Abstract
ZIF-8 has been rapidly developed as a potential candidate for CO2 capture due to its low density, high surface area, and robust structure. Considering the electron-donating effect of amino functional groups, amino-modification is expected to be an efficient way to improve CO2 adsorption of ZIF-8. In this work, grand canonical Monte Carlo (GCMC) simulation was performed to study the CO2 adsorption isotherm based on ZIF-8, ZIF-8-NH2, and ZIF-8-(NH2)2. ZIF-8 was synthesized and CO2 adsorption isotherms based on ZIF-8 was measured. The experimental surface area, pore volume, and CO2 adsorption isotherm were used to validate the force field. Adsorptive capacity of ZIF-8-NH2, and ZIF-8-(NH2)2 were first estimated. The GCMC simulation results indicated that the order of increasing CO2 capacity of the ZIF-8 in the lower pressure regime is: ZIF-8 < ZIF-8-NH2 < ZIF-8-(NH2)2, and in the high pressure is: ZIF-8 < ZIF-8-(NH2)2 < ZIF-8-NH2. New adsorption sites can be generated with the existence of-NH2 groups. In addition, for non-modified and amino-modified ZIF-8, it was the first time to use density functional theory (DFT) calculations to investigate their CO2 adsorption sites and CO2 binding energies. The present work indicates that appropriate amine-functionalized can directly enhanced CO2 capacity of ZIF-8.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Attempts to solve the greenhouse problem have led to increasing interest in removing CO2 from the exhaust streams of fossil fuel combustion, which is the first step in carbon sequestration (Sayari et al. 2011; Aaron and Tsouris 2005; Bai and Yeh 1997). However, this step is very difficult to accomplish because of the high temperature and low CO2 partial pressure flue gas post combustion. Adsorption-based separation technologies exhibit many advantages in dealing with these unfavorable conditions. Therefore, several kinds of materials have been studied for CO2 adsorption, such as carbon-based materials (Agnihotri et al. 2005; Lu et al. 2008; Su et al. 2009; Choi et al. 2009) and zeolites (Xu et al. 2003; Belmabkhout et al. 2010; Hicks et al. 2008; Krishna and van Baten 2009; Su et al. 2010; Zukal et al. 2010).
Recently, a new class of porous materials known as metal organic frameworks (MOFs) has been synthesized and studied because of its well-defined pores, high surface areas, and desired chemical functionalities (Chen et al. 2010; Tranchemontagne et al. 2008). Additional new MOFs have been synthesized and used in CO2 adsorption and CO2 separation process. The so-called zeolitic imidazolate frameworks (ZIFs), most of which have topologies analogous to zeolite structures, are particularly interesting members of the MOF family. They have exceptional chemical and thermal stabilities, and are considered as the promising materials for gas storage and separation (Park et al. 2006; Phan et al. 2009). In addition, several studies have reported the experimental adsorption results of CO2 on ZIFs. For example, Banerjee et al. (2008) investigated the adsorption of CO2 on ZIF-69 and found that ZIF-69 had a large adsorption capacity of 82.6 L/L at a low pressure of 1 atm at 298 K. Banerjee et al. (2009) studied the CO2 adsorption performance of ZIF-78, and found that ZIF-78 exhibits an extraordinary capacity of 60.2 cm3/g at 273 K and 1 atm. In fact ZIF-78, one of the best-performing ZIFs, displays a superior ability of storing CO2 compared to the industrially utilized adsorbent BPL carbon. However, the cost of synthesizing ZIF-78 is too high, especially comparing with its commercial potential. Pérez-Pellitero et al. (2010) reported that the quantity of CO2 adsorbed on ZIF-8 at lower pressure was lower than that on ZIF-69. But at higher pressure, ZIF-68 achieves a higher CO2 capacity. These phenomenon could be explained by the fact that the total pore volumes of the ZIF-8 is larger than ZIF-69. It indicated that ZIF-8 is a promising candidate for CO2 capture.
One feature provoking interest in MOFs is the possibility of modifying desired chemical functionalities to MOF structure. Tuning the affinity of the framework functionalities toward CO2 is crucial for optimization of the adsorptive properties. Metal-organic frameworks functionalized with amine functional groups have been intensively studied for their enhanced CO2 adsorption properties (Eddaoudi et al. 2002; Ahnfeldt et al. 2009; Devic et al. 2012; Bauer et al. 2008; Couck et al. 2009; Vaidhyanathan et al. 2009).
In recent years, many researchers, such as Babarao and Jiang (2009), Babarao et al. (2008, 2009), Zhong (Xu et al. 2010; Xue and Zhong 2009; Zheng et al. 2009), Snurr (Bae et al. 2008), Karra and Walton (2010) have investigated the CO2 adsorption capability of MOF materials using the molecular simulation method. Some attempts have been made to investigate amine modification of IRMOFs (Babarao and Jiang 2008; Mu et al. 2010) and MILs (Arstad et al. 2008; Stavitski et al. 2011; Couck et al. 2012). However, few studies have been performed on molecular simulation regarding amine functionalized ZIFs for CO2 capture (Morris et al. 2010). Moreover, the mechanism of how the amine-functionalized affects the CO2 adsorption capability of ZIFs has not been fully understood so far.
