Abstract
Deep brain stimulation (DBS) involves the implantation of electrodes into specific central brain structures for the treatment of Parkinson’s disease. Image guidance and robot-assisted techniques have been developed to assist in the accuracy of electrode placement. Traditional DBS is performed with the patient awake and utilizes microelectrode recording for feedback, which yields lengthy operating room times. Asleep DBS procedures use imaging techniques to verify electrode placement. The objective of this study is to demonstrate the validity of an asleep robot-assisted DBS procedure that utilizes intraoperative imaging techniques for precise electrode placement in a large, inclusive cohort. Preoperative magnetic resonance imaging (MRI) was used to plan the surgical procedure for the 128 patients that underwent asleep DBS. During the surgery, robot assistance was used during the implantation of the electrodes. To verify electrode placement, intraoperative CT scans were fused with the preoperative MRIs. The mean radial error of all final electrode placements is 0.85 ± 0.38 mm. MRI-CT fusion error is 0.64 ± 0.40 mm. The average operating room time for bilateral and unilateral implantations are 139.3 ± 34.7 and 115.4 ± 42.1 min, respectively. This study shows the validity of the presented asleep DBS procedure using robot assistance and intraoperative CT verification for accurate electrode placement with shorter operating room times.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Deep brain stimulation (DBS) is a common treatment option for symptoms associated with Parkinson’s Disease (PD) including essential tremor, rigidity, and dystonia.11,12,16,27 DBS involves the implantation of electrodes into specific central brain structures. The electrodes deliver pulsed, high frequency electrical currents that help regulate pathological local synchronous firing patterns of local stimulatory activity. The primary target structures in the treatment of PD are the subthalamic nucleus (STN), globus pallidus interna (GPi), and ventralis intermedius (Vim). While the STN treats most symptoms of PD, the GPI and Vim are targeted to reduce the effects of dystonia and essential tremor, respectively.28
A DBS procedure begins with preoperative planning to determine the target location within the brain and trajectory required to reach that location. Magnetic resonance images (MRI) of the patient are acquired and used to identify the target location. Traditional DBS procedures can be referred to as awake DBS because they involve the patient being under local anesthesia, aware of what is happening in the operating room. DBS was performed awake so that feedback could be obtained in the operating room on the effects of the implanted electrodes from the patients themselves, from microelectrode recordings (MER), or sometimes a combination of both. MER involves incrementally inserting electrodes smaller than the permanent one along the planned trajectory to measure the electrical signals coming from neurons. The electrode is advanced until reaching the target structure, and based upon both individual and local area neuronal firings, the sensorimotor regions along the trajectory can be mapped, which are used to verify that the target location is the optimal placement within the target structure. The use of MER can be associated with longer operating room times that may lead to additional surgical complications and infections,5,10,21 including an increased risk for hemorrhage.13 The average operating room time for an awake DBS procedure ranges from 4 to 6 h.1,6 There are a number of limitations associated with awake DBS. Lengthy procedures are taxing for the patient and operating room staff. The patient can undergo fatigue, and although they can provide feedback, it cannot include full motor function assessment such as standing or walking. There is a proportionally higher economic burden associated with the surgeon, OR staff, and facilities time required for these lengthy procedures.15 Typically, the longer the operating room time, the longer the recovery time, which is challenging for the patient and can create additional costs.
