Abstract
The crystallization of zeolite A was monitored by measuring the adsorption capacities of synthetic products. The influences of organic additives on the crystallization process and adsorption performances of zeolite were investigated. SDS (sodium dodecyl sulphonate), TWEEN (Tween-80), and PEG (poly(ethylene glycol)) shorten the induction period by reducing the interfacial energy while SCMC (sodium carboxymethylcellulose) can prolong the induction period by increasing the interfacial energy. TEA (triethanolamine) can also suppress the nucleation through reducing the effective supply of aluminum. All the organic additives but SCMC diminish the rate of crystal growth. CTAB (cetyltrimethylammonium bromide) causes the destruction of crystal structure and reduce the concentration of OH− ions. As a result, the rate of crystal growth is significantly reduced. Meanwhile, PAM (poly(acrylamide)), SDS, TWEEN, HMTA (hexamethylenetetramine), and PEG increase the viscosities of synthesis systems, thus, diminish the growth rate. PAM restrains the transformation of zeolite A crystal into hydroxysodalite one, therefore, tremendously improves the stability of crystals of zeolite A. In addition, PAM can promote the rates of n-hexane adsorption on zeolite 5A because of the impact of PAM on the crystal-size distributions of zeolite 5A.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Due to the extensive applications in catalytic, separation and ion exchange processes (Breck 1974), the synthesis control of zeolite has received continuous attention. In general, zeolite crystallization is focused on because of its notable influence on the performances of the synthetic zeolite products. Various materials including crystal seeds (Edelman et al. 1989), aged mother liquor (Gora and Thompson 1997), inorganic compounds (Sun et al. 2009) as well as organic compounds (Charnell, 1971; Basaldella and Tara, 1998; Kim et al., 2009) were used for governing the crystallization process or varying the crystal sizes of zeolite products. Controlled addition of organic additive to zeolite synthesis systems was most extensively investigated (Barrer 1981). Charnell (1971) first reported that the addition of triethanolamine to zeolite A synthesis gels led to the growth of larger crystals. Scott et al. (1990) and Morris et al. (1991, 1993) found that tertiary alkanolamines could chelate aluminum with its hydroxyl groups being directly involved in bonding to the metal ion, thus, affect the nucleation and crystallization rates. Moreover, the crystal sizes of zeolite A could be enlarged by controlling the number of nuclei. Basaldella and Tara (1998) also confirmed that triethanolamine could help produce larger crystal while studied the transformation of metakaolinite into zeolite A. Recent study on synthesis of large and uniform crystals of zeolite A was conducted by Yang et al. (2006) and concluded that triethanolamine increased the viscosity of the reaction system, and reduced the reactivity of aluminum species towards nucleation as well as crystal growth through forming chelated complex compounds. In addition, the crystallization of zeolite A with sodium dodecyl sulphate, cetyltrimethylammonium bromide, Nonidet P40, and poly(ethylene glycol) was investigated and the crystalline-growth curves were determined (Myatt et al. 1994).
In this paper, we study in detail the effect of different organic additives on the three crystallization stages with respect to induction stage, crystal growth stage, and stabilization stage, respectively. In addition, we attempt to exploit the effect of organic additives on adsorption performances of synthesized zeolite 5A.
2 Experimental
2.1 Materials
Sodium hydroxide, aluminum hydroxide, sodium silicate nonahydrate, calcium chloride, n-hexane, cyclohexane, triethanolamine (TEA), cetyltrimethylammonium bromide (CTAB), hexamethylenetetramine (HMTA), sodium dodecyl sulphonate (SDS) and sodium carboxymethylcellulose (SCMC) were obtained from Lingfeng Chemical Reagent Corporation (Shanghai, China) at the purities of >97 %. Tween-80 (Tween), p-octyl polyethylene glycol phenyl ether (OP), poly(ethylene glycol) (average molecular weight of 10,000) (PEG) and poly(acrylamide) (PAM) were provided by Sinopharm Chemical Reagent Co., Ltd (Shanghai, China) with >95 % purities.
