Abstract
Tricuspid annuloplasty is a surgical procedure that cinches the valve’s annulus in order to reduce regurgitant blood flow. One of its critical parameters is the degree of downsizing. To provide insight into the effect of downsizing, we studied the annulus of healthy sheep during suture annuloplasty. To this end, we implanted fiduciary markers along the annulus of sheep and subsequently performed a DeVega suture annuloplasty. We performed five downsizing steps in each animal while recording hemodynamic and sonomicrometry data in beating hearts. Subsequently, we used splines to approximate the annulus at baseline and at each downsizing step. Based on these approximations we computed clinical metrics of annular shape and dynamics, and the continuous field metrics height, strain, and curvature. With these data, we demonstrated that annular area reduction during downsizing was primarily driven by compression of the anterior annulus. Similarly, reduction in annular dynamics was driven by reduced contractility in the anterior annulus. Finally, changes in global height and eccentricity of the annulus could be explained by focal changes in the continuous height profile and changes in annular curvature. Our findings are important as they provide insight into a regularly performed surgical procedure and may inform the design of transcatheter devices that mimic suture annuloplasty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tricuspid valve annuloplasty is a surgical procedure that is frequently performed to address leaking tricuspid heart valves.15,21 In patients with so-called functional tricuspid regurgitation, right ventricular remodeling dilates the tricuspid annulus and increases the valve’s orifice area.5,7,39,40 Due to the limited redundancy of the tricuspid valve leaflets, continued annular dilation eventually prevents full leaflet coaptation and allows for retrograde or regurgitant blood flow. The intention of tricuspid annuloplasty is to counteract the pathological dilation of the tricuspid valve annulus by cinching it via implantation of an annuloplasty device9,24 or via DeVega suture annuloplasty.6 Naturally, when repairing the tricuspid valve, the surgeon does not know the original size or shape of the native annulus and must estimate the proper degree of downsizing in the attempt to re-establish normal valve function.10 Some recommendations go so far as to suggest downsizing the annulus beyond the native annular orifice area during so-called “overcorrected” or “undersized” tricuspid valve annuloplasty.34 The consequence of too little downsizing is an incomplete repair with residual leakage. The consequences of too much downsizing are currently not clear but could be multi-fold by affecting any of the valvular components, e.g., the valve annulus, the valve leaflets and chordae tendineae, as well as the peri-valvular tissue. Studying the effect of annular downsizing is particularly pertinent provided (i) that recent guidelines are asking for a more aggressive approach to tricuspid valve repair,4,21 resulting in more frequent procedures, and (ii) the recent, surgical trend toward larger degrees of downsizing.9,18 Given the central role of the tricuspid annulus as the interface between the tricuspid leaflets12,36 and the peri-valvular myocardium of the right ventricle23 and the right atrium,16 it is a natural starting point to study the consequences of annular downsizing.
The objective of our present work is to use DeVega suture annuloplasty as an experimental platform that allows us to study the effect of annular downsizing using a within-subject design.19 We have previously performed similar experiments in an animal model of acute right heart failure with significant tricuspid regurgitation.20 However, there were limitations to our previous study, which our present work will address. First, we previously performed only two cinching steps, which did not allow evaluating the potentially non-linear relationship between the degree of downsizing and mechanical effects on the annulus. Second, we performed these experiments in animals with tricuspid regurgitation, which did not allow studying the effect of annular downsizing in isolation. Specifically, during downsizing, in our previous study, we simultaneously altered the size of the annulus and diminished tricuspid regurgitation. Thus, changes in annular shape and dynamics may have been due to mechanical and/or hemodynamic effects. Here, we perform experiments on healthy animals and use five cinching steps. Therefore, the data arising from this current work will allow us to separate mechanical and hemodynamic effects and make direct observations on the mechanical effects of downsizing on the annulus alone.
Methods
Animal Experiments
We performed all animal procedures in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for Care and Use of Laboratory Animals prepared by the National Academy of Science and published by the National Institutes of Health. All procedures were approved by our local Institutional Animal Care and Use Committee (Spectrum Health IACUC #: 18-01).
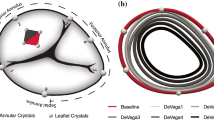
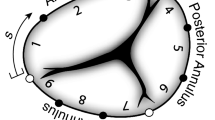
Detailed descriptions of the surgical procedures are available in our previous publications.19,20 In short, we intravenously anesthetized ten (n=10) Dorset male sheep (\(60 \pm 3\) kg) with propofol before intubating and mechanically ventilating the animals. Under anesthetic maintenance via isoflurane and fentanyl, we performed a mid-sternotomy and exposed the heart. While on cardiopulmonary bypass and on the beating heart, we performed an atriotomy and placed the suture annuloplasty around the tricuspid annulus as described before,20 see Fig. 1. To this end, we used 2-0 polypropylene sutures in two layers around the tricuspid valve orifice and anchored the sutures with pledgets at the antero-septal commissure and the mid-septal annulus. For external control of the degree of downsizing, we externalized the annuloplasty suture through the antero-posterior commissure to a tourniquet. Next, we implanted six, 2mm sonomicrometry crystals (Sonometrics Corp, London, Ontario, Canada) around the tricuspid annulus with one crystal at each of the three commissural and mid-commissural points, subdividing the annulus into six regions, see also Fig. 2. For a separate study, we also implanted 1mm sonomicrometry crystals on each tricuspid valve leaflet. We externalized the crystal wires for data acquisition through an atriotomy. For hemodynamic measurements, we additionally placed pressure transducers (PA4.5-X6; Konigsberg Instruments, Inc, Pasadena, CA) in the left ventricle, right ventricle, and right atrium. After these procedures, we weaned the animal off cardiopulmonary bypass and recovered them for 30 min under anesthesia while their hemodynamics stabilized. During this period and during data acquisition, we also intravenously administered lidocaine to prevent ventricular ectopic beats.
