the test heavy metals on the biosorbents change based on the adsorption mechanisms. For CM, the maximum adsorption amounts were in the order: Cu>Ni≈Cd>Pb. The results indicated that the complexations are the primary mechanism of CM relative to the test heavy metals. For SB, the maximum adsorption amounts indicated the following order: Cu>Ni≈Cd>Pb. This shows that the adsorption capacities resulting from the complexations are higher than those resulting from the ion exchange. For PF, the maximum adsorption amounts follow the order Cd>Pb>Cu>Ni. The ion exchange may be more important than the complexation. The results also demonstrated relatively higher adsorption capacities, mainly from the complexations. However, the ion exchange may be the primary adsorption mechanism for some biosorbents. The primary adsorption mechanism varies with the surface functional groups of biosorbents. In this study, the major functional groups on the biosorbent surface are OH and COOH. The COOH group may lead to complexations. The OH and COOH groups may generate exchangeable cation sites. The adsorption mechanisms are also affected by the pH value of the solution.
Table 2 shows the appropriated results from the Freundlich and Langmuir adsorption equilibrium equations of biosorbents uptaking the heavy metals of copper(II), cadmium(II), nickel(II), and lead(II) in the pH 5.0 solutions. The results indicated that the obtained data generate a better fitting for the Langmuir equation. Therefore, the maximum adsorption capacities estimated from the Langmuir adsorption equilibrium equations are also listed in Table 2. All of the Henry adsorption constants for the test heavy metals indicated the following order: CM>SB>PF. The result was ascribed to the difference in the pretreatment process. The pretreatment of PF without the degrease process led to a lower affinity between the heavy metals and adsorbent surfaces at a low equilibrium concentration. For the CM and PF, the sequences of the test heavy metals for Henry adsorption constants agree with those for the adsorption capacities. The main adsorption mechanisms of heavy metals on the CM and PF at the low equilibrium concentration are the complexations and ion exchanges, respectively. For the SB, the primary adsorption mechanism of the test heavy metals at the high equilibrium concentration is the ion exchange. However, the primary mechanism is the complexation at the low equilibrium concentration.
The results indicated that the biosorbents from CM and PF possess higher adsorption capacities. The adsorption capacities of SB for the test heavy metals are lower than in other biosorbents. The main reason is ascribed to low CEC values and low oxygen content. In this study, the adsorption capacities of heavy metals increased with the increasing oxygen contents of the biosorbents. For the 2 adsorption mechanisms presented, the contents of COOH and OH groups are the important factors in determining the adsorption capacities. The OH and COOH groups are dependent of the oxygen atom content. It is reasonable that biosorbents with high oxygen content can be regarded as excellent adsorbents to obtain high adsorption capacities. In addition, the adsorption capacities of CM and PF for the test heavy metals are similar to those of the biosorbents in other studies. For copper, the adsorption capacities of the biosorbents in other reports were approximately 1300 mmol/kg (Pavan et al. 2006). The result obtained for CM is similar to those of ponkan mandarin peels and pecan nutshells. The adsorption capacity of PF for lead is also similar to that of yellow passion-fruit shells reported (Jacques et al. 2007). The selected biosorbents including CM and PF can be applied to become new biosorbents.
4 Conclusions
In this study, we selected 3 agricultural wastes to produce biosorbents and examined their maximum adsorption capacities for the heavy metal ions of copper, lead, nickel, and cadmium. For test heavy metals, the adsorption capacities do not correspond to the ion radii. The results demonstrated that both the ion exchange and complexation were the adsorption mechanisms of biosorbents relative to the heavy metals. Moreover, the results emphasized that the primary adsorption mechanism is the complexation. The biosorbents with high oxygen content may possess relatively higher adsorption capacities. Among the selected biosorbents, CM and PF possess higher adsorption capacities, which can be developed to become new biosorbents.