In this work, we present an amine-functionalized study for a typical nitrogen containing heterocycles MOF: ZIF-8. The CO2 adsorption properties of ZIF-8 and amine-functionalized ZIFs [ZIF-8-NH2 and ZIF-8-(NH2)2] were investigated by using grand canonical Monte Carlo (GCMC) molecular simulations and density functional theory (DFT) calculations. ZIF-8 was synthesized and characterized by CO2 adsorption-desorption isotherms, powder XRD, TEM, and Micromeritics ASAP adsorption porosimeter. CO2 adsorption isotherm was measured to validate the force field. By GCMC calculation, the adsorption site and CO2 capacity trends of ZIF-8, ZIF-8-NH2 and ZIF-8-(NH2)2 were predicted. With the DFT calculation, the electrostatic fields of ZIF-8 and ZIF-8-(NH2)2 were studied, and the binding energy between CO2 and these two materials were investigated.
2 Models and simulation method
2.1 ZIF structure
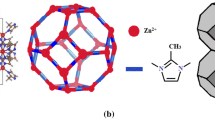
In the present computational study, the structures of ZIF-8 were constructed based on X-ray diffraction (XRD) (Park et al. 2006) patterns using Materials Studio Visualizer (Materials Studio 2009). ZIF-8 has a primitive Zn topology linked by an imidazolate linker. To investigate the amine modification of ZIF-8 simultaneously, two kinds of structures were used in the present study. Different numbers of amino groups were added at two available positions of the imidazolate linker in ZIF-8, near the C=C bond. Then the models were optimized by energy minimization and structure optimization with DFT calculation. In these calculations, the periodic boundary conditions were performed to optimize the structures of their unit cells and the gradient corrected (GGA) correlation functional of PW91 was used with the precise numerical basis set double numerical plus d-functions (DND). The above calculations were carried out with Accelrys Dmol3 code (Materials Studio 2009). All structures are shown in Fig. 1.
2.2 Force field
In the present work, CO2 was modeled as a rigid linear molecule with three charged Lennard-Jones (LJ) sites located on each atom. A combination of the site–site LJ and Coulomb potentials was used to calculate the CO2–CO2 intermolecular interactions. The LJ potential parameters for O and C atoms in CO2 were taken from the universal force field (UFF) (Rappe et al. 1992). The partial point charges centered at each LJ site (i.e., q O=−0.272 e and q C=0.544 e) represent the first-order electrostatic and second-order conduction interaction, respectively. This potential model was recently used to successfully simulate the adsorption of CO2 on ZIF materials (Liu et al. 2009).
For the studied MOFs, an atomistic representation similar to CO2 was used to model ZIF-8. The same potential model, a combination of the site–site LJ and Coulomb potentials, was used to calculate the interactions between adsorbate molecules and adsorbents. The UFF adopted in this work is able distinguish the type of atoms in the frameworks of the ZIFs materials in detail (Liu et al. 2009). In order to better represent the adsorption isotherms of pure CO2 on these ZIFs, the calculated part of the atomic partial charges of ZIF-8 by DFT and LJ parameters of the UFF were refined.
The LJ parameters based on this force field were readapted by scaling cohesive energy on the dispersion–repulsion interactions as in equation:
σ ij and ε ij are the crossed LJ parameters obtained between the solid parameters and the CO2 model of Liu et al. (2009). Crossed LJ interactions were calculated by applying standard Lorentz-Berthelot combining rules. The parameter ζ was then determined by an iterative procedure to fit the experimental CO2 isotherm of ZIF-8. A scaling factor of ζ≈0.899 was obtained. We then used this factor to adjust the dispersion repulsion parameters for the additional atoms needed to describe host-guest interactions of different gases adsorbed on different ZIFs materials as described in equation (Pérez-Pellitero et al. 2010):
The parameters used are shown in Table 1. The Lorentz-Berthelot combining rules were adopted to calculate the LJ cross-parameters and describe the interaction of the CO2 molecules with the framework atoms in the ZIF-8 for consistency.
2.3 DFT calculations
The partial charges of the ZIFs were extracted by DFT calculations using the Accelrys Dmol3 code (Materials Studio 2009). The calculations were performed on the periodic models of ZIF-8 using the PW91 GGA density functional (Perdew and Wang 1992), as well as the double numerical basis set plus polarization (DNP), which is a viable alternative to ab initio methods and can provide reasonable accuracy for computation (Tsuzuki and Luthi 2001). The atomic charges were estimated by the electrostatic potential (ESP) charge method (Mulliken 1955), which has been successfully employed to study the adsorption of CO2 on ZIFs (Liu et al. 2009; Sirjoosingh et al. 2010). The partial atomic charges of ZIF-8 required in the simulations were showed in Fig. 2.
An important computational study by Torrisi et al. (2010), in which the binding energy (BE) between CO2 molecule and benzene…X (X=F, Cl, Br, CH3) molecular complexes were estimated, according to the following schematic equation, in which E optim represents the energy of the system after full geometry relaxation,
shows that intermolecular interactions between the CO2 molecule and a range of functionalized aromatic molecules can be calculated using the DFT theory. Similarly, in this study the BE between CO2 and the imidazole ring was computed using the same method.