To address some of the concerns surrounding awake DBS, a procedure utilizing general anesthesia, known as asleep DBS has been developed.5 Asleep DBS still involves the preoperative MRI planning, but relies on high-resolution imaging, image guidance, and sometimes robotic-assistance to validate the placement of the electrode within the target location. Imaging and robotic assistance provides the accuracy and precision required to remove the dependency on physiological feedback relied on during awake DBS to determine electrode placement. MER can still be used in asleep DBS procedures to provide electrical-signal feedback, but due to the additional risk and the controversy regarding the efficacy of MER,5,10,13,19 intra-operative imaging techniques are used instead. These techniques involve computed tomography (CT) or MR images being taken during the procedure to verify electrode placement within the anatomical target. If CT is used intraoperatively, it is fused to preoperative MRI because only the MRI can accurately show the grey matter within the brain to view the target structure. The merged MRI-CT scans can then be used to assess the placement of the electrode (captured from the CT) compared to the target location (identified on the MRI). This does introduce a source of error to the procedure, as MRI and CT both have individual errors, along with error involved when merging the two types of scans. MRI has a nonuniform magnetic field generated from the main magnet in the equipment that leads to non-linearities in the gradients generated, which makes straight lines appear curved or distorted at the edge of MRI scans.18 CT scans have a low soft tissue contrast which makes it hard to visualize target structures and any metal in the image can lead to streaking distortion.3 By combining the two imaging modalities, the electrode placement can be properly planned and verified after insertion. The operating room time is drastically shorter for asleep procedures, and is reported to range from 2 to 3.17 h.5,8,24
The primary measure of success for a DBS procedure within the operating room before clinical outcomes can be determined is electrode placement accuracy. This is measured as the radial error between the center of the target location determined preoperatively and the center of the implanted electrode. If the surgeon is concerned about the accuracy of initial placement, the electrode may be re-implanted; however, there is debate as to what constitutes sufficient accuracy. Some studies use the standard of re-implanting the electrode if it is farther than 2 to 3 mm away from the target22 while others state simply 3 mm as the standard for re-implantation.5 Asleep DBS procedures have reported placement accuracies comparable to those of awake DBS,5,23 with the lowest report radial error for asleep DBS being 0.6 ± 0.3 mm.26
To improve the precision of asleep DBS, robots, such as SurgiScope, NeuroMate, Renaissance, and ROSA, are being utilized within the operating room.7 The safety and effectiveness of using a robot for stereotactic neurosurgery has been shown previously.7,14,17,20,29,31 A study of a frame-based DBS procedure using the NeuroMate robot for the implantation of 30 leads reported a Euclidean error of 0.86 ± 0.32 mm measured using orthogonal radiographs in Stereoplan.30 Neudorfer and colleagues found that there were statistically significant improvements for a cohort of 80 patients (40 implanted using each method) in lateral deviation and operating room time when performing robot-assisted DBS compared to conventional frame-based implantation methods.25 The addition of robot-assistance within a DBS asleep procedure has been shown to have the same clinical improvement as awake surgeries when Unified Parkinson’s Disease Rating Scale (UPDRS) motor scores were compared.21 The Mazor Renaissance robot is used in this study, which is an FDA approved system for electrode/implant placement and brain biopsies. It is a small, frameless platform with 360° working volume for highly accurate access to planned trajectories.
The objective of this study is to demonstrate the validity of an asleep robot-assisted DBS procedure that utilizes intraoperative imaging techniques for precise electrode placement in a large, inclusive cohort. Electrode placement accuracy, fusion error associated with intraoperative CT to preoperative MRI, operating room times, and adverse effects are quantified for a cohort of 128 patients with 241 lead placements.
Materials and Methods
Patient Inclusion and Demographics
A total of 128 consecutive patients were included in this study, of which 113 underwent bilateral implantation and 15 unilateral implantation (total 241 lead placements). The target location was the STN in 162 cases, Vim in 42 cases, and GPi in 37 cases. Of the 128 patients, 68 were female, 48 were male, and 12 did not have information recorded. The mean age of the patients was 64.6 ± 13.2 years. All surgeries were performed by the same surgeon (DVS) at Littleton Adventist Hospital in Littleton, CO between August 2014 and October 2017. This study was approved by the Porter Adventist Hospital Institutional Review Board.
Surgical Procedure
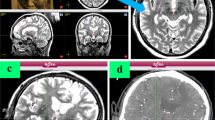
All patients received a preoperative T1 MRI with gadolinium and a T2 MRI using a GE LX, 60-cm bore, 1.5 Tesla MRI under general anesthesia (Fig. 1a) (1 mm slice, matrix 512 × 512, 0.487 × 0.487 mm in-plane resolution). The MRI was calibrated using the American College of Radiology standard phantom tests.2 For the first twelve patients, the MRI and the electrode placement procedure were performed on the same day and under the same anesthetic; however, following a practice change, the MRI and trajectory planning were performed under general anesthesia the day prior to surgery for the remaining patients. On the day of surgery, the patient is positioned using a head clamp (Doro 4002-20, Pro Med Instruments, Freiburg, Germany) customized for use with intraoperative CT, which would not be necessary for an awake DBS procedure. The surgical plan is verified and measurements are taken for the placement of the Renaissance robot (Mazor Robotics, Caesarea, Israel) attachment base. High precision of the robot base in not required as long as the robot is attached on the skull in a location where the robot can reach the target trajectories. The robotic software calculates a series of possible mounting locations. Calipers are used to triangulate from known anatomic landmarks or fiducial markers to the selected base location. A sterile field is then created and a fiducial frame, known as the Star Marker, is attached to the base that allows the planning software to orient the Renaissance system to the patient and intraoperative CT scan (Fig. 2a). The intraoperative CT (2 s. rotation, 120kv, 7 mA, 1.25 mm slice thickness, 0.494 × 0.494 mm in-plane resolution; CereTom™, Neurologica Corp., Danvers, MA.) is obtained in a sterile fashion (Fig. 1b) and then fused with the preoperative MRI (Fig. 1). When the CT is fused to the MRI, the robot base location is known relative to the target trajectories. This intraoperative CT is referred to in this study as the fiducial CT. The MRI-CT fusion process is completed in the Mazor Renaissance software. It involves an initial manual alignment performed by the surgeon, followed by the software registration algorithm completing the six-degree-of-freedom fusion. Once completed, the fusion is visually inspected by the surgeon for success.