(a) We implanted one sonomicrometry crystal at each commissure and mid-commissural point, dividing the annulus into six segments. Markers 1–3, 3–5, 5–1 span the anterior, posterior, and septal annulus, respectively. The end-points for the suture annuloplasty were the antero-septal commissure and the mid-septal crystal (dashed line). (b) Illustrates the average annular shape for baseline and for each subsequent downsizing step, i.e., DeVega1–5.
Data Acquisition
After the hemodynamics normalized, we recorded hemodynamic data, sonomicrometry data, and echocardiographic data for at least three cardiac cycles under baseline conditions. Once recorded, we snared the externalized sutures by 5 mm to reduce the annular orifice area in the first downsizing step (DeVega1). After hemodynamics had stabilized once again, we recorded an additional set of data. We repeated this sequence for a total of five downsizing steps (DeVega1–5) each time snaring the sutures by 5 mm, see Fig. 3. Upon completion of the experiments, we euthanized the animals by administering sodium pentothal and potassium chloride. Postmortem, we dissected the hearts and verified proper crystal positioning.
Annular downsizing progressively increased the transducer-derived transvalvular gradient during filling (p < 0.001). (a) The average transvalvular gradient throughout the cardiac cycle calculated as the right ventricular pressure minus the right atrial pressure with standard error. (b) Zoom-in on the gradient during filling where it is clear that progressive downsizing increased the transvalvular gradient. Note, errors were omitted for visual clarity. End-diastole (ED), end-isovolumetric contraction (EIVC), end-systole (ES), end-isovolumetric relaxation (EIVR).
Spatial Annular Approximation and Temporal Interpolation
Sonomicrometry uses a least-squares triangulation procedure to determine the temporospatial distribution of small ultrasonic crystals in the beating heart of our experimental animals. We have extensively reported on our methodology to fit least-squares cubic splines to approximate the annulus based on these discrete marker coordinates, both in the mitral valve29,30,33 and the tricuspid valve.31,32 In our previous and present work, we used splines to represent the tricuspid annulus because, (i) we have shown that spline approximations provide more accurate clinical metrics of annular shape than simple linear interpolation of inter-crystal spaces, and (ii) through the order of the splines we can ensure sufficient continuity to compute strain and curvature fields along the annulus.8
In brief, we performed the following minimization problem,
where n is the number of annular crystals, \(\varvec{c}_n(s,t)\) are cubic spline segments, \(\chi _n\) are our physical marker coordinates, s is the normalized arc-length parameter, t is time, and \(\lambda\) is a penalty parameter with which we can control the smoothness of the resulting curve. Thus, once performed, the minimization problem provided a smooth cubic spline representing the tricuspid annulus at each time point throughout the cardiac cycle.
Because heart rates varied between animals while the data acquisition rate remained constant at 128 Hz, we aligned data sets for all clinical metrics and field data before averaging them between animals, see the “Clinical and Field Metrics” section for specifics on how we computed those metrics. To this end, we divided these temporal data into four segments throughout the cardiac cycle based on the following time points: end-diastole (ED), end-isovolumic contraction (EIVC), end-systole (ES), and end-isovolumic relaxation (EIVR). Subsequently, we normalized the length of each segment, resampled the data uniformly, averaged the segments between animals, rescaled the data, and reassembled the segments into full temporal evolutions.
The annular area decreased with each downsizing step and the dynamics became suppressed. Curves show the average area change throughout the cardiac cycle with standard error for baseline and each downsizing step, i.e., DeVega1–5. End-diastole (ED), end-isovolumetric contraction (EIVC), end-systole (ES), end-isovolumetric relaxation (EIVR).
Clinical and Field Metrics
We report on annular area, perimeter, global height, and eccentricity as classic, clinical metrics of annular shape and dynamics. We computed area as the inscribed area of the cubic spline, \(\varvec{ c}(s,t)\), approximating the six annular crystals as described in “Spatial Annular Approximation and Temporal Interpolation” section. To this end, we first projected the spatial curves representing the annulus onto the curves’ best-fit planes. On those same projected curves, we computed the perimeter via the arc-length integral and eccentricity by means of identifying a best-fit ellipse and using standard formulae. Finally, we calculated global height as the maximum orthogonal distance (to the best-fit plane) between any two points of the spatial annulus curve.38
To report continuous height changes along the tricuspid annulus in response to annular downsizing, we first computed distance vectors between the annuli at DVn (with \(n \in [1,5]\)) and baseline (BSL) as a function of the arc-length parameter s and time t, i.e.,
Next, we projected those vectors onto the surface normal to the curve’s least-squares plane and reported their lengths.
We computed DeVega strain, \(E_{DVn}(s,t)\), between DVn and BSL, by first determining the tangent vectors to each annulus, again as a function of t and s, i.e., \(\varvec{t}(s,t) = \partial _s \varvec{c}(s,t)\). Next, we computed DeVega strain as
where \(\lambda _{DVn}=|\varvec{t}_{DVn}(s,t)|/|\varvec{t}_{BSL}(s,t)|\). Similarly, we computed cycle strain (\(E_{CC}\)), i.e., strain throughout the cardiac cycle, as
where \(\lambda _{CC}=|\varvec{t}(s,t)|/|\varvec{t}(s,t_{ED})|\) with \(\varvec{t}(s,t)\) being the tangent vectors to \(\varvec{c}_{DVn/BSL}(s,t)\). Thus, for cycle strain, we chose ED as the reference configuration.