The calculations were performed using PW91/DNP method, as implemented in the Dmol3 program version (Materials Studio 2009). A real-space orbital global cutoff of 3.7 Å was applied, and the convergence threshold parameters for the optimization were 1×10−6 (energy), 2×10−4 (gradient), and 5×10−3 (displacement). Frequency calculation was performed to determine whether the structure achieved the energy minimization and the distinguishing cases of real energy minima from transition states (TS). A new optimization was performed with more convergence parameters: 1×10−6 (energy), 1×10−4 (gradient), and 5×10−4 (displacement).
2.4 GCMC simulation
The conventional GCMC simulation technique was employed for calculating gas adsorption isotherms of the CO2 on ZIF-8, ZIF-8-NH2, and ZIF-8-(NH2)2. The simulation box representing ZIFs contained eight (2×2×2) unit cells, and periodic boundary conditions were applied in all three dimensions. The simulation with larger boxes showed that no finite-size effects existed. The ZIFs were treated rigidly, and a cut-off radius of 12.8 Å was applied to calculate the LJ interactions. The long-range electrostatic interactions were handled using the Ewald summation technique with a tin-foil boundary condition (Zhang et al. 2007). For each state point, the GCMC simulation consisted of 1×106 steps to guarantee equilibration, and the data were collected for another 5×106 GCMC steps to obtain the average amount adsorbed.
3 Experimental
3.1 Synthesis of ZIF-8
First, a solid mixture of zinc nitrate hexahydrate Zn(NO3)2⋅6H2O (0.956 g, 3.2 mmol) and 2-methylimidazole (H-MeIM) (0.24 g, 3.4 mmol) was dissolved in 70 mL of DMF solvent. The mixture was quickly transferred to a 100 mL autoclave and sealed. The autoclave was heated at a rate of 5 K/min to 413 K in a programmable oven and held at this temperature for 24 h under autogenous pressure by solvothermal synthesis. Autoclave was then cooled to room temperature at a rate of 0.3 K/min. Chloroform (40 mL) was then added into the autoclave after the removal of the mother liquor from the mixture. The as-synthesized ZIF-8 crystals were isolated by filtration, and the colorless polyhedral crystals were collected from the upper layer, washed thrice with DMF (10 mL), and dried at 383 K overnight (Park et al. 2006). Finally, the as-synthesized ZIF-8 crystals were placed in a quartz tube, which in turn was placed inside a tubular furnace. The samples were maintained under an atmosphere of H2 (40 mL/min) at 573 K for 120 min, and then cooled to room temperature.
3.2 Characterization
The powder X-ray diffraction (PXRD) patterns of ZIF-8 were taken on a D/max-IIIA (0.5 kW) instrument using Cu-KR radiation of 40 kV and 30 mA at 5° and 25°. The adsorption–desorption isotherms of CO2 on the ZIF-8 samples were analyzed using a magnetic suspension balance method from Rubotherm, Germany. The surface morphology and particle size of the crystalline ZIF-8 samples were observed by a Philips FEI XL-30 scanning electron microscope at an accelerating voltage of 20 kV. The pore textural properties of the ZIF-8 samples were measured by a Micromeritics ASAP 2020 adsorption porosimeter at a liquid nitrogen temperature of 77 K. The Brunauer-Emmet-Teller (BET) area, Langmuir surface area, and pore volume were obtained.
4 Results and discussion
4.1 Structure stability and force field validation
From the structure optimization results, the unit cell lengths of ZIF-8-NH2 and ZIF-8-(NH2)2 are 16.81 and 16.72, which are very close to that of ZIF-8 (16.99 Å). Figure 1 shows the optimized unit cell crystal structures of ZIF-8-NH2 and ZIF-8-(NH2)2. As can be seen from Fig. 1, the optimized crystal structures do not collapse and keep the similar SOD topologies as that of ZIF-8. These results indicate that the structures of amine-modified ZIF-8 are stable and can be further used for the following investigations in this work.
GCMC simulations were performed to calculate the adsorption isotherm of CO2 on ZIF-8 in the pressure range from 0 to 30 atm at 298 K. The calculated isotherms and the experimental values are shown in Fig. 3. The simulated results calculated with the UFF LJ parameters are much higher than the experimental results. In regards to the accuracy of the measurement, the results calculated with new parameters are in better agreement with the experimental results for CO2 isotherm. The simulated surface area and pore volume (showed in Table 3) calculated with simple GCMC and the new LJ parameters also present acceptable accuracy. These results demonstrate that the model and the new force field parameters used in the present work accurately describe the CO2 adsorption behavior on ZIF-8.
4.2 CO2 adsorption isotherm simulation of ZIF-8 and amine-modified ZIF-8
To investigate the adsorption enhancement of amine-modified ZIFs produced by the grafting of amino functional groups, additional GCMC simulations were performed on the ZIF-8-(NH2)2 and CO2 molecules. The calculated isotherms, expressed in Nmolecules/eight unit cell, are shown in Fig. 4 at low and high pressure. The data indicates that the capacity for CO2 adsorption on ZIF-8-(NH2)2 and ZIF-8-NH2 is much better than that on ZIF-8. This phenomenon is attributed to two reasons. First, the basic amino groups have a high affinity toward acidic CO2 mainly through the interaction with the lone electron pair on nitrogen. Second, quadrupole-π electron interaction between CO2 molecule and imidazole ring is strengthened by the electron-donating group (amino functional groups). In essence, the more the electron-donating groups (amino functional groups) are added, the stronger the quadrupole-π electron interaction is. Thus, at lower pressure, the capacity of CO2 adsorption on ZIF-8-(NH2)2 is better than on ZIF-8-NH2. Moreover, at higher pressure, the capacity for CO2 adsorption of ZIF-8-(NH2)2 is nearly the same as that on ZIF-8-NH2. That is because at higher pressure, the adsorption sites near the methyl contribute to the CO2 adsorption. However these adsorption sites are weakened by the addition of amino functional groups.