(a) Preoperative T2 MRI acquired with a 1.5 Tesla machine under general anesthesia (top), with preoperative cannula trajectory plan for the right STN shown in blue (bottom). (b) Intraoperative CT (including fiducial frame for orientation of the renaissance system). (c) Fusion of MR and CT scans; transparent overlay of T2 MRI and intraoperative CT (top), intraoperative CT with T2 MRI shown within window (bottom). (d) Intraoperative CT with preoperative plan mapped from the fused MRI shown in blue. Sagittal and axial images that pass through the right STN are shown in each instance.
The robotic arm is attached to the base and the arm of the robot commanded to move to the target insertion point (Fig. 2b), where the location is marked and the robot is removed in order to create a sterile incision. After the incision is made, the robot is reattached to locate the site of the planned burr-hole. The robot is removed once again for the actual burring procedure and attached a third time for placement of the to-target cannula. The dura is not opened at this stage of the procedure. An FHC (Bowdoin, ME) ST-DS-MA drive system is attached to the robotic arm for to-target cannula depth measurement. The dura is perforated using monopolar electrocautery. The size of the penetration matches the size of the cannula to prevent cerebral spinal fluid loss and subsequent brain shift. A secondary intraoperative CT is performed with the robot attached and cannula in place; this intraoperative CT is referred to in this study as the verification CT (Fig. 3). In order to verify accurate placement of the cannula, the verification CT is fused with the fiducial CT and the deviation between cannula placement and the preoperative trajectory plan is assessed. Acceptance of the cannula position is based upon the accuracy of the placement, a radial error of less than 2 mm, and a verification that the 1.8 mm diameter cannula is wholly within the target structure, so unwanted stimulation to surrounding structures does not occur, both of which are at the surgeon’s discretion. Any adjustment is made by use of an X–Y stage (Alpha Omega, Nazareth, Israel). For any surgery that requires adjustment of the cannula position, an additional verification CT is performed with the cannula in its final position. If an adjustment needed to be made only to the depth of the cannula for the final electrode placement, it was adjusted accordingly and no additional verification CT was taken. For bilateral surgeries, this process is repeated (Fig. 4).
Electrode Accuracy
Deviation from the intended target is measured when looking down the view of the planned trajectory on the verification CT for a given side (Fig. 5). The electrode placement accuracy is the radial distance between the center of the implanted electrode and the center of the target location (Fig. 6). Errors in depth of the cannula after implantation were also calculated and reported, however, this study focuses primarily on radial errors as errors related to the depth of the cannula measured by the verification CT were subsequently corrected by using the micro-drive system to adjust the depth placement to eliminate this depth error. Unless otherwise stated, the errors reported in this study refer to radial errors. An algorithm was developed in MATLAB 2017b (The Mathworks, Inc., Natick, MA) to automate the electrode placement accuracy measurement process. It utilizes image processing tools to locate the center of the electrode and target. It then quantifies and converts the accuracy to standard units of mm. The development of the automated measurement process eliminates human variance in measurement and bias. A comparison of 27 patients with 53 electrodes implanted measured both manually and using the algorithm shows a statistically significant difference (p = 0.008) between the final placement accuracies of 0.79 ± 0.36 and 0.85 ± 0.35 mm for the manual and automatic measurement systems, respectively. The automated process also saves computational time, which is beneficial when analyzing large cohorts.