Annular area and area changes decreased significantly after downsizing via DeVega (DV). (a) Average diastolic annular area decreased linearly with each downsizing step (p < 0.001). (b) Using annular area change throughout the cardiac cycle as a surrogate for quantifying annular dynamics, annular dynamics decreased non-linearly as a function of degree of downsizing (p < 0.001). After the first two downsizing steps (DeVega1 and DeVega2) further downsizing did not reduce annular dynamics more. Note, bars indicate standard error and multiple comparisons were performed using Tukey–Kramer.
Finally, we computed curvature change as the relative change in curvature at DVn relative to BSL. To this end, we calculated the curvature
for \(X \in [BSL,DVn]\) and compute curvature change as
Statistics
We report all data in tables as \({\rm mean} \pm 1\) standard deviation, while we report data in figures as mean ± standard error. We performed all statistical analyses via 1-way or 2-way, repeated measure ANOVA in Matlab (R2017b) and the multi-comparisons using Tukey–Kramer, also in Matlab. We considered p < 0.05 as statistically significant.
Results
All animals recovered well from surgery and were successfully weaned off cardiopulmonary bypass. Table 1 summarizes hemodynamic data at baseline and after each downsizing step.
Figure 4 shows the average transducer-derived, trans-valvular pressure gradient between animals for each downsizing step. Note, that we used atrial pressure as the reference. Thus, during diastole, a pressure drop across the valve results in a negative gradient. The figure illustrates that hemodynamics were stable and comparable between steps. However, during diastole it becomes apparent that downsizing increased the transvalvular gradient. Specifically, the gradient increased from \(-\,7.43 \,\pm\) 2.87 to \(-\,11.36\, \pm\) 5.47 mmHg between baseline and DeVega5. Most of this gradient is due to the DeVega3-5 downsizing steps, while the first two downsizing steps affect the gradient only marginally. Although ANOVA shows that the gradient changed with downsizing (p < 0.001), the increase in gradient reached significance only between baseline and DeVega5 (p = 0.048). The transducer-derived transvalvular gradient is not to be confused with the echo-derived transvalvular gradient, which we report among other hemodynamic metrics in Table 1. Those values are smaller with the largest value reaching 3.343 ± 1.78 mmHg at DeVega5.
Figure 5 depicts annular area as a function of time for baseline and each downsizing step. Annular area under baseline conditions changed throughout the cardiac cycle with the area being the largest during diastole and the smallest during systole. For a more complete discussion on normal annular dynamics, see Ref. 32. After downsizing, annular size and annular dynamics markedly decreased (p < 0.001 for both, via 1-Way ANOVA).
We quantitatively analyze both diastolic area and changes in annular area throughout the cardiac cycle in Figs. 6a and 6b, respectively. We found that diastolic area decreased significantly (p < 0.05) between all steps, except for the second downsizing step. Additionally, using annular area change throughout the cardiac cycle as a surrogate for annular dynamics, we found significant difference due to the second and third downsizing step only. Thus, the effect of downsizing on annular dynamics diminished after the third downsizing step.
In addition to area changes, we also report on three other clinical metrics in Table 2: annular perimeter, annular eccentricity, and global annular height. Of the three, annular perimeter and eccentricity changed with annular downsizing (p < 0.001 and p = 0.01, respectively). Global annular height did not change significantly according to 1-way ANOVA (p = 0.15). In detail, perimeter decreased monotonically with each downsizing step, i.e., DeVega1–5. On the other hand, eccentricity underwent non-linear changes with increased downsizing. Eccentricity decreased for DeVega1–3, which implies that the annulus first became more circular, but then increased for DeVega4-5, implying that the annulus became more elliptical again.
We identified the mechanisms of annular downsizing by computing DeVega strain, i.e., the strain between each downsizing step and baseline at ED. Figure 7 demonstrates the average strain for the anterior, posterior, and septal annulus. We found that annular strain was significantly different between regions (p < 0.001) and between downsizing steps (p < 0.001). Specifically, the annulus was compressed during each DeVega step, with the anterior segment shortening the most. On the other hand, the septal and posterior regions compressed approximately the same, but less than the anterior region.
Annular downsizing via DeVega (DV) was driven primarily by compressive strains in the anterior annulus and secondarily by compressive strains in the posterior and septal annulus. Regional strains were measured relative to baseline at end-diastole. 2-Way ANOVA reveals difference with degree of downsizing (p < 0.001) and region (p < 0.001).
We identified the mechanism of reduced annular dynamics by depicting tangential strain over the cardiac cycle for baseline and each DeVega step. Figure 8 illustrates that annular contraction under baseline conditions was driven primarily by the anterior and posterior segments. Moreover, we found that the magnitude of the anterior segment compression progressively increased for each downsizing step to the point where it became positive in DeVega3–5. It is this change in anterior dynamics that rendered the annulus less contractile overall, see Fig. 6b. In contrast, the septal and posterior dynamics changed only marginally with each downsizing step.
Reduction in annular dynamics was predominantly driven by reduced contractility of the anterior annulus. (a) Annular contraction was driven by contraction of the anterior and posterior annulus as indicated by negative, i.e., compressive strains, in those regions. On the other hand, the septal annulus expanded during systole. (b–f) Progressive downsizing suppressed primarily the contraction of the anterior annulus.