The simulated CO2 adsorption isotherm on ZIF-8 and the simulated CO2 adsorption isotherm on ZIF-8-(NH2)2 and ZIF-8-NH2 at 298 K are shown in Fig. 5. All adsorption quantities in adsorption isotherms are expressed in mmol/g. Results of the adsorption isotherm obtained from the simulation shows that, at lower pressure (below 12 bar), the CO2 adsorption quantity on ZIF-8-(NH2)2 is higher than that on ZIF8-NH2. The order of CO2 capacity of ZIF-8 is: ZIF-8 < ZIF-8-NH2 < ZIF-8-(NH2)2. However at higher pressure (above 12 bar), the CO2 adsorption quantity on ZIF-8-(NH2)2 is much lower than ZIF-8-NH2, which differs from the result shown in Fig. 4. The order of CO2 capacity of the ZIF-8’s is, ZIF-8 < ZIF-8-(NH2)2 < ZIF-8-NH2. This is likely because the numbers of adsorption sites on the ZIFs increased with the addition of amino functional groups, which also increased the framework mass (11.7 %). At lower pressure, the more amino functional groups added into ZIF-8 structure, the larger the adsorption quantity. However, the opposite occurs at higher pressure, indicating that the adsorption quantity can be improved only when appropriate numbers of amino functional groups are added.
4.3 Adsorption sites in ZIFs
Knowledge of adsorption sites is important in understanding the adsorption mechanism. Therefore, the center of mass (COM) probability distributions of adsorbate CO2 on ZIF-8 was calculated based on all the configurations recorded during the GCMC simulations. The COM map of ZIF-8 and ZIF-8-(NH2)2 near the Zn-hexagon opening was also sliced to form the unit cell of ZIF-8 and ZIF-8-(NH2)2 with certain rules (Zhou et al. 2009). The sliced images [(111) view] of different positions are plotted in Fig. 6. Several color fields (blue–green–yellow) are used to indicate the adsorption sites of CO2 on ZIF-8 and ZIF-8-(NH2)2. The blue represents the highest quantity of CO2 adsorbed and the yellow represents the least adsorbed. The most important adsorption site in ZIF-8 is above the three methyl rings (Plane A) and near the six imidazole rings (Plane B). Besides the three methyl rings (Plane C) and near the imidazole ring (Plane D) in ZIF-8-(NH2)2, another adsorption site can be found in the middle of six amidogen rings (Plane E); this new adsorption site likely appeared when the electron-donating group (amino functional groups) was added into the ZIF-8.
Sliced images [(111) view] of the different positions of the ZIF-8 and ZIF-8-(NH2)2 structure. (A) above the three methyl rings; (B) near the six imidazole rings of the ZIF-8 structure; (C) the three methyl rings of ZIF-8-(NH2)2; (D) near the imidazole ring of ZIF-8-(NH2)2; and (E) in the middle of the six amidogen rings of ZIF-8-(NH2)2
4.4 Relationship between the electrostatic fields in ZIFs and the CO2 adsorption capacity
To investigate the effects of the electron-donating groups on the adsorption site, the electrostatic potentials (Babarao and Jiang 2008; Mu et al. 2010) for ZIF8, ZIF8-NH2, and ZIF8-(NH2)2 were studied to determine the enhanced electrostatic interactions of CO2 molecules within the frameworks. The contour maps of the ESPs were calculated using Dmol3, as implemented in the Materials Studio package (Materials Studio 2009), to obtain the gradient of potential changes and reflect the strength of the electrostatic field in the pore of the ZIFs. The values should influence the polarization of the CO2 molecules within the ZIFs cavities, as shown in Fig. 7. An ESP with a larger gradient and higher absolute values is near the organic linker and imidazole ring, reflecting a stronger electrostatic field in the pores of ZIF8-(NH2)2 than that in ZIF8-NH2 and ZIF8. The reason is that amino functional groups not only provide the lone pair electrons, but also significantly enhance the π electrons interaction on the imidazole ring. According to the adsorption isotherms and adsorption sites obtained from the GCMC simulation, higher CO2 adsorption and new adsorption sites on ZIF8-(NH2)2 and ZIF8-NH2 were caused by the electrostatic field enhancement from the amino functional groups. Overall, these observations are in good agreement with the results shown in Fig. 5.
4.5 DFT calculation of BE
Figure 8 shows three different stable configurations for the complex C4H6N2⋯CO2. The most stable conformation has the CO2 on the imidazole ring top position (Fig. 8, dimer A, BE=−7.741 kJ mol−1), with C(CO2) at a distance of 3.556 and 3.958 Å from two imidazole ring N atoms and 4.494 Å from the methyl C atom. The stability of the conformation is caused by the quadrupole-π electron interaction between the π electrons on the imidazole ring and the electron-deficient carbon of the CO2 molecule, as well as by the weak interaction between the methyl and CO2 molecule.