2.2 Synthetic procedure
The hydrothermal syntheses of zeolite were carried out in a series of 100 ml batch Teflon autoclaves using sodium silicate and sodium metaaluminate as the silica and alumina sources, respectively. The sodium silicate and sodium metaaluminate solutions were prepared and then mixed with stirring (100 rpm) to form the hydrogels with the overall batch compositions of 2.5Na2O⋅Al2O3⋅2SiO2⋅150H2O⋅xM, where x is 0 for additive-free synthesis system and 0.03 for additive systems, M is TEA, CTAB, OP, PEG, HMTA, SDS, SCMC, TWEEN, or PAM. The preparation details of the samples of zeolites 4A and type 5A can be found in Ref. (Sun 2009).
2.3 Characterization
The adsorption capacities were determined after undergoing calcium ion exchanging by measuring the concentration changes of n-hexane in an n-hexane-cyclohexane-zeolite 5A system at 25 °C with the initial n-hexane concentration of around 0.03 % in weight (Sun et al. 2009). The adsorption capacities Q were expressed as m g g−1, indicating that the sample is of m grams adsorption capacity for n-hexane per gram zeolite 5A.
Powder X-ray diffraction (XRD) patterns of zeolite 4A products were measured on a Ricoh D/Max2550 X-ray diffractometer with CuKα radiation (40 kV and 100 mA). Data collection was carried out in the 2 theta ranging from 3 to 50°, with a step size of 0.02°. Crystal morphologies of zeolite 4A samples were determined by a JEOL JSM-6360LV scanning electron microscope (SEM), operating at 15 kV.
3 Results and discussion
3.1 General crystallization behavior of zeolite A
A synthesis experiment was carried out in the additive-free system, and the adsorption capacities of zeolite 5A products corresponding to different crystallization times were measured and presented in Fig. 1. It is clearly shown that the curve is in accordance with typical S-shaped crystallization curve (Myatt et al. 1994) and can be divided into three time regions.
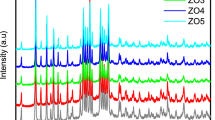
According to available mechanism, the crystallization of zeolite A can be explained as two stages: the initial induction period as well as the crystal growth period (Breck 1974). The induction period corresponds to the first region (R1) in Fig. 1. A linear increase in adsorption capacities of zeolite 5A products can be observed from R2 shown in Fig. 1. The SEM morphologies of zeolite 4A products at different crystallization times are also illustrated as well in Fig. 1 and the XRD patterns are indicated in Fig. 2. As far as the third region (R3) in Fig. 1 is concerned, further SEM and XRD analysis results prove that a part of zeolite A is converted to another phase. In this stage, the adsorption capacities show overall decrease of 15.85 % in following 240 min synthesis time. At 360 min, some plate crystals can be observed growing from the surfaces of cubic crystals. Moreover, four more diffraction peaks at 2θ=13.94°, 24.30°, 31.54°, and 34.64°, which are assigned to hydroxysodalite (S) crystals (Walton et al. 2001), appear in the XRD pattern from Fig. 2(e). In the present study, we define the third region as the stabilization period for zeolite A. During the stabilization period, the metastable zeolite A tends to convert to the thermodynamically more stable hydroxysodalite crystals in the hot alkaline crystallization solution. For optimizing the adsorption capacity of zeolite A, the zeolite products should be isolated from the crystallization solutions once the crystallization steps into the stabilization stage.
3.2 Effects of organic additives on the crystallization process of zeolite A
Three kinds of organic compounds such as surfactants (i.e., SDS, CTAB, TWEEN, and OP), organic amines (TEA and HMTA) as well as water-soluble polymer (PAM, SCMC, and PEG) were selected as additives. All the organics were introduced to the synthesis systems through dissolving them in the sodium metaaluminate solutions under heating. At last, the additives could be removed by calcination in air at higher than 400 °C in a muffle oven (Ruthven 1998). A thermogravimetric analysis also has been carried out as to confirm the removal of additives from zeolite products. In order to identify the weight change during TG measurement clearly, a sample of zeolite 4A was synthesized with larger amount of PAM (the overall compositions of 2.5Na2O⋅Al2O3⋅2SiO2⋅150 H2O⋅0.1PAM) and used for the test. The TG test result is shown in Fig. 3. From Fig. 3, the weight of zeolite 4A declines with an increase of temperature and finally reaches a platform under N2 flow and 410 °C. After the O2 flow being introduced, the weight of sample declines again because of the oxidative removal of the organic additive.