To analyze changes from baseline to DeVega1–5 in a spatially continuous manner, we computed continuous DeVega strain, height change, and curvature change at ED for an averaged annulus between all animals. Figure 9a depicts DeVega strain along the annulus for each downsizing step. These data confirm that downsizing was driven by changes in the anterior annulus (between markers 1 and 3) and to a lesser degree by changes at the posterior-septal commissure (around marker 5).
Furthermore, we found that the annulus changed its height profile during downsizing. In Fig. 9b we show relative height changes, i.e., where the annulus increased and decreased in height relative to its best-fit plane. We found that the annulus raised its profile at the antero-posterior annulus and along the septum, while dropping it everywhere else. Those changes were qualitatively consistent between DeVega steps and increased in magnitude between DeVega 1–5. In other words, the annulus folds out of plane as its perimeter is reduced.
Field metrics strain, height change, and curvature change (all relative to baseline) reveal distinct spatial patterns that are driven by annular downsizing. (a) Compressive tangential strain in the anterior and posterior annulus drive annular downsizing. (b) During downsizing, relative height changes revealed a periodic out-of-plane bending of the annulus with peaks in the anterior and posterior annulus and valleys in the mid-septal annulus and the antero-posterior commissure. (c) Curvature overall increases during downsizing owing to the overall size reduction of the annulus. Focal increases in curvature at markers 3 and 5 may be interpreted as the hinges of the annular shape changes during downsizing.
Similarly, we found that the annulus changed its shape between baseline and each downsizing step. To characterize these changes, we depict change in curvature between baseline and DeVega1–5 in Fig. 9c. Negative change in curvature means that the annulus straightened locally, while positive change in curvature means that the annulus became more acute. We identified the antero-posterior (marker 3) and the postero-septal commissures (marker 5) as notable regions of increased curvature; they essentially function like hinges at which the annulus “kinks” as it is being downsized. Data in Figs. 9b and 9c are supported by quantitative, reduced data in Table 3 and 4, respectively.
In Table 3, we identify average height for each annular segment. We found that height changes with DeVega (p < 0.001) by increasing in some regions (2–3, 5–6) and decreasing in all others, reflecting our findings in Fig. 9. The magnitude of those changes increased consistently (with few exceptions) with each downsizing step. This finding contradicts that of global height, where we did not find a significant difference. This difference is due to the global vs. local nature of the two metrics and illustrates the need for detailed, spatially-resolved metrics. In Table 4, we list the average curvature for each annular segment, i.e., between marker 1 and 2, 2 and 3, etc. First, we found that average curvature differed significantly between regions (p < 0.001) and between downsizing steps (p < 0.001). Specifically, we found that average curvature increased in all regions as annular size decreased. Focal changes at markers 3 and 5, as seen in Fig. 9, were averaged out by this current analysis, again, illustrating the value of spatially-resolved metrics.
Discussion
Tricuspid annuloplasty is a frequent, but imperfect surgical procedure that aims at re-establishing the function of leaking tricuspid valves. Here, we studied how the degree of downsizing affects the tricuspid annulus. To this end, we used suture annuloplasty as a technique that allowed us to externally cinch the annulus in the beating hearts of ten sheep. We downsized the annulus in five steps, i.e., DeVega1–5, while recording sonomicrometry data and hemodynamic data. All animals recovered well from the procedure and we were able to collect complete data on all subjects.
The Effect of Downsizing Via Hemodynamic Metrics
One major concern of annular downsizing is creating significant transvalvular gradients during ventricular filling that would oppose diastolic function. We found that downsizing indeed increased transducer-derived pressure gradients by as much as 4 mmHg for the largest downsizing step (DeVega5). In contrast, we found that when the transvalvular gradient was measured via echo, it increased by approximately 2.3 mmHg, leading to a total gradient of 3.3 mmHg. Thus, even for the most aggressive downsizing step, transvalvular gradients remained below the clinically significant mark of 5 mmHg.
The Effect of Downsizing Via Clinical Metrics
The annulus provides the interface between the tricuspid valve leaflets and the surrounding peri-valvular myocardium of the right ventricle and the right atrium. Thus, the shape and dynamics of the annulus are strongly reflective of changes in the boundary conditions of the leaflets and thus likely affect the leaflets’ mechanics.2,3,26 Similarly, the shape and dynamics of the annulus reflect the local mechanics of the surrounding myocardium. Hence, knowledge of the annular changes during downsizing is also a critical determinant of right heart function. Indeed, we recently reported that annular downsizing affects right ventricular epicardial mechanics in normal sheep.19 Here, we first identified changes in the tricuspid annulus via classic, clinical metrics: area, perimeter, global height, and eccentricity. Naturally, we found that annular downsizing reduced annular area. Importantly, we could demonstrate that our successive cinching of the suture produced a strictly linear reduction in area. This area reduction was accompanied by a marked reduction in annular dynamics, which depended non-linearly on the degree of area reduction. This finding is important as it implies that annular reduction may come at the cost of losing annular dynamics and may thus disrupt the natural dynamics of the right ventricle and the tricuspid leaflets; the latter of which we recently reported on in healthy animals.22 Not unexpectedly so, changes in annular perimeter closely resembled those of area. In contrast, we found that global annular height and eccentricity changed non-monotonically with area reduction. Again, this is important because it may imply that annular downsizing disrupts the natural state of the right ventricle and leaflets and, through mechanobiological pathways, may induce (mal-)adaptive remodeling in those tissues.11 In summary, we showed via clinical metrics that annular downsizing does not only change the shape of the tricuspid annulus, but also reduces its dynamics in healthy animals.