A second, less stable configuration has the CO2 positioned beside the methyl group with the shortest distance O(CO2)⋯H(methyl) of 3.239 Å (Fig. 8, dimer B, BE=−3.43 kJ mol−1) because there is a hydrogen bond-like interaction between O(CO2) and H(methyl).
The least stable configuration (Fig. 8, dimer C, BE=−4.85 kJ mol−1) has the CO2 located at the side of the molecule, which is roughly perpendicular to the imidazole ring and on the opposite side to the methyl group. There is O(CO2)-H(imidazole ring) at distances of 3.252 and 3.236 Å, likely stabilized by a very weak electrostatic interaction between one of the oxygens of CO2 (roughly in the plane of the ring) and the positive electrostatic potential of the imidazole hydrogen.
Three stable configurations of the C4H8N4⋯CO2 complex were identified. The most stable one (Fig. 9, dimer A, BE=−9.76 kJ mol−1) shows that CO2 is positioned between two amino functional groups, with a C(CO2)⋯N(amino functional groups) interactions at 3.726 and 3.039 Å. This configuration is stabilized by the electrostatic interaction between the lone pair nitrogen electrons of two amino groups and the electron-deficient carbon of the CO2 molecule.
Another stable configuration with BE of −7.90 kJ mol−1 (Fig. 9, dimer B) corresponds to CO2 on top of the imidazole ring position with a C(CO)2⋯N(imidazole ring) distance of 4.147 and 3.931 Å, and with a C(CO)2⋯N(amino functional groups) distance of 3.481 and 3.891 Å. This configuration is considered a quadrupole-π electron interaction between the CO2 molecule and imidazole ring.
The third stable configuration with BE of −2.16 kJ mol−1 (Fig. 9, dimer C) corresponds to CO2 positioned beside the methyl group because of the interaction between O(CO2) and methyl H; the shortest distance of O(CO2)⋯C(methyl) is 3.133 Å.
The present work studies the BEs of above stable configurations. When the amino functional groups were added into the imidazole ring, the BE between CO2 and methyl was reduced, indicating that the CO2 adsorption capacity near the methyl was also reduced. The configuration of CO2, when positioned between two amino functional groups, has the largest BE, indicating that the CO2 adsorption ability between two amino functional groups was much stronger than the configuration with no amino functional groups. In addition, the quadrupole-π electron interaction between CO2 and imidazole ring can be improved by an electron-donating group (e.g. amino functional groups).
Recently, several research groups reported that amine groups anchored on linkers of MIL structures have no direct effect on CO2 adsorption (Arstad et al. 2008; Serra-Crespo et al. 2011; Stavitski et al. 2011). The most typical result is reported by Stavitski et al. (2011), they demonstrated that the adsorption of CO2 moleculars on MIL-53 is directed by the formation of hydrogen bonding between CO2 and bridging –OH groups located at the [AlO6] unit. However, it was easier to form the hydrogen bond between CO2 and bridging –OH groups due to the interaction between the adjacent [AlO6] unit and –NH2 group. They also found that hydrogen bond between –NH2 groups and [AlO6] made the flexible NH2-MIL-53(Al) much more easy to form a narrow pore structure (np NH2-MIL-53(Al)). Both of the above interactions reduce the electron-donating effect of –NH2 group. So no direct strong interaction between CO2 and the amino group are observed. Compared with the present results, the major adsorption site of CO2 on ZIF-8 is near imidazole rings instead of metal oxides cluster (ZIFs have no metal oxides cluster, such as [AlO6] or [CrO6]). The main interaction is between organic linker and CO2. For ZIF-8-NH2 and ZIF-8-(NH2)2, there is no hydrogen bond between –NH2 groups and metal oxides cluster. Therefore, –NH2 groups on imidazolate ring can directly affect the CO2 molecules. Moreover, the –NH2 groups also contribute to quadrupole-π electron interaction of imidazolate rings and the interaction with CO2. Hence, interaction between CO2 and –NH2 groups are still strong.
4.6 Experimental section of ZIF-8
The single-crystal PXRD images of the synthesized ZIF-8 sample are shown in Fig. 10. The well-formed peaks imply the high crystallinity of the ZIF-8 samples. Table 2 shows that the main peak in PXRD and the main peaks of the ZIF-8 sample are in conformity with the XRD patterns for the sample synthesized by Yaghi et al. (Park et al. 2006).
The SEM picture in Fig. 11 shows that the particles were micron scale with sharp hexagonal facets. The mean particle size is ∼2 μm. The N2 adsorption measurement in Fig. 12 shows the type I isotherm. The increase in the volume adsorbed at low relative pressures is due to the presence of micropores; while a second uptake at high relative pressure indicates existence of textural meso/macroporosity formed by packing of nanoparticles. The microporous pore volume is about 0.47 cm3 g−1, and the BET (showed in Table 3) and Langmuir surface areas are 1070 and 1243 m2 g−1, respectively. The BET surface area is near the values that were reported for other ZIF-8 samples (Park et al. 2006).