The influences of various additives on the adsorption capacities of zeolite 5A samples during crystallization process are illustrated in Fig. 4. These organics show different influences on the adsorption capacities of 5A samples. The same as additive-free system, all the additive systems undergo similar three-stage crystallization process. With the additions of different organics, the SEM images and XRD patterns of zeolite 4A samples in the stabilization period are shown in Figs. 5 and 6. The SEM images show no significant morphological difference. In Fig. 6, the diffraction peaks of hydroxysodalite crystal are highlighted with dashed line frames. The hydroxysodalite crystals also can be identified in XRD patterns of zeolite samples synthesized from several additive systems. According to the definition of three-stage crystallization process, we attempt to explain the effects of different organic additives on the induction period, growth period, and stabilization period, respectively.
3.2.1 Effects on the induction period
According to the simplified nucleation theory recommended by Van Hook (1961), the free energy change involved in the nucleation step can be expressed as:
where ΔG is the free energy change of the nucleation, σ is the interfacial energy, ν is the molar volume, and μ is the chemical potential.
Then, the rate of critical nuclei formation is expressed as Eq. (2):
where r n is the nuclei formation rate, c is the constant, α is the supersaturation, k is the Boltzmann constant.
The various influences of additives on induction periods are demonstrated in Fig. 7. Compared to the additive-free system, the synthesis systems in the presence of SDS, TWEEN, or PEG show shorter induction periods. Some consistent results about the influences of SDS and PEG on the induction have been reported by Myatt et al. (1994). According to Myatt et al., the effects on induction time of SDS or PEG can be increased as increase the amount of additive in synthesis system. With the low amount of SDS, TWEEN, or PEG, the induction time is reduced from 75 min to 60 min. In order to reveal the change of interfacial energy with the addition of organics, the surface tensions of all the synthesis systems were determined at 40 °C. Table 1 shows the influences of additives on the surface tensions of synthesis systems. SDS, TWEEN, and PEG reduce the surface tensions of synthesis systems, suggesting the decrease in the interfacial energy σ. In addition, the nuclei precursors are easier to generate in the micelle centers of some organics than the clear system due to the decrease in the interfacial energy. Many organic additives have been used for inducing the nucleation. In the presence of some surfactants, the surfactant micelles in synthesis system enable the zeolite nanoclusters to be assembled because of the affinity (Gu et al. 2010). Under the action of affinity, some parts of the micelles can insert into the barrier layer and reduce the nucleation free energy (Hu et al. 2011). TEA and SCMC, in contrast, suppress the nucleation of zeolite A, hence, prolong the induction period. The increases in surface tensions can be found as for the additions of TEA and SCMC. TEA can affect the nucleation by chelating aluminum with its hydroxyl groups being directly involved in bonding to the metal ions. The reduction in effective supply of aluminum for nuclei, in other words, reduction in c suppresses the nucleation. The existence of the complex has been confirmed by solution-phase 13C NMR spectroscopy (Scott, 1990) as well as 27Al NMR spectroscopy (Morris et al. 1991). As for SCMC, the longer nucleation period can be attributed to the increase in interfacial energy.
3.2.2 Effects on the rates of crystallization
In order to reveal the influences of additives on the rates of crystallization, the slopes of different crystallization curves corresponding to the crystal growth period were firstly obtained through the linear correlation. Assuming the slope for additive-free system is of 1, the slope ratios of additive systems to the additive-free system were calculated and shown in Fig. 8. A larger slope represents the larger rate of crystal growth. From the present study, all organic additives but SCMC reduce the rate of crystal growth to some extent. Some investigations concerned the influences of TEA on crystal growth of zeolite A have been done since 1970s (Charnell, 1971; Scott et al., 1990; Morris et al., 1991, 1993; Yang et al., 2006). TEA can significantly affect the rate of crystal growth by complexing, hereafter, releasing slowly aluminum in the synthesis system. As shown in Fig. 8, however, TEA displays a smaller effect in the growth rate than other compounds. The lowest growth rate can be found in the presence of CTAB and the influence mechanism can be explained from two aspects. On one hand, the quaternary ammonium cations cause the destruction as well as deformation of crystal structure which is formed in the growth period (Iwasaki et al. 1998; Roozeboom et al. 1983). On the other, OH− ions also take part in the crystal growth of zeolite A as the reactive species (Barthomeuf and Holderich 1990). The quaternary ammonium cations in crystallization system can reduce the effective concentration of OH− ions for the crystal growth. The overall rate of crystal growth, therefore, declines. A decrease in adsorption capacity of synthetic product with CTAB addition has been found and can also be attributed to the same destruction behavior of quaternary ammonium cations.