Overlay of the preoperative MRI with target trajectory (blue) and verification CT cannula placement (red). Close-up with 5.0 mm reference scale bar shown on the right—this image was used to measure the difference between center of the target site and the center of the implanted cannula. Images are shown looking along the target cannula trajectory.
A source of potential error that adds uncertainty to the accuracy of the electrode placement is the MRI-CT fusion process. To our knowledge, the error involved in fusing the two scans has not previously been quantified. In the operating room, the verification CT scans are fused with the original fiducial CT; the fiducial CT is the only CT which is fused directly with the preoperative MRI. In order to quantify the error associated with the MRI-CT fusion process, in post-operative analysis each verification CT was independently fused with the preoperative T1 MRI. The target location from the MRI was mapped to each CT scan (fiducial plus verification CTs). When the CT scans are compared, the target location appear in slightly different locations in each scan. While it is not possible to determine the exact location of the target with respect to the CT images, the difference between the electrode centers in each CT is the deviation that results from fusing the CT and MRI scans (Fig. 7). The deviation analysis includes all first, second, and third passes for unilateral and bilateral implantations since fusion order does not play a role in calculating the fusion error.
(a) Measurement of MRI-CT fusion error. Preoperative MRI with target cannula trajectory (blue dashed line). (b) Close-up showing apparent location of the center of the cannula from verification CT fused with fiducial CT (red) and apparent location of the center of the cannula from verification CT fused directly with MRI (green). Fusion error is defined as the distance between these locations.
Statistical comparisons between the first and second side implanted and initial and final placement accuracies were quantified using a student’s paired t test. The effect of target location (STN, GPi, Vim) was evaluated used a one-way ANOVA. A p-value below 0.05 was considered statistically significant.
Results
A total of 241 electrodes were implanted, of which 226 were for bilateral implantations, 7 for unilateral right, and 8 for unilateral left. The placement accuracy for all initial passes of the 241 implants is 1.06 ± 0.60 mm. The mean initial pass placement accuracies of the first and second sides implanted are 0.91 ± 0.46 and 1.20 ± 0.65 mm, respectively. There is a significant difference (p < 0.001) between the initial pass placement accuracy of side one and side two. Of the 241 electrodes implanted, 51 were re-implanted a second time (21%) and 3 were re-implanted a third time (1%). Re-implantation was determined based on a variety of factors including a radial error greater than 2 mm, the electrode not being positioned optimally in the target structure either because the cannula is not wholly within the structure or there is a better location possible that could only be observed once the electrode was implanted, or a combination of the aforementioned reasons. A total of 14 electrodes (6%) were re-implanted a second time based upon a radial error greater than 2 mm.
The placement accuracy for all final placements for the 241 implants is 0.85 ± 0.38 mm. There is a statistical significance (p < 0.001) between the total initial and final placement accuracy values. The final placement accuracy for the first and second implanted sides are 0.82 ± 0.36 and 0.87 ± 0.38 mm, respectively, which have no statistical difference. The initial and final placement accuracies based on target location are shown in Table 1. There is no statistical difference between the placement accuracies of the three locations. There is a statistical difference between the initial and final placement accuracies in each location independently: STN (p < 0.001), VIM (p = 0.027), and GPi (p = 0.020).
When the errors in cannula depth along the planned trajectory were calculated, the initial and final placement absolute depth errors were 0.57 ± 0.62 and 0.64 ± 0.62 mm, respectively. In the initial placement, 41% of implants were located at the target depth, 38% were located shallower than the target by 0.98 ± 0.50 mm, and 21% were located deeper than the target by 0.92 ± 0.54 mm. Similar results in depth error were measured from the verification CT after final placement; 34% of implants were located at the target depth, 39% were located shallower than the target by 0.99 ± 0.48 mm, and 27% were located deeper than the target by 0.95 ± 0.54 mm. However, the micro-drive system was subsequently used to adjust the depth placement to eliminate this depth error.
By using all of the implanted electrode fusions, including re-implants, the MRI-CT fusion error was calculated for 292 fusions. The mean deviation is 0.64 ± 0.40 mm. There was no statistical difference in fusion deviation between first and second side implants.
The operating room time, defined as skin-to-skin contact time, for 97 bilateral implantation procedures is 139.3 ± 34.7 min. For 11 unilateral implantations, the operating room time is 115.4 ± 42.1 min. Operating time was not available for the remaining 20 procedures.