The Effect of Downsizing Via Field Metrics
While clinical metrics of annular shape and dynamics have been and are useful, they are inherently limited in their information content. We used field metrics to identify mechanistically how downsizing changes annular shape and dynamics. Specifically, we took advantage of the C2-continuity of our cubic spline approximations to calculate the continuous metrics: height, strain, and curvature of the annulus. We have used these techniques previously to characterize the mitral and the tricuspid annulus in healthy sheep models and disease sheep models.29,31,32 Using these metrics, again, in our current study we were able to identify the local annular changes that led to a reduction in area and perimeter, changes in height, and changes in shape. In detail, we were able to use strain to identify the anterior annulus as the primary source of area reduction and reduced contractility. Similarly, we found that very focal changes in annular curvature resulted in shape changes during downsizing. Knowing the locations of what we called “hinges” may be critical for designing medical devices that mimic suture annuloplasty, where those regions may become areas of increased stress and thus potential fatigue failure loci. Finally, we found that the annulus changed its height profile through periodic reduction and increase in height, i.e., peaks and valleys, along its perimeter. These changes, as pointed out earlier, likely affect the mechanics of the tricuspid leaflets as the annulus may be considered the proximal boundary of the leaflets. Also, similar to focal regions of increased/decreased curvature, knowing where those peaks and valleys are may help optimize medical devices and surgical procedures.
Correlation Between Our Findings and Annular Microstructure
The tricuspid valve annulus is a heterogeneous structure whose composition varies along its length. Its makeup depends on the peri-annular tissue, with which the annular tissue shares characteristics, e.g., septal myocardium differs from lateral wall myocardium and so does the annular tissue bordering those muscles.1,37,25 Additionally, the annular tissue, through mechanobiological pathways, has likely evolved to meet and accommodate the local mechanical demands, which may additionally explain variations in its structure and composition. For example, Basu et al found in porcine tissue that annular tension, a measure for the normalized kinetic interaction between leaflets and annulus, is highest in the septal segment of the annulus.2 Such higher demand may translate into variations of the annulus’ mechanical properties along its length as measured in vitro. Again in porcine tissue, Basu et al found that the stiffness of the annular tissue varies spatially.3 They found that the annulus is stiffest in the septal region, which the authors successfully correlated to the highest collagen content of all annular regions. Also, Paul et al and Madukauwa-David et al, in sheep and humans, respectively, found that suture pull-out forces are highest in the septal and antero-septal regions, again, demonstrating a correlation between mechanical metrics and the collagen content of the tissue.17,28 The reported variations in annular composition along the annulus length and the associated increased stiffness and strength, may partially explain our findings. We found that the anterior annulus underwent the largest compression during annular downsizing, which may be related to its smaller collagen content when compared to the septal annulus. This increased compression may subsequently be theorized to also lead to lower dynamics. However, it is important to keep in mind that the DeVega procedure, as studied in our work, spares portions of the septal annulus, which may also be responsible for the reduced shortening of the septal annulus when compared to the anterior and posterior segments, see Fig. 1.20
Comparison to Our Previous Study in Animals with Acute Right Heart Failure
Before, we have studied the effect of annular downsizing on tricuspid annular shape and dynamics in animals with acute right heart failure and tricuspid regurgitation.20 In this previous study, we found that annular downsizing did not affect annular dynamics or 3D geometry (as measured via the clinical metric annular height). Importantly, in both our previous study and our current study, the size of the tricuspid valve annulus was reduced significantly beyond the normal annulus. Thus, both studies report on comparable degrees of downsizing. One important difference being that in our previous study, hemodynamic effects, due to the diminishing of tricuspid valve regurgitation, and mechanical effects could not be isolated. On the other hand, in our current study, because the animals were healthy, only the mechanical effects were present. Interestingly, in our current study, we did find that annular dynamics changed (decreased) in contrast to our earlier finding in diseased animals. This reduction was reflected in both percentage area change throughout the cardiac cycle and strain throughout the cardiac cycle. We also identified the anterior annulus as the region where most dynamics were lost. The non-congruency of our data may imply that the reduction of tricuspid regurgitation via annuloplasty may have a positive effect on annular dynamics and overcome the diminishing effect of compressing the annulus during downsizing.
Potential Impact on Tricuspid Valve Repair Techniques and Devices
DeVega annuloplasty has been repeatedly criticized for its sub-par performance when compared to device-based annuloplasty.13,27 However, recent reports indicate that lack of standardization and too conservative downsizing may have negatively biased these data.34 More recent publications suggest that DeVega annuloplasty, after various incremental improvements, may be more successful than previously thought. In the future, surgical tricuspid valve repair will likely be replaced with less invasive, catheter-based approaches, which will likely resemble DeVega suture annuloplasty. Thus, improvements in suture annuloplasty and information gathered on the technique are going to be pivotal to the successful design of catheter-based technologies. The information presented in our current work will serve the medical device community in two ways: i) our data illustrates the spatially-heterogeneous mechanisms through which DeVega suture annuloplasty successfully downsizes the annulus, and ii) our data identifies areas of increased strain and curvature that may indicate locations of heightened risk for device failure. Thus, our data may be important not only to our understanding of DeVega suture annuloplasty, but also to future, catheter-based repair technologies for the tricuspid valve annulus.