Furthermore, the viscosity of synthesis mixture can be improved by adding some kinds of organic compounds. The viscosities of different synthesis systems have been measured at 40 °C and the results are listed in Table 2. As conducted the synthesis experiments, we found that the synthesis system with additions of PAM, SDS, TWEEN, HMTA, and PEG showed higher viscosity than others. It is reasonable that an increasing in viscosity will reduce the transfer rate of silica and aluminum species reaching to the nuclei, as a result, reduce the crystal growth rate.
3.2.3 Effects on the stabilization period
When thermodynamically metastable zeolite A is kept in the mother liquor for extensive periods of time, it converts to the more stable hydroxysodalite (Walton et al., 2001; Subotić et al., 1980). The stability of zeolite A in crystallization mother liquor is evaluated by the decrease in adsorption capacity (D ac). D ac of zeolite products from different synthesis systems are defined using Eq. (3) and the results are shown in Fig. 9.
where D ac is the decrease in adsorption capacity, %, Q m is the adsorption capacity of the zeolite product with the highest relative crystallinity, and \(Q_{\mathrm{2\,h}}\) is the adsorption capacity of the product after 2 h of stabilization time.
As shown in Fig. 9, the D ac of SCMC and PEG adding systems are indeed similar to that of additive-free system. It should be noted that zeolite A products show smaller D ac, in other word, have higher stabilities in other seven organic additive systems as compared to additive-free system. Among these seven additives, PAM increases the stability of zeolite A to the greatest extent. Furthermore, we determined the adsorption capacities of two zeolite products at 16 h stabilization time from PAM and additive-free systems respectively. The adsorption capacity decreases for PAM and additive-free systems are 11.29 % and 31.71 % after 16 h of stabilization time. In addition, XRD patterns of 4A samples from PAM system are analyzed and presented in Fig. 10 for comparison with additive-free system. As indicated in Fig. 2 and Fig. 10, the zeolite sample from PAM system shows weaker peak intensities of hydroxysodalite crystal than that from additive-free system after the same stabilization time. It can be concluded that PAM show the significant inhibition effect on the transformation of zeolite A crystal into hydroxysodalite one in crystallization mother liquor. The stabilization effect of PAM on zeolite A is manifested in two ways: Firstly, we speculate that some zeolite A crystals with some structure defects are formed, during the competitive growing process, because of the temporary lacking of reactive species. The crystal structures with defects are instable and easier to deform or rearrange than the perfect crystal structures in the mother liquor. In accordance with what is observed in crystal growth period, the rate of crystal growth is suppressed and the amount of zeolite A crystals with structure defects is reduced by adding PAM. As a result, the stability of zeolite A in the mother liquor is improved. Secondly, the solvent effect of the synthesis system with addition of PAM (Coetzee and Ritchie 1969) changes the ion strength of the system which can influence the conversion rate of zeolite A to hydroxysodalite. As shown in Fig. 5, the PAM system seems to lead to more perfect crystals than other systems. As for CTAB, the influences on stability of zeolite A can be interpreted that the destruction effect of CTAB on crystal structures of zeolite A leads to the deformation of some crystal structures with defects and hereafter re-dissolution into the synthesis system. Similarly, the stability of zeolite A increase with a decrease in the amount of crystals with structure defects. According to the research results reported by Cheng et al. (2009), OP also plays a restraining role in the co-crystallization of MOR zeolite impurity during the FER zeolite crystallization.
3.3 Effects of organic additives on the adsorption performances of zeolite 5A
3.3.1 Effects on the adsorption capacities (Q m)
For each synthesis system, the adsorption capacity, Q m, is obtained by monitoring the adsorption capacities of crystallized products in system. All Q m corresponding to nine synthesis systems are illustrated in Fig. 11. Three synthesis systems with the additions of SDS, CTAB, or TWEEN show smaller Q m than that of additive-free system.