Adverse events that were complications of robotic surgery occurred in four (3.1%) patients. One patient had a lead repositioning due to movement in contact position while another had an erosion of a DBS lead extension on a single side. In the operating room, one patient experienced an intraparenchymal hemorrhage that led to symptoms of a stroke which resolved, and a deep vein thrombosis in the left arm. Two weeks postoperatively, one patient had a pulmonary embolism. In all cases, a diagnostic post-operative CT was performed. No significant intraprechymal hemorrhage was present. Complications unrelated to the robotic surgery occurred in two patients who had a DBS pulse generator repositioned within the pocket due to migration, which was causing discomfort. There were no battery infections outside the 2 week period.
Discussion
The application of intraoperative imaging techniques to DBS have been revolutionary in modifying the procedure to where it is today with near real-time electrode placement verification within the operating room. For a traditional awake procedure, the reported average placement accuracy of McClelland and colleagues for a cohort of 26 patients (52 leads) is 1.4 mm in the lateral/medial direction and 1.2 mm in the anterior/posterior direction.22 A recent study utilized the Renaissance Mazor robot and MER for electrode implantation in 20 patients (40 leads), which included both awake and asleep DBS procedures, and measured a radial error of 1.40 ± 0.11 mm.14 The final electrode placement accuracy of the current study is comparable to other reports of asleep DBS procedures; radial errors reported in the literature include 1.24 ± 0.87 mm on a cohort of 60 patients (119 leads),5 0.9 ± 0.5 mm on 48 patients (94 leads) using the NexFrame and intraoperative CT verification,23 and 0.6 ± 0.3 mm on twenty patients (40 leads).26 In the procedure described by Ostrem et al.,26 the surgery is performed entirely within a MRI suite which can be costly and not feasible at all hospitals. The presented surgical procedure has the advantage of using the CereTom portable CT scanner which is available in a standard operating room with lower costs.15
The accuracy of the system being reported is a culmination of numerous factors including the to-target cannula that prevents deviation of the electrode, immobilization of the head during surgery, and the robot being affixed to the skull. It should be also noted that there are numerous other factors that may contribute to the accuracy of electrode placement, apart from the use of a robot-assisted technique. These factors include, amongst others, the experience of the surgeon and surgical team, learning curve associated with the surgical procedure, or different surgical priorities in awake as compared to asleep DBS procedures. The patient being under general anesthesia for the preoperative MRI is also critical to the placement accuracy as even a 1–2 mm shift during image acquisition would become the dominant source of error for the procedure.
The automated measurement algorithm eliminates human bias when determining the electrode primary contact center that may subsequently affect radial error values. Previous studies have measured placement error on the Stealth Station5,22 or using FrameLink software.23,26 One study using the Stealth Station analyzed the interobserver reliability of determining the coordinates of the principal contact on post-operative MRI images and found that there were statistically significant differences in three of eight measured coordinates.22 Alternatively, Mirzadeh et al. found no significant difference in measurements following independent principal contact coordinate selections from two surgeons on post-operative MRIs.23 Although both of these studies analyzed manual coordinate determination on MRIs, CT images also have artifact around the implanted cannula that can make determining the precise center of the cannula difficult. The automated measurement process accounts for the electrode not being perfectly circle and removes human variability that may impact the determination of the circle center for more precise radial error measurements.
The significant difference between the initial placement accuracies of the first and second sides implanted could be caused by CT artifact distortion from the electrode previously implanted on the verification CT check for the second side. This indicates the need to further understand and quantify CT artifact caused by the electrodes.
Previous studies have looked at MRI-CT fusion as it applies to DBS surgery. Mirzadeh et al. fused intraoperative CT with preoperative MRI and target location from the MRI was mapped to the CT.23 Then, postoperative MRI was used to independently identify the target location. The error differences between the target location identified on intraoperative CT and postoperative MRI were quantified, thereby calculating a combination of plan-to-CT fusion error plus MRI measurement variance. Geevarghese et al. measured stereotactic fusion error in a different way.9 They identified the stereotactic coordinate system through fiducial markers on the MRI, and then fused the intraoperative CT with the MRI. Using an unfused version of the same CT scan, they identified the stereotactic coordinate system through fiducial markers on the CT. The error measured in their study is the difference in location of the electrode tip between these two coordinate systems. In the study presented here, two, or more in the case of reimplantation, CT scans were independently fused with preoperative MRI and the target location superimposed on each CT. CT scans were subsequently merged and difference in target location of the target between CT scans was used to quantify a MRI-CT error.