References
Bessbousse, H., Rhlalou, T., Verche, J.F., Lebrun, L.: Removal of heavy metal ions from aqueous solutions by filtration with a novel complexing membrane containing poly (ethyleneimine) in a poly (vinyl alcohol) matrix. J. Membr. Sci. 307, 249–259 (2008)
Bhainsa, K.C., D’Souza, S.F.: Removal of copper ions by the filamentous fungus, Rhizopus oryzae from aqueous solution. Bioresour. Technol. 99, 3829–3835 (2008)
Deng, L., Su, Y., Su, H., Wang, X., Zhu, X.: Biosorption of copper(II) and lead(II) from aqueous solutions by nonliving green algae Cladophora fascicularis: equilibrium, kinetics and environmental effects. Adsorption 12, 267–277 (2006)
Deng, L., Zhu, X., Wang, X., Su, Y., Su, H.: Biosorption of copper(II) from aqueous solutions by green alga Cladophora fascicularis. Biodegradation 18, 393–402 (2007)
Dursun, A.Y.: A comparative study on determination of the equilibrium, kinetic and thermodynamic parameters of biosorption of copper(II) and lead(II) ions onto retreated Aspergillus niger. Biochem. Eng. J. 28, 187–195 (2006)
Febrianto, J., Kosasih, A.N., Sunarso, J., Ju, Y.-H., Indraswati, N., Ismadji, S.: Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J. Hazard. Mater. 162, 616–645 (2009)
González-Muñoz, M.J., Rodríguez, M.A., Luque, S., Álvarez, J.R.: Recovery of heavy metals from metal industry wastewaters by chemical precipitation and nanofiltration. Desalination 200, 742–744 (2006)
Hanif, M.A., Nadeem, R., Bhatti, H.N., Ahmad, N.R., Ansari, T.M.: Ni(II) biosorption by Cassia fistula (Golden Shower) biomass. J. Hazard. Mater. B 139, 345–355 (2007)
Jacques, R.A., Lima, E.C., Dias, S.L.P., Mazzocato, A.C., Pavan, F.A.: Yellow passionfruit shell as biosorbent to remove Cr(III) and Pb(II) from aqueous solution. Sep. Purif. Technol. 57, 193–198 (2007)
Kurniawan, T.A., Chan, G.Y.S., Lo, W., Babel, S.: Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 366, 409–426 (2006)
Ofomaja, A.E., Naidoo, E.B.: Biosorption of lead(II) onto pine cone powder: studies on biosorption performance and process design to minimize biosorbent mass. Carbohydr. Polym. 82, 1031–1042 (2010)
Pavan, F.A., Lima, I.S., Lima, E.C., Airoldi, C., Gushikem, Y.: Use of ponkan mandarinpeels as biosorbent for toxic metals uptake from aqueous solutions. J. Hazard. Mater. 137, 527–533 (2006)
Preetha, B., Viruthagiri, T.: Batch and continuous biosorption of chromium(VI) by Rhizopus arrhizus. Sep. Purif. Technol. 57, 126–133 (2007)
Reddy, D.H.K., Ramana, D.K.V., Seshaiah, K., Reddy, A.V.R.: Biosorption of Ni(II) from aqueous phase by Moring a oleifera bark, a low cost biosorbent. Desalination 268, 150–157 (2011)
Rocha, C.G., Zaia, D.A.M., Ventura da Silva Alfaya, R., da Silva Alfaya, A.A.: Use of rice straw as biosorbent for removal of Cu(II), Zn(II), Cd(II) and Hg(II) ions in industrial effluents. J. Hazard. Mater. 166, 383–388 (2009)
Satapathy, D., Natarajan, G.S.: Potassium bromate modification of the granular activated carbon and its effect on nickel adsorption. Adsorption 12, 147–154 (2006)
Vaghetti, J.C.P., Lima, E.C., Royer, B., Cunha, B.M., Cardoso, N.F., Brasil, J.L., Dias, S.L.P.: Pecan nutshell as biosorbent to remove Cu(II), Mn(II) and Pb(II) from aqueous solutions. J. Hazard. Mater. 162, 270–280 (2009)
Vilar, V.J.P., Botelho, C.M.S., Boaventura, R.A.R.: Modeling equilibrium and kinetics of metal uptake by algal biomass in continuous stirred and packed bed adsorbers. Adsorption 13, 587–601 (2007)
Zheng, J.-C., Feng, H.-M., Lam, M.H.-W., Lam, P.K.-S., Ding, Y.-W., Yu, H.-Q.: Removal of Cu(II) in aqueous media by biosorption using water hyacinth roots as a biosorbent material. J. Hazard. Mater. 171, 780–785 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chao, HP., Chang, CC. Adsorption of copper(II), cadmium(II), nickel(II) and lead(II) from aqueous solution using biosorbents. Adsorption 18, 395–401 (2012). https://doi.org/10.1007/s10450-012-9418-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-012-9418-y