Figure 13 shows the CO2 adsorption-desorption isotherm on ZIF-8. The desorption isotherm of ZIF-8 material is nearly the same as the adsorption isotherm. The CO2 adsorption–desorption isotherm indicates that ZIF-8 material show micropore adsorption behavior.
To further understand the adsorption properties, the enthalpy of adsorption (ΔHads) of CO2 was determined by measuring a series of isotherms at 298 K, 308 K, 318 K, 328 K and fitting the data to the virial model. The enthalpy of adsorption (ΔHads) for ZIF-8 is 26.7 kJ/mol (see Figs. 14 and 15).
5 Conclusion
GCMC simulations were carried out to investigate the CO2 adsorption isotherms and adsorption sites on ZIF-8 and amine-modified ZIF-8. Results of the CO2 adsorption isotherm simulation of ZIF-8 are consistent with the experimental results obtained. It shows that the parameters applied in the present work are in agreement with the experimental data. The GCMC simulation results also show that the CO2 adsorption ability of ZIF-8 can be enhanced by the addition of amino functional groups. The results of CO2 adsorption sites on ZIF-8 and ZIF-8-(NH2)2 indicated that the adsorption sites on ZIF-8 are near the imidazole rings and above the three methyl rings. On ZIF-8-(NH2)2, there is a new adsorption site in the middle of six amidogen rings beside the two adsorption sites above, as supported by the electrostatic field and BE investigations. We find that the quadrupole-π electron interaction between the CO2 molecule and the imidazole ring in the ZIF-8 structure is strengthened by the electron donating amino groups. We also propose that –NH2 groups on imidazolate ring can directly affect the CO2 molecules. Hence, CO2 adsorption capacity on ZIF-8 can be increased by the addition of –NH2 groups.
References
Aaron, D., Tsouris, C.: Separation of CO2 from flue gas: a review. Sep. Sci. Technol. 40(1–3), 321–348 (2005)
Agnihotri, S., Rood, M.J., Rostam-Abadi, M.: Adsorption equilibrium of organic vapors on single-walled carbon nanotubes. Carbon 43(11), 2379–2388 (2005). doi:10.1016/j.carbon.2005.04.020
Ahnfeldt, T., Gunzelmann, D., Loiseau, T., Hirsemann, D., Senker, J., Ferey, G., Stock, N.: Synthesis and modification of a functionalized 3D open-framework structure with MIL-53 topology. Inorg. Chem. 48(7), 3057–3062 (2009)
Arstad, B., Fjellvåg, H., Kongshaug, K., Swang, O., Blom, R.: Amine functionalised metal organic frameworks (MOFs) as adsorbents for carbon dioxide. Adsorption 14(6), 755–762 (2008). doi:10.1007/s10450-008-9137-6
Babarao, R., Jiang, J.: Molecular screening of metal–organic frameworks for CO2 storage. Langmuir 24(12), 6270–6278 (2008). doi:10.1021/la800369s
Babarao, R., Jiang, J.: Unprecedentedly high selective adsorption of gas mixtures in rho zeolite-like metal–organic framework: a molecular simulation study. J. Am. Chem. Soc. 131(32), 11417–11425 (2009). doi:10.1021/ja901061j
Babarao, R., Jiang, J., Sandler, S.I.: Molecular simulations for adsorptive separation of CO2/CH4 mixture in metal-exposed, catenated, and charged metal–organic frameworks. Langmuir 25(9), 5239–5247 (2008). doi:10.1021/la803074g
Babarao, R., Tong, Y.H., Jiang, J.W.: Molecular insight into adsorption and diffusion of alkane isomer mixtures in metal-organic frameworks. J. Phys. Chem. B 113(27), 9129–9136 (2009). doi:10.1021/jp902253p
Bae, Y.-S., Mulfort, K.L., Frost, H., Ryan, P., Punnathanam, S., Broadbelt, L.J., Hupp, J.T., Snurr, R.Q.: Separation of CO2 from CH4 using mixed-ligand metal−organic frameworks. Langmuir 24(16), 8592–8598 (2008). doi:10.1021/la800555x
Bai, H., Yeh, A.C.: Removal of CO2 greenhouse gas by ammonia scrubbing. Ind. Eng. Chem. Res. 36(6), 2490–2493 (1997). doi:10.1021/ie960748j
Banerjee, R., Phan, A., Wang, B., Knobler, C., Furukawa, H., O’Keeffe, M., Yaghi, O.M.: High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 319(5865), 939–943 (2008). doi:10.1126/science.1152516
Banerjee, R., Furukawa, H., Britt, D., Knobler, C., O’Keeffe, M., Yaghi, O.M.: Control of pore size and functionality in isoreticular zeolitic imidazolate frameworks and their carbon dioxide selective capture properties. J. Am. Chem. Soc. 131(11), 3875–3877 (2009). doi:10.1021/ja809459e