The key aspect of asleep DBS is the use of intraoperative imaging to verify electrode placement location without MER or patient feedback. This can be accomplished using either intraoperative MRI or CT, but regardless of which is used, the fusing of two images together has inherent error. This error is an additional source of variability to the placement accuracy values stated above. The precise location is unknown due to the MRI-CT fusion error, but the deviation analysis allows quantification of this uncertainty across the patient population. This uncertainty metric can be utilized in the operating room to help surgeons determine how close the electrode must be to the target location to be confident that it is actually within the boundary of the target structure (Fig. 8).
Implication of MRI-CT fusion error. To ensure accurate placement, the target (blue) must be wholly within the cannula region (red). When the average MRI-CT fusion deviation (blue dashed) is accounted for, the probability that the placement is not fully with the target region can be calculated (green striped region).
The mean operating room time of this study (2.3 h) is on the lower side of the range of previous asleep DBS studies, which is significantly lower than that of awake DBS procedures. The shorter surgery time is more comfortable for the patient, surgeon, and operating room staff. There is no significant change in OR time from the first cases to the last, which seems to indicate a shorter learning curve for this technique. The low rate of infection of 3.1% compares favorably to 5.6% reported in prior studies,4 and adverse effects postoperatively can in part be attributed to the shorter amount of time spent within the operating room. With the shorter operating room time, a surgeon can more easily fit multiple surgeries in a day. While the necessity of robotic-assistance in asleep DBS requires capital investment in equipment and maintenance costs that add to the overall economic impact of the procedure, these costs are offset by the reduced OR time per surgery—reduced OR time may facilitate increased volume of procedures which reduced the “per surgery” capital costs, which are typically in the range of $60–$100 per minute of OR time. Additionally, a corresponding reduction in infection and adverse effects rates may reduce the hospital stay length for these patients further reducing the overall cost of the procedure.
An advantage to using the Mazor robotic system over other commercially available options is the autoregistration that the system utilizes. Most other frameless systems require the manual registration of fiducial markers, whereby a probe attached to the robot or followed by a 3D camera system is sequentially placed by hand into bone mounted fiducials. The Mazor system embeds the fiducials directly into the Star Marker at fixed positions relative to the robotic attachment base, making the manual registration step unnecessary. This saves OR time and leads to more accuracy in electrode placement.
Although the placement accuracy with this procedure using the Renaissance robot with intraoperative CT verification is comparable to that of awake procedures and other asleep procedures, the relationship between placement accuracy and patient functional outcomes is beyond the scope of the current study. Error in placement accuracy may be compensated for during calibration of electrode voltage and the introduction of directional electrodes; however, optimizing placement accuracy may facilitate minimizing voltage magnitude and localizing the effects of electrical stimulation. An analysis must be performed to evaluate the effect placement accuracy has on the clinical outcomes of the patient, such as UPDRS scores and mobility tests. Additionally, future work should involve determining if there is a correlation between placement accuracy and the stimulation parameters set up postoperatively for the patient.
This study analyzing a cohort of such a large magnitude shows the validity of this asleep DBS procedure that uses the Renaissance robot for precise electrode implantation with the convenience of intraoperative verification CTs using the portable CereTom CT scanner. The workflow of this procedure allows a shorter operating room time that benefits the patient while obtaining the necessary electrode accuracy within the target structure, which can be pinpointed more accurately within the operating room using the now quantified MRI-CT fusion error. Future work will provide the link between electrode placement and clinical efficacy.
References
Abosch, A., L. Timmermann, S. Bartley, H. G. Rietkerk, D. Whiting, P. J. Connolly, D. Lanctin, and M. I. Hariz. An international survey of deep brain stimulation procedural steps. Stereotact. Funct. Neurosurg. 91:1–11, 2013.
American College of Radiology. MR accreditation program testing instructions. Revised 2018. https://www.acraccreditation.org/~/media/ACRAccreditation/Documents/MRI/MRAccreditationTestingInstructions.pdf
Barrett, J. F., and N. Keat. Artifacts in CT: recognition and avoidance. Radiographics. 24:1679–1691, 2012.