Limitations and Future Directions
This study is naturally limited by a number of shortcomings. First off, this study was performed on animals, not human patients. Thus, any extrapolation of our findings must be done with care. Furthermore, we performed this study on healthy animals without tricuspid regurgitation. Hence, we did not evaluate the surgical efficacy of DeVega suture annuloplasty. Instead, our goal was to identify the effects of suture annuloplasty on the tricuspid annulus only, without the confounding effects of valvular or right heart disease. Importantly, in disease, the tricuspid annulus dilates primarily in the anterior, antero-posterior regions. Thus, DeVega annuloplasty may have spatially heterogeneous effects beyond what we observed in healthy animals in our current study.20 Because, we limited our analysis to the annulus only, we did not report or identify changes in the right ventricle or the leaflets. Those analyses are reserved for future studies. Specifically, we suggest numerical studies of full valve complexes which explore the effect of annular changes on the leaflets.14,35 From a technological perspective, we based our analysis on spline approximations to six sonomicrometry crystals. Although, our technique provides significant advantages over other, non-invasive imaging-based techniques with higher spatial resolution (due to the fiduciary character of our markers), we had to make assumptions about the inter-marker spaces. Specifically, here we assumed that the annulus behaved smoothly between markers and may thus be represented with cubic splines. While this assumption is supported by visual and echocardiographic observations, it should be kept in mind when interpreting our data.
Conclusion
We reported for the first time on the shape and dynamics of the healthy tricuspid annulus following downsizing via DeVega suture annuloplasty. We found that annular downsizing via DeVega suture annuloplasty resulted in complex shape changes measured via clinical metrics: annular area, perimeter, global height, and eccentricity. Employing the field metrics height, strain, and curvature, we identify the local mechanisms that resulted in the annular changes. Specifically, we found that annular downsizing was primarily driven by compressive strains in the anterior annulus, and secondarily by compressive strains in the septal and posterior annulus. Changes in annular dynamics, which we observed via reduced annular area contraction throughout the cardiac cycle following downsizing, were explained by a reduction in contractility of the anterior annulus. Finally, we found that non-linear changes in global annular height and eccentricity with degree of downsizing were driven by focal changes in annular height and curvature. Our findings will be critical to our basic understanding of DeVega suture annuloplasty, which is regularly performed in clinical practice. Moreover, understanding the role of the annulus during downsizing will provide critical information for future studies on right ventricular mechanics and leaflet mechanics during annuloplasty. Finally, our findings may be important to the design of transcatheter technologies that aim to mimic DeVega suture annuloplasty. Specifically, our data provide targets for technologies aimed toward mimicking traditional suture annuloplasty and may inform device design by highlighting areas of increased deformation and thus potential damage nucleation sites.
References
Anderson, R. H., S. Y. Ho, and A. E. Becker. Anatomy of the human atrioventricular junctions revisited. Anat. Rec. 260(1):81–91, 2000. https://doi.org/10.1002/1097-0185(20000901)260:1<81::AID-AR90>3.0.CO;2-3.
Basu, A., and Z. He. Annulus tension on the tricuspid valve: an in-vitro study. Cardiovasc. Eng. Technol. 7(3):270–279, 2016. https://doi.org/10.1007/s13239-016-0267-9.
Basu, A., C. Lacerda, and Z. He. Mechanical properties and composition of the basal leaflet-annulus region of the tricuspid valve. Cardiovasc. Eng. Technol. 9(2):217–225, 2018. https://doi.org/10.1007/s13239-018-0343-4.
Benedetto, U., G. Melina, E. Angeloni, S. Refice, A. Roscitano, C. Comito, and R. Sinatra. Prophylactic tricuspid annuloplasty in patients with dilated tricuspid annulus undergoing mitral valve surgery. J. Thorac. Cardiovasc. Surg. 143(3):632–638, 2012. https://doi.org/10.1016/j.jtcvs.2011.12.006.
Casa, L. D. C., J. R. Dolensky, E. M. Spinner, E. Veledar, S. Lerakis, and A. P. Yoganathan. Impact of pulmonary hypertension on tricuspid valve function. Ann. Biomed. Eng. 41(4):709–724, 2013. https://doi.org/10.1007/s10439-012-0713-2.
de Vega Sanromán, N. G. La anuloplastia selectiva, regulable y permanente. Una técnica original para el tratamiento de la insuficiencia tricúspide. Cirugía Cardiovasc. 19(4):349–350, 2012. https://doi.org/10.1016/s1134-0096(12)70015-3.
Dreyfus, G. D., R. P. Martin, K. M. J. Chan, F. Dulguerov, and C. Alexandrescu. Functional tricuspid regurgitation: a need to revise our understanding. J. Am. Coll. Cardiol. 65(21):2331–2336, 2015. https://doi.org/10.1016/j.jacc.2015.04.011.
Eckert, C. E., B. Zubiate, M. Vergnat, J. H. Gorman, R. C. Gorman, and M. S. Sacks. In vivo dynamic deformation of the mitral valve annulus. Ann. Biomed. Eng. 37(9):1757–1771, 2009. https://doi.org/10.1007/s10439-009-9749-3.
Ghoreishi, M., J. M. Brown, C. E. Stauffer, C. A. Young, M. J. Byron, B. P. Griffith, and J. S. Gammie. Undersized tricuspid annuloplasty rings optimally treat functional tricuspid regurgitation. Ann. Thorac. Surg. 92(1):89–96, 2011. https://doi.org/10.1016/j.athoracsur.2011.03.024.
Huffman, L. C., J. S. Nelson, A. N. Lehman, M. C. Krajacic, and S. F. Bolling. Identical tricuspid ring sizing in simultaneous functional tricuspid and mitral valve repair: a simple and effective strategy. J. Thorac. Cardiovasc. Surg. 147(2):611–614, 2014. https://doi.org/10.1016/j.jtcvs.2013.01.027.
Humphrey, J. D., E. R. Dufresne, and M. A. Schwartz. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 15(12):802–812, 2014. https://doi.org/10.1038/nrm3896.