Bauer, S., Serre, C., Devic, T., Horcajada, P., Marrot, J., Ferey, G., Stock, N.: High-throughput assisted rationalization of the formation of metal organic frameworks in the iron(III) aminoterephthalate solvothermal system. Inorg. Chem. 47(17) (2008). doi:10.1021/ic800538r
Belmabkhout, Y., Serna-Guerrero, R., Sayari, A.: Adsorption of CO2-containing gas mixtures over amine-bearing pore-expanded MCM-41 silica: application for gas purification. Ind. Eng. Chem. Res. 49(1), 359–365 (2010)
Chen, B., Wang, X., Zhang, Q., Xi, X., Cai, J., Qi, H., Shi, S., Wang, J., Yuan, D., Fang, M.: Synthesis and characterization of the interpenetrated MOF-5. J. Mater. Chem. 20(18) (2010). doi:10.1039/B922528E
Choi, S., Drese, J.H., Jones, C.W.: Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2(9), 796–854 (2009)
Couck, S., Denayer, J.F.M., Baron, G.V., Remy, T., Gascon, J., Kapteijn, F.: An amine-functionalized MIL-53 metal-organic framework with large separation power for CO2 and CH4. J. Am. Chem. Soc. 131(18), 6326–6327 (2009)
Couck, S., Gobechiya, E., Kirschhock, C.E.A., Serra-Crespo, P., Juan-Alcaniz, J., Joaristi, A.M., Stavitski, E., Gascon, J., Kapteijn, F., Baron, G.V., Denayer, J.F.M.: Adsorption and separation of light gases on an amino-functionalized metal-organic framework: an adsorption and in situ XRD study. ChemSusChem 5(4), 740–750 (2012)
Devic, T., Vimont, A., Greneche, J., Moreau, F., Magnier, E., Filinchuk, Y., Marrot, J., Lavalley, J., Daturi, M., Ferey, G.: Functionalization in flexible porous solids: effects on the pore opening and the host-guest interactions. J. Am. Chem. Soc. 132(3), 1127–1136 (2012)
Duren, T., Snurr, R.Q.: Assessment of isoreticular metal-organic frameworks for adsorption separations: a molecular simulation study of methane/n-butane mixtures. J. Phys. Chem. B. 108(40) (2004)
Eddaoudi, M., Kim, J., Rosi, N., Vodak, D., Wachter, J., O’Keeffe, M., Yaghi, O.M.: Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 295(18), 469–471 (2002)
Hicks, J.C., Drese, J.H., Fauth, D.J., Gray, M.L., Qi, G.G., Jones, C.W.: Designing adsorbents for CO2 capture from flue gas-hyperbranched aminosilicas capable of capturing CO2 reversibly. J. Am. Chem. Soc. 130(10), 2902–2903 (2008)
Karra, J.R., Walton, K.S.: Molecular simulations and experimental studies of CO2, CO, and N2 adsorption in metal organic frameworks. J. Phys. Chem. C 114(37), 15735–15740 (2010)
Krishna, R., van Baten, J.M.: Comment on comparative molecular simulation study of CO2/N2 and CH4/N2 separation in zeolites and metal–organic frameworks. Langmuir 26(4), 2975–2978 (2009). doi:10.1021/la9041875
Liu, D., Zheng, C., Yang, Q., Zhong, C.: Understanding the adsorption and diffusion of carbon dioxide in zeolitic imidazolate frameworks: a molecular simulation study. J. Phys. Chem. C 113(12), 5004–5009 (2009). doi:10.1021/jp809373r
Lu, C., Bai, H., Wu, B., Su, F., Hwang, J.F.: Comparative study of CO2 capture by carbon nanotubes, activated carbons, and zeolites. Energy Fuels 22(5), 3050–3056 (2008). doi:10.1021/ef8000086
Materials Studio: Materials Studio 4.4. v. Accelrys, Inc., San Diego (2009)
Morris, W., Leung, B., Furukawa, H., Yaghi, O.K., Hayashi, H., Houndonougbo, Y., Asta, M., Laird, B.B., Yaghi, O.M.: A combined experimental-computational investigation of carbon dioxide capture in a series of isoreticular zeolitic imidazolate frameworks. J. Am. Chem. Soc. 132(32) (2010)
Mu, W., Liu, D., Yang, Q., Zhong, C.: Computational study of the effect of organic linkers on natural gas upgrading in metal–organic frameworks. Microporous Mesoporous Mater. 130(1–3), 76–82 (2010). doi:10.1016/j.micromeso.2009.10.015
Mulliken, R.S.: Electronic population analysis on LCAO-MO molecular wave functions. J. Chem. Phys. 23, 1833–1840 (1955)
Myers, A.L.: Adsorption in porous materials at high pressure: theory and experiment. Langmuir 18(26) (2002)
Pérez-Pellitero, J., Amrouche, H., Siperstein, F.R., Pirngruber, G., Nieto-Draghi, C., Chaplais, G., Simon-Masseron, A., Bazer-Bachi, D., Peralta, D., Bats, N.: Adsorption of CO2, CH4, and N2 on zeolitic imidazolate frameworks: experiments and simulations. Chem., Eur. J. 16(5), 1560–1571 (2010). doi:10.1002/chem.200902144
Park, K.S., Ni, Z., Côté, A.P., Choi, J.Y., Huang, R., Uribe-Romo, F.J., Chae, H.K., O’Keeffe, M., Yaghi, O.M.: Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 103(27), 10186–10191 (2006). doi:10.1073/pnas.0602439103