Bjerknes, S., I. M. Skogseid, T. Saehle, E. Dietrichs, and M. Toft. Surgical site infections after deep brain stimulation surgery: frequency, characteristics and management in a 10-year period. PLoS ONE 9:e105288, 2014.
Burchiel, K. J., S. McCartney, A. Lee, and A. M. Raslan. Accuracy of deep brain stimulation electrode placement using intraoperative computed tomography without microelectrode recording. J. Neurosurg. 119:301–306, 2013.
Chen, T., Z. Mirzadeh, K. M. Chapple, M. Lambert, H. A. Shill, G. Moguel-Cobos, A. I. Tröster, R. Dhall, and F. A. Ponce. Clinical outcomes following awake and asleep deep brain stimulation for Parkinson disease. J. Neurosurg. 2018. https://doi.org/10.3171/2017.8.JNS17883.
Faria, C., W. Erlhagen, M. Rito, E. de Momi, G. Ferrigno, and E. Bicho. Review of robotic technology for stereotactic neurosurgery. IEEE Rev. Biomed. Eng. 8:125–137, 2015.
Foltynie, T., L. Zrinzo, I. Martinez-Torres, E. Tripoliti, E. Petersen, E. Holl, I. Aviles-Olmos, M. Jahanshahi, M. Hariz, and P. Limousin. MRI-guided STN DBS in Parkinson’s disease without microelectrode recording: efficacy and safety. J. Neurol. Neurosurg. Psychiatry. 82:358–363, 2011.
Geevarghese, R., R. O’Gorman Tuura, D. E. Lumsden, M. Samuel, and K. Ashkan. Registration accuracy of CT/MRI fusion for localization of deep brain stimulation electrode position: an imaging study and systematic review. Stereotact. Funct. Neurosurg. 94:159–163, 2016.
Hariz, M. I. Safety and risk of microelectrode recording in surgery for movement disorders. Stereotact. Funct. Neurosurg. 78:146–157, 2002.
Hariz, G.-M., M. Lindberg, and A. T. Bergenheim. Impact of thalamic deep brain stimulation on disability and health-related quality of life in patients with essential tremor. J. Neurol. Neurosurg. Psychiatry. 72:47–52, 2002.
Herzog, J., J. Volkmann, P. Krack, F. Kopper, M. Pötter, D. Lorenz, M. Steinbach, S. Klebe, W. Hamel, B. Schrader, D. Weinert, D. Müller, H. M. Mehdorn, and G. Deuschl. Two-year follow-up of subthalamic deep brain stimulation in Parkinson’s disease. Mov. Disord. 18:1332–1337, 2003.
Ho, A. L., R. Ali, I. D. Connolly, J. M. Henderson, R. Dhall, S. C. Stein, and C. H. Halpern. Awake versus asleep deep brain stimulation for Parkinson’s disease: a critical comparison and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 89:687–691, 2018.
Ho, A. L., A. V. Pendharkar, R. Brewster, D. L. Martinez, R. A. Jaffe, L. W. Xu, K. J. Miller, and C. H. Halpern. Frameless robot-assisted deep brain stimulation surgery: an initial experience. Oper. Neurosurg. (Hagerstown) 2019. https://doi.org/10.1093/ons/opy395.
Jacob, R. L., J. Geddes, S. McCartney, and K. J. Burchiel. Cost analysis of awake versus asleep deep brain stimulation: a single academic health center experience. J. Neurosurg. 124:1517–1523, 2016.
Jankovic, J. Parkinson’s disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry. 79:368–376, 2008.
Joskowicz, L., R. Sharmir, M. Freiman, M. Shoham, E. Zehavi, F. Umansky, and Y. Shoshan. Image-guided system with miniature robot for precise positioning and targeting in keyhole neurosurgery. Comput. Aided Surg. 11:181–193, 2006.
Khoo, V. S., D. P. Dearnaley, D. J. Finnigan, A. Padhani, S. F. Tanner, and M. O. Leach. Magnetic resonance imaging (MRI): considerations and applications in radiotherapy treatment and planning. Radiother. Oncol. 42:1–15, 1997.
Krack, P., A. Batir, N. Van Blercom, S. Chabardes, V. Fraix, C. Ardouin, A. Koudsie, P. D. Limousin, A. Benazzouz, J. F. LeBas, A.-L. Benabid, and P. Pollak. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N. Engl. J. Med. 349:1925–1934, 2003.