Khoiy, K., D. Biswas, T. N. Decker, K. T. Asgarian, F. Loth, and R. Amini. Surface strains of porcine tricuspid valve septal leaflets measured in ex vivo beating hearts. J. Biomech. Eng. 138(11):111006, 2016. https://doi.org/10.1115/1.4034621.
Khorsandia, M., A. Banerjeeb, H. Singhb, and A. R. Srivastava. Is a tricuspid annuloplasty ring significantly better than a de Vega’s annuloplasty stitch when repairing severe tricuspid regurgitation? Interact. Cardiovasc. Thorac. Surg. 15(1):129–135, 2012. https://doi.org/10.1093/icvts/ivs070.
Kong, F., T. Pham, C. Martin, R. McKay, C. Primiano, S. Hashim, S. Kodali, and W. Sun. Finite element analysis of tricuspid valve deformation from multi-slice computed tomography images. Ann. Biomed. Eng. 46(8):1112–1127, 2018. https://doi.org/10.1007/s10439-018-2024-8.
Lee, C. H., D. W. Laurence, C. J. Ross, K. E. Kramer, A. R. Babu, E. L. Johnson, M. C. Hsu, A. Aggarwal, A. Mir, H. M. Burkhart, R. A. Towner, R. Baumwart, Y. Wu, C. H. Lee, D. W. Laurence, C. J. Ross, K. E. Kramer, A. R. Babu, E. L. Johnson, M. C. Hsu, A. Aggarwal, A. Mir, H. M. Burkhart, R. A. Towner, R. Baumwart, and Y. Wu. Mechanics of the tricuspid valve-from clinical diagnosis/treatment, in-vivo and in-vitro investigations, to patient-specific biomechanical modeling. Bioengineering 6(2):47, 2019. https://doi.org/10.3390/bioengineering6020047.
Leng, S., M. Jiang, X. D. D. Zhao, J. C. Allen, G. S. Kassab, R. Z. Z. Ouyang, J. L. L. Tan, B. He, R. S. S. Tan, and L. Zhong. Three-dimensional tricuspid annular motion analysis from cardiac magnetic resonance feature-tracking. Ann. Biomed. Eng. 44(12):3522–3538, 2016. https://doi.org/10.1007/s10439-016-1695-2.
Madukauwa-David, I. D., E. L. Pierce, F. Sulejmani, J. Pataky, W. Sun, and A. P. Yoganathan. Suture dehiscence and collagen content in the human mitral and tricuspid annuli. Biomech. Model. Mechanobiol. 2018. https://doi.org/10.1007/s10237-018-1082-z.
Maghami, S., M. Ghoreishi, N. Foster, M. Y. Dawood, G. R. Hobbs, P. Stafford, D. Adawal, I. Mohammed, E. D. Z. van Rilland, X. Y. Diao, M. Walterhoefer, B. S. Taylor, B. P. Griffith, and J. S. Gammie. Undersized rigid nonplanar annuloplasty: the key to effective and durable repair of functional tricuspid regurgitation. Ann. Thorac. Surg. 102(3):735–742, 2016. https://doi.org/10.1016/j.athoracsur.2016.02.084.
Malinowski, M., T. Jaźwiec, M. Goehler, J. Bush, N. Quay, H. Ferguson, M. K. Rausch, and T. A. Timek. Impact of tricuspid annular size reduction on right ventricular function, geometry and strain. Eur. J. Cardio Thorac. Surg. 2019. https://doi.org/10.1093/ejcts/ezy484.
Malinowski, M., H. Schubert, J. Wodarek, H. Ferguson, L. Eberhart, D. Langholz, T. Jazwiec, M. K. Rausch, and T. A. Timek. Tricuspid annular geometry and strain after suture annuloplasty in acute ovine right heart failure. Ann. Thorac. Surg. 106(6):1804–1811, 2018. https://doi.org/10.1016/j.athoracsur.2018.05.057.
Mangieri, A., C. Montalto, M. Pagnesi, R. J. Jabbour, J. Rodés-Cabau, N. Moat, A. Colombo, and A. Latib. Mechanism and implications of the tricuspid regurgitation: from the pathophysiology to the current and future therapeutic options. Circ. Cardiovasc. Interv. 10(7):1–13, 2017. https://doi.org/10.1161/CIRCINTERVENTIONS.117.005043.
Mathur, M., T. Jazwiec, W. D. Meador, M. Malinowski, M. Goehler, H. Ferguson, T. A. Timek, and M. K. Rausch. Tricuspid valve leaflet strains in the beating ovine heart. Biomech. Model. Mechanobiol. 2019. https://doi.org/10.1007/s10237-019-01148-y.
Meador, W. D., M. Malinowski, T. Jazwiec, M. Goehler, N. Quay, T. A. Timek, and M. K. Rausch. A fiduciary marker-based framework to assess heterogeneity and anisotropy of right ventricular epicardial strains in the beating ovine heart. J. Biomech. 80:179–185, 2018. https://doi.org/10.1016/S0263-4368(02)00017-3.
Meador, W. D., M. Mathur, and M. K. Rausch. Tricuspid valve biomechanics: a brief review. In: Advances in heart valve biomechanics, edited by M. Sacks, and J. Liao. Berlin: Springer, 2018, pp. 105–114. https://doi.org/10.1007/978-3-030-01993-8_5.
Messer, S., E. Moseley, M. Marinescu, C. Freeman, M. Goddard, and S. Nair. Histologic analysis of the right atrioventricular junction in the adult human heart. J. Heart Valve Dis. 21(3):368–373, 2012.