Perdew, J.P., Wang, Y.: Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 45(23), 13244–13249 (1992)
Phan, A., Doonan, C.J., Uribe-Romo, F.J., Knobler, C.B., O’Keeffe, M., Yaghi, O.M.: Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc. Chem. Res. 43(1), 58–67 (2009). doi:10.1021/ar900116g
Rappe, A.K., Casewit, C.J., Colwell, K.S., Goddard, W.A., Skiff, W.M.: UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 114(25), 10024–10035 (1992). doi:10.1021/ja00051a040
Sayari, A., Belmabkhout, Y., Serna-Guerrero, R.: Flue gas treatment via CO2 adsorption. Chem. Eng. J. 171(3), 760–774 (2011)
Serra-Crespo, P., Ramos-Fernandez, E.V., Gascon, J., Kapteijn, F.: Synthesis and characterization of an amino functionalized MIL-101(Al): separation and catalytic properties. Chem. Mater. 23, 2565–2572 (2011)
Sirjoosingh, A., Alavi, S., Woo, T.K.: Grand-canonical Monte Carlo and molecular-dynamics simulations of carbon-dioxide and carbon-monoxide adsorption in zeolitic imidazolate framework materials. J. Phys. Chem. C 114(5), 2171–2178 (2010). doi:10.1021/jp908058n
Stavitski, E., Pidko, E.A., Couck, S., Remy, T., Hensen, E.J.M., Weckhuysen, B.M., Denayer, J., Gascon, J., Kapteijn, F.: Complexity behind CO2 capture on NH2-MIL-53(Al). Langmuir 27, 3970–3976 (2011)
Su, F., Lu, C., Cnen, W., Bai, H., Hwang, J.F.: Capture of CO2 from flue gas via multiwalled carbon nanotubes. Sci. Total Environ. 407(8), 3017–3023 (2009)
Su, F., Lu, C., Kuo, S.-C., Zeng, W.: Adsorption of CO2 on amine-functionalized Y-type zeolites. Energy Fuels 24(2), 1441–1448 (2010). doi:10.1021/ef901077k
Torrisi, A., Mellot-Draznieks, C., Bell, R.G.: Impact of ligands on CO(2) adsorption in metal-organic frameworks: first principles study of the interaction of CO(2) with functionalized benzenes. II. Effect of polar and acidic substituents. J. Chem. Phys. 132(4) (2010). doi:Artn 044705
Tranchemontagne, D.J., Hunt, J.R., Yaghi, O.M.: Room temperature synthesis of metal-organic frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 64(36), 8553–8557 (2008). doi:10.1016/j.tet.2008.06.036
Tsuzuki, S., Luthi, H.P.: Interaction energies of van der Waals and hydrogen bonded systems calculated using density functional theory: assessing the PW91 model. J. Chem. Phys. 114(9), 3949–3957 (2001)
Vaidhyanathan, R., Iremonger, S.S., Dawson, K.W., Shimizu, G.K.H.: An amine-functionalized metal organic framework for preferential CO2 adsorption at low pressures. Chem. Commun. 35, 5230–5232 (2009)
Xu, Q., Liu, D., Yang, Q., Zhong, C., Mi, J.: Li-modified metal-organic frameworks for CO2/CH4 separation: a route to achieving high adsorption selectivity. J. Mater. Chem. 20(4) (2010)
Xu, X.C., Song, C.S., Andresen, J.M., Miller, B.G., Scaroni, A.W.: Preparation and characterization of novel CO2 “molecular basket” adsorbents based on polymer modified mesoporous molecular sieve MCM-41. Microporous Mesoporous Mater. 62(1–2), 29–45 (2003)
Xue, C., Zhong, C.: Molecular simulation study of hexane diffusion in dynamic metal-organic frameworks. Chin. J. Chem. 27(3), 472–478 (2009). doi:10.1002/cjoc.200990077
Zhang, L., Wang, Q., Liu, Y.-C.: Design for hydrogen storage materials via observation of adsorption sites by computer tomography. J. Phys. Chem. B 111(17), 4291–4295 (2007). doi:10.1021/jp0713918
Zheng, C., Liu, D., Yang, Q., Zhong, C., Mi, J.: Computational study on the influences of framework charges on CO2 uptake in metal–organic frameworks. Ind. Eng. Chem. Res. 48(23), 10479–10484 (2009). doi:10.1021/ie901000x
Zhou, M., Wang, Q., Zhang, L., Liu, Y.-C., Kang, Y.: Adsorption sites of hydrogen in zeolitic imidazolate frameworks. J. Phys. Chem. B 113(32), 11049–11053 (2009). doi:10.1021/jp904170s
Zukal, A., Mayerova, J., Cejka, J.: Alkali metal cation doped Al-SBA-15 for carbon dioxide adsorption. Phys. Chem. Chem. Phys. 12(20) (2010)
Acknowledgements
Financial support from the National Natural Science Foundation of China (Nos. 20936001 and 21176084), and Guangdong Natural Science Foundation (S2011030001366) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, D., Wu, Y., Xia, Q. et al. Experimental and molecular simulation studies of CO2 adsorption on zeolitic imidazolate frameworks: ZIF-8 and amine-modified ZIF-8. Adsorption 19, 25–37 (2013). https://doi.org/10.1007/s10450-012-9407-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-012-9407-1