Lefranc, M., C. Capel, A. S. Pruvot-Occean, A. Fichten, C. Desenclos, P. Toussaint, D. Le Gars, and J. Peltier. Frameless robotic stereotactic biopsies: a consecutive series of 100 cases. J. Neurosurg. 122:342–352, 2015.
Lefranc, M., Y. Zouitina, M. Tir, P. Merle, M. Ouendo, J.-M. Constans, O. Godefroy, J. Peltier, and P. Krystkowiak. Asleep robot-assisted surgery for the implantation of subthalamic electrodes provides the same clinical improvement and therapeutic window as awake surgery. World Neurosurg. 106:602–608, 2017.
McClelland, III, S., B. Ford, P. B. Senatus, L. M. Winfield, Y. E. Du, S. L. Pullman, Q. Yu, S. J. Frucht, G. M. McKhann, and R. R. Goodman. Subthalamic stimulation for Parkinson disease: determination of electrode location necessary for clinical efficacy. Neurosurg. Focus. 19:1–12, 2005.
Mirzadeh, Z., K. Chapple, M. Lambert, R. Dhall, and F. A. Ponce. Validation of CT-MRI fusion for intraoperative assessment of stereotactic accuracy in DBS surgery. Mov. Disord. 29:1788–1795, 2014.
Mirzadeh, Z., K. Chapple, M. Lambert, V. G. Evidente, P. Mahant, M. C. Ospina, J. Samanta, G. Moguel-Cobos, N. Salins, A. Lieberman, A. I. Tröster, R. Dhall, and F. A. Ponce. Parkinson’s disease outcomes after intraoperative CT-guided “asleep” deep brain stimulation in the globus pallidus internus. J. Neurosurg. 124:902–907, 2016.
Neudorfer, C., S. Hunsche, M. Hellmich, F. El Majdoub, and M. Maarouf. Comparative study of robot-assisted versus conventional frame-based deep brain stimulation stereotactic neurosurgery. Stereotact. Funct. Neurosurg. 96:327–334, 2018.
Ostrem, J. L., N. Ziman, N. B. Galifianakis, P. A. Starr, M. S. Luciano, M. Katz, C. A. Racine, A. J. Martin, L. C. Markun, and P. S. Larson. Clinical outcomes using ClearPoint interventional MRI for deep brain stimulation lead placement in Parkinson’s disease. J. Neurosurg. 124:908–916, 2016.
Rodriguez-Oroz, M. C., J. A. Obeso, A. E. Lang, J. L. Houeto, P. Pollak, S. Rehncrona, J. Kulisevsky, A. Albanese, J. Volkmann, M. I. Hariz, N. P. Quinn, J. D. Speelman, J. Guridi, I. Zamarbide, A. Gironell, J. Molet, B. Pascual-Sedano, B. Pidoux, A. M. Bonnet, Y. Agid, J. Xie, A. L. Benabid, A. M. Lozano, J. Saint-Cyr, L. Romito, M. F. Contarino, M. Scerrati, V. Fraix, and N. Van Blercom. Bilateral deep brain stimulation in Parkinson’s disease: a multicenter study with 4 years follow-up. Brain 128:2240–2249, 2005.
Schrader, B., W. Hamel, D. Weinert, and H. M. Mehdorn. Documentation of electrode localization. Mov. Disord. 17:S167–174, 2002.
Vadera, S., A. Chan, T. Lo, A. Gill, A. Morenkova, N. M. Phielipp, N. Hermanowicz, and F. P. Hsu. Frameless stereotactic robot-assisted subthalamic nucleus deep brain stimulation: case report. World Neurosurg. 97:762.e11–762.e14, 2015.
von Langsdorff, D., P. Paquis, and D. Fontaine. In vivo measurement of the frame-based application accuracy of the Neuromate neurosurgical robot. J. Neurosurg. 122:191–194, 2015.
Zimmermann, M., R. Krishnan, A. Raabe, and V. Seifert. Robot-assisted navigated neuroendoscopy. Neurosurgery. 51:1446–1452, 2002.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Joel Stitzel oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
VanSickle, D., Volk, V., Freeman, P. et al. Electrode Placement Accuracy in Robot-Assisted Asleep Deep Brain Stimulation. Ann Biomed Eng 47, 1212–1222 (2019). https://doi.org/10.1007/s10439-019-02230-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02230-3