Pant, A. D., V. S. Thomas, A. L. Black, T. Verba, J. G. Lesicko, and R. Amini. Pressure-induced microstructural changes in porcine tricuspid valve leaflets. Acta Biomater. 67:248–258, 2017. https://doi.org/10.1016/j.actbio.2017.11.040.
Parolari, A., F. Barili, A. Pilozzi, and D. Pacini. Ring or suture annuloplasty for tricuspid regurgitation? A meta-analysis review. Ann. Thorac. Surg. 98:2255–2263, 2014. https://doi.org/10.1016/j.athoracsur.2014.06.100.
Paul, D. M., A. Naran, E. L. Pierce, C. H. Bloodworth, S. F. Bolling, and A. P. Yoganathan. Suture dehiscence in the tricuspid annulus: an ex vivo analysis of tissue strength and composition. Ann. Thorac. Surg. 104(3):820–826, 2017. https://doi.org/10.1016/j.athoracsur.2017.02.040.
Rausch, M. K., W. Bothe, J. P. E. Kvitting, J. C. Swanson, N. B. Ingels, D. C. Miller, and E. Kuhl. Characterization of mitral valve annular dynamics in the beating heart. Ann. Biomed. Eng. 39(6):1690–1702, 2011. https://doi.org/10.1007/s10439-011-0272-y.
Rausch, M. K., W. Bothe, J. P. E. Kvitting, J. C. Swanson, D. C. Miller, and E. Kuhl. Mitral valve annuloplasty: a quantitative clinical and mechanical comparison of different annuloplasty devices. Ann. Biomed. Eng. 40(3):750–761, 2012. https://doi.org/10.1007/s10439-011-0442-y.
Rausch, M. K., M. Malinowski, W. D. Meador, P. Wilton, A. Khaghani, and T. A. Timek. The effect of acute pulmonary hypertension on tricuspid annular height, strain, and curvature in sheep. Cardiovasc. Eng. Technol. 9(3):365–376, 2018. https://doi.org/10.1007/s13239-018-0367-9.
Rausch, M. K., M. Malinowski, P. Wilton, A. Khaghani, and T. A. Timek. Engineering analysis of tricuspid annular dynamics in the beating ovine heart. Ann. Biomed. Eng. 9(3):365–376, 2017. https://doi.org/10.1007/s10439-017-1961-y.
Rausch, M. K., F. A. Tibayan, N. B. Ingels, D. C. Miller, and E. Kuhl. Mechanics of the mitral annulus in chronic ischemic cardiomyopathy. Ann. Biomed. Eng. 41(10):2171–2180, 2013. https://doi.org/10.1007/s10439-013-0813-7.
Shinn, S. H., V. Dayan, H. V. Schaff, J. A. Dearani, L. D. Joyce, B. Lahr, K. L. Greason, J. M. Stulak, and R. C. Daly. Outcomes of ring versus suture annuloplasty for tricuspid valve repair in patients undergoing mitral valve surgery. J. Thorac. Cardiovasc. Surg. 152(2):406– 415, 2016. https://doi.org/10.1016/j.jtcvs.2016.04.068.
Singh-Gryzbon, S., V. Sadri, M. Toma, E. L. Pierce, Z. A. Wei, and A. P. Yoganathan. Development of a computational method for simulating tricuspid valve dynamics. Ann. Biomed. Eng. 47(6):1422–1434, 2019. https://doi.org/10.1007/s10439-019-02243-y.
Singh-Gryzbon, S., A. W. Siefert, E. L. Pierce, and A. P. Yoganathan. Tricuspid valve annular mechanics: interactions with and implications for transcatheter devices. Cardiovasc. Eng. Technol. 2019. https://doi.org/10.1007/s13239-019-00405-6.
Skwarek, M., J. Hreczecha, J. Jerzemowski, and M. Grzybiak. Microscopic study of right fibrous annulus. Folia Morphol. 68(1):32–35, 2009.
Spinner, E. M., D. Buice, C. H. Yap, and A. P. Yoganathan. The effects of a three-dimensional, saddle-shaped annulus on anterior and posterior leaflet stretch and regurgitation of the tricuspid valve. Ann. Biomed. Eng. 40(5):996–1005, 2012. https://doi.org/10.1007/s10439-011-0471-6.
Spinner, E. M., S. Lerakis, J. Higginson, M. Pernetz, S. Howell, E. Veledar, and A. P. Yoganathan. Correlates of tricuspid regurgitation as determined by 3D echocardiography: pulmonary arterial pressure, ventricle geometry, annular dilatation and papillary muscle displacement. Circ. Cardiovasc. Imaging 5(1):43–50, 2011. https://doi.org/10.1161/CIRCIMAGING.111.965707.
Spinner, E. M., P. Shannon, D. Buice, J. H. Jimenez, E. Veledar, P. J. Del Nido, D. H. Adams, and A. P. Yoganathan. In vitro characterization of the mechanisms responsible for functional tricuspid regurgitation. Circulation 124(8):920–929, 2011. https://doi.org/10.1161/CIRCULATIONAHA.110.003897.
Acknowledgments
We appreciate the support by the internal grant from Meijer Heart and Vascular Institute at Spectrum Health and by the American Heart Association (18CDA34120028). The authors also acknowledge Jeanette Binkowski for the preparation of Fig. 1.
Conflict of interest
None of the authors have conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Arash Kheradvar oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mathur, M., Meador, W.D., Jazwiec, T. et al. The Effect of Downsizing on the Normal Tricuspid Annulus. Ann Biomed Eng 48, 655–668 (2020). https://doi.org/10.1007/s10439-019-02387-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02387-x