3.3.2 Effects on the adsorption rates
During each synthesis, the zeolite product which has the largest adsorption capacity was crushed and sieved. The rate of n-hexane adsorption on the sieved sample with particle-size of 100–120 mesh was determined as:
where r a is the adsorption rate, % min−1, Q 5 is the measured adsorption amount in the first five minutes of adsorption time.
The average adsorption rates of the zeolites 5A crystallized from different systems are presented in Fig. 12. SDS, TEA, TWEEN, OP and HMTA reduce adsorption rates of zeolites A. In contrast, PAM promotes the adsorption rates of zeolites A, therefore, can be introduced into synthesis system for optimizing the adsorption performance of zeolites 5A. The rates of normal paraffins adsorption on zeolites 5A can be governed by micropore diffusion or macropore diffusion depending on the pore diameter distributions of the zeolite samples (Ruthven 1984). The adsorption rates are obviously influenced by adding certain organic additives mainly because that these compounds change the crystal-size or the pore-size distributions of the crystal agglomerates. The crystal-size distributions of zeolite 5A samples are presented in Fig. 13. It is indicated that the sample synthesized from OP system has wider distribution range as compared to other synthesis systems. Meanwhile, the narrowest distribution range has been found as PAM is used as the additive.
4 Conclusions
SDS, TWEEN, and PEG are helping in shortening nucleation of zeolite A because they can reduce the interfacial energy of synthesis system. In contrast, SCMC can lengthen the induction period through increasing the interfacial energy and TEA can suppress the nucleation by reducing the effective supply of aluminum. All the organic compounds but SCMC diminish the rate of crystal growth. Among these organics, CTAB leads to the most reduction in rate of crystal growth because of the destruction of crystal structure and the decrease in the concentration of OH− ions. PAM, SDS, TWEEN, HMTA, and PEG diminish the growth rate by increasing the viscosities of synthesis systems. The stability of crystals of zeolite A can be improved by adding SDS, CTAB, TEA, TWEEN, OP, or HMTA. Especially, PAM shows a significant stabilization effect on zeolite A crystals. Several additives also have effect on crystal-size distributions of zeolite 5A. As a result, the n-hexane adsorption rate of the synthesized zeolite 5A is promoted by PAM.
Abbreviations
- SDS:
-
Sodium dodecyl sulphonate
- TWEEN:
-
Tween-80
- PEG:
-
Poly(ethylene glycol)
- TEA:
-
Triethanolamine
- SCMC:
-
Sodium carboxymethylcellulose
- PAM:
-
Poly(acrylamide)
- CTAB:
-
Cetyltrimethylammonium bromide
- HMTA:
-
Hexamethylenetetramine
- OP:
-
p-Octyl polyethylene glycol phenyl ether
- XRD:
-
X-Ray diffraction
- SEM:
-
Scanning electron microscope
- TG:
-
Thermogravimetric
References
Barrer, R.M.: Zeolites. Academic Production, London (1981)
Barthomeuf, D.E.G., Holderich, W.: Guidelines for Mastering the Proprieties of Molecular Sieves: Relationship Between the Physicochemical Properties of Zeolitic Systems and Their Low Dimensionality. Plenum, New York (1990)
Basaldella, E.I., Tara, J.C.: Modification of crystallite morphology during synthesis of LTA zeolite using triethanolamine as additive. Mater. Lett. 34, 119–123 (1998)
Breck, D.W.: Zeolite Molecular Sieves. Wiley, New York (1974)
Charnell, J.F.: Gel growth of large crystals of sodium A and sodium X zeolites. J. Cryst. Growth 8, 291–294 (1971)
Cheng, X.W., Wang, J., Guo, J., He, H.Y., Long, Y.C.: FER zeolite crystallized in THF–Na2O–SiO2–Al2O3–H2O reactant system containing catalytic amount of organic additives. Microporous Mesoporous Mater. 119, 60–67 (2009)
Coetzee, J.F., Ritchie, C.D.: Solute-Solvent Interactions. Dekker, New York (1969)
Edelman, R.D., Kudalkar, D.V., Ong, T., Warzywoda, J., Thompson, R.W.: Crystallization phenomena in seeded zeolite syntheses. Zeolites 9, 496–502 (1989)
Gu, F.N., Wei, F., Yang, J.Y., Lin, N., Lin, W.G., Wang, Y., Zhu, J.H.: New strategy to synthesis of hierarchical mesoporous zeolites. Chem. Mater. 22, 2442–2450 (2010)
Gora, L., Thompson, R.W.: Controlled addition of aged mother liquor to zeolite NaA synthesis solutions. Zeolites 18, 132–141 (1997)
Hu, J., Gao, F., Shang, Y.Z., Peng, C.J., Liu, H.L., Hu, Y.: One-step synthesis of micro/ mesoporous material templated by CTAB and imidazole ionic liquid in aqueous solution. Microporous Mesoporous Mater. 142, 268–275 (2011)
Iwasaki, A., Sano, T., Kiyozumi, Y.: Effect of additives on the growth behavior of silicalite crystal. Microporous Mesoporous Mater. 25, 119–126 (1998)
Kim, S.H., Park, M.B., Min, H.K., Hong, S.B.: Zeolite synthesis in the tetraethylammonium-tetramethylammonium mixed-organic additive system. Microporous Mesoporous Mater. 123, 160–168 (2009)
Morris, M., Dixon, A.G., Sacco, A. Jr., Thompson, R.W.: Investigation on the relative effectiveness of some tertiary alkanolamines in the synthesis of large-crystal zeolite NaA. Zeolites 13, 113–121 (1993)
Morris, M., Sacco, A. Jr., Dixon, A.G., Thompson, R.W.: The role of an aluminum-tertiary alkanolamine chelate in the synthesis of large crystal zeolite NaA. Zeolites 11, 178–183 (1991)
Myatt, G.J., Budd, P.M., Price, C., Hollway, F., Carr, S.W.: The influence of surfactants and water-soluble polymers on the crystallization of zeolite NaA. Zeolites 14, 190–197 (1994)
Roozeboom, F., Robson, H.E., Chan, S.S.: Laser Raman study on the crystallization of zeolites A, X and Y. Zeolites 3, 321–328 (1983)
Ruthven, D.M.: Principles of Adsorption & Adsorption Processes. Wiley, New York (1984)
Ruthven, D.M.: Characterization of zeolites by sorption capacity measurements. Microporous Mesoporous Mater. 22, 537–541 (1998)
Scott, G., Thompson, R.W., Dixon, A.G., Sacco, A., Jr.: The role of triethanolamine in zeolite crystallization. Zeolites 10, 44–50 (1990)
Subotić, B., Škrtić, D., Šmit, I.: Transformation of zeolite A into hydroxysodalite. I. An approach to the mechanism of transformation and its experimental evaluation. J. Cryst. Growth 50, 498–508 (1980)
Sun, H.: Study on high-performances adsorbent material for improving molecular scale management functions of naphtha. Ph.D. thesis, East China University of Science and Technology, Shanghai (2009)
Sun, H., Shen, B.X., Liu, J.C.: Liquid-phase adsorption kinetics of n-paraffins on 5A zeolite. Pet. Sci. Technol. 27, 1704–1714 (2009)
Van Hook, A.: Crystallization: Theory and Practice. Chapman & Hall, London (1961)
Walton, R.I., Millange, F., O’Hare, D., Davies, A.T., Sankar, G., Catlow, C.R.A.: An in situ energy-dispersive X-ray diffraction study of the hydrothermal crystallization of zeolite A. 1. Influence of reaction conditions and transformation into sodalite. J. Phys. Chem. B 105, 83–90 (2001)
Yang, X.B., Albrecht, D., Caro, J.: Revision of Charnell’s procedure towards the synthesis of large and uniform crystals of zeolites A and X. Microporous Mesoporous Mater. 90, 53–61 (2006)
Acknowledgements
The authors are grateful to the Ministry of Education of Republic of China for the financial support under the Research Fund for the Doctoral Program of Higher Education of China(RFDP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, H., Shen, B. Effects of organic additives on crystallization process and the adsorption performances of zeolite A. Adsorption 18, 103–111 (2012). https://doi.org/10.1007/s10450-012-9385-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-012-